Abstract
A 59-year-old female with history of progressive scleroderma and pulmonary fibrosis was referred for biopsy of a pulmonary nodule that was discovered on computed-tomography (CT) chest surveillance, not present on prior CT- scan. Imaging was suspicious for granuloma, malignancy or aneurysm. CT- Angiography (CTA), performed immediately before the procedure, did not show enhancement of the mass, followed by placement of coaxial-needle into the mass. Suspicion of aneurysm was again raised and repeat CTA demonstrated contrast filling of the aneurysm. With the coaxial-needle in the aneurysm, embolization of the sac was performed using microfibrillar collagen, followed by confirmation of containment and thrombosis with CT.
Keywords: pulmonary artery aneurysm, lung nodule, percutaneous embolization, scleroderma
CASE REPORT
The patient is a 59-year-old woman with scleroderma manifested by severe pulmonary hypertension (105/55 mm Hg) with right heart failure, large pericardial effusion, severe pulmonary fibrosis and Raynaud’s phenomenon requiring 2–3 liters/minute oxygen at home, with saturations ranging from 86–90%. She was being considered for bilateral lung transplant, and on pre-transplant surveillance noncontrast CT scan, she was found to have interval development (from CT chest 6 months prior) of a well-defined nodule in the left lower lobe measuring 22 × 18mm, with compression of adjacent bronchi. The differential at the time included granuloma, malignancy or aneurysm. Biopsy was suggested to only follow a negative CTA.
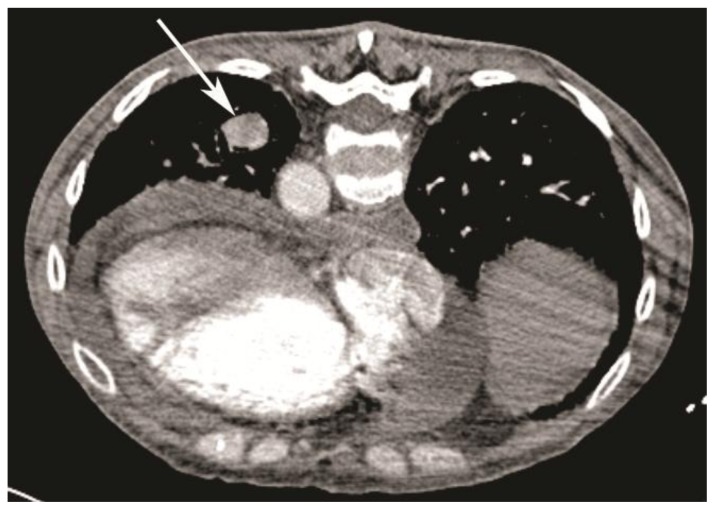
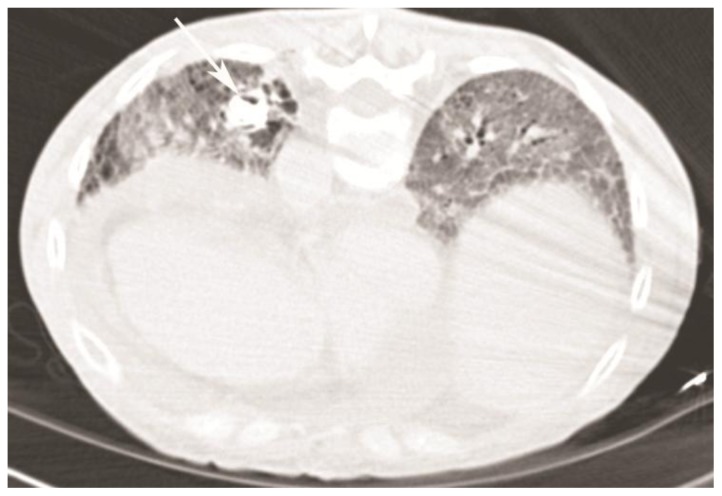
Thereafter, the patient was brought in for percutaneous transthoracic biopsy. CT angiogram performed just before the procedure in order to rule out an aneurysm demonstrated only 10 Hounsfield units (HU) of enhancement and no significant change in size in comparison to the diagnostic scan performed 1 week prior (Fig 1).
Figure 1.
59-year-old woman with scleroderma and a solitary lung nodule, which turned out to be an aneurysm after biopsy. CT angiogram (64-Channel CT scanner. Sensation 64, Siemens Medical Solutions, 120 kV, 286 mAs, 3 mm reformation, 100 c.c. of 300 mg/ml iodine concentration non-ionic contrast) demonstrates fibrosis from scleroderma lung disease, with a 22 × 18mm nodule in left lower lobe (white arrow, patient is in prone position) compressing the adjacent bronchi and showing only 10 HU of enhancement. Also note right ventricular enlargement and large pericardial effusion, secondary to severe pulmonary hypertension.
At this time, with the patient in prone position, a 19-gauge coaxial biopsy needle (AngioTech, Gainesville, FL, USA) was placed via a posterior approach into the center of the left lower lobe nodule using a 64-channel CT scanner (Sensation 64; Siemens Medical Solutions, Malvern, Pa, USA ) for guidance. Two attempts were made to enter the nodule, with the first attempt bouncing off the nodule, and in the second attempt there was an unusual tactile feel as the nodule was entered by the operator. Subsequent CT demonstrated a partially air filled nodule with thick walls (Fig 2). This was followed by injection of 2 cc of air to better delineate the walls. At this point, with the needle at the center of the nodule, the stylet was removed and pulsatile flow of blood was observed from the hub of the needle. Repeat CT angiogram was performed, which demonstrated contrast filling and pooling into the cavity suggestive of a patent aneurysm (Fig 3). At this point, to avoid aneurysmal leak and to induce thrombosis, a mixture of 5 cc of contrast, 2 cc of normal saline and a small amount of microfibrillar collagen (MFC; trade name Avitene) was prepared into a thin slurry. A small amount was gently injected through the needle into the aneurysmal sac. Repeat CT scan demonstrated the high density Avitene mixture within the aneurysmal sac and extending into the feeding vessels and distal and proximal branches (Fig 4). Thereafter the needle was removed, and immediate post biopsy scan showed no evidence of contrast leak or hemorrhage (Fig 5).
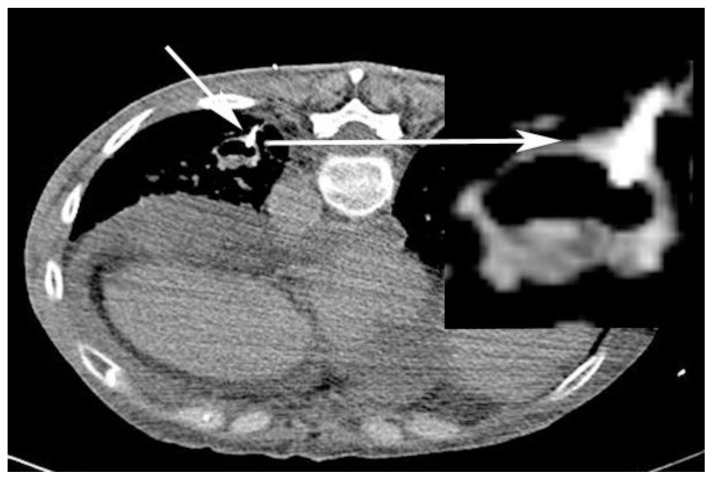
Figure 2.
59-year-old woman with scleroderma and a solitary lung nodule, which turned out to be an aneurysm after biopsy. CT angiogram (64-Channel CT scanner. Sensation 64, Siemens Medical Solutions, 120 kV, 210 mAs, 3 mm reformation, 100 c.c. of 300 mg/ml iodine concentration non-ionic contrast) shows needle tip confirmed in the nodule (short white arrow; magnified view - long white arrow; patient is in prone position). Allowing for the “popping” feel before entering the nodule, no biopsy was performed; But, aspiration was performed which in turn demonstrated partially air filled nodule with thick walls.
Figure 3.
59-year-old woman with scleroderma and a solitary lung nodule, which turned out to be an aneurysm after biopsy. Repeat CT angiogram (64-Channel CT scanner. Sensation 64, Siemens Medical Solutions, 120 kV, 306 mAs, 3 mm reformation, 80 c.c. of 300 mg/ml iodine concentration non-ionic contrast) after injection of 2 cc of air to better delineate the walls (white arrow, patient is on prone position) demonstrating contrast filling and pooling into the cavity suggestive for a patent aneurysm.
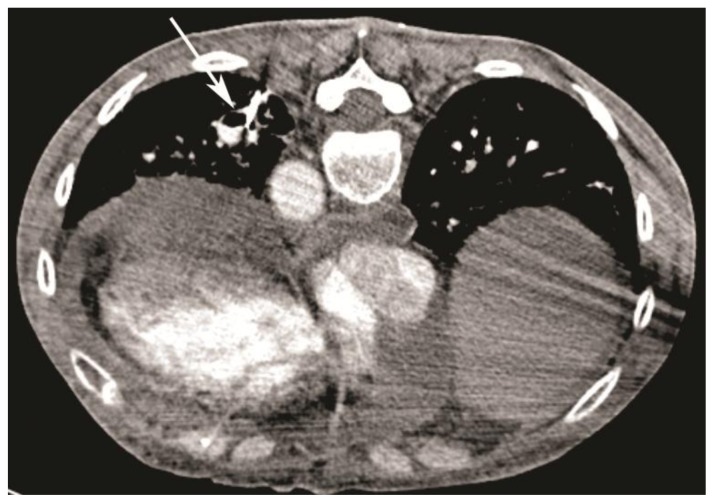
Figure 4.
59-year-old woman with scleroderma and a solitary lung nodule, which turned out to be an aneurysm after biopsy. CT scan (64-Channel CT scanner. Sensation 64, Siemens Medical Solutions, 120 kV, 200 mAs, 3 mm reformation) demonstrating high density Avitene mixture, after injection of the aneurysm, within the aneurysmal sac (white arrow, patient is in prone position) and extending into the feeding vessels and distal and proximal branches.
Figure 5.
59-year-old woman with scleroderma and a solitary lung nodule, which turned out to be an aneurysm after biopsy. Immediate post procedure CT scan (64-Channel CT scanner. Sensation 64, Siemens Medical Solutions, 120 kV, 250 mAs, 3 mm reformation) after removing the needle demonstrates no evidence of hemorrhage in the aneurysmal sac (white arrow, patient is in prone position).
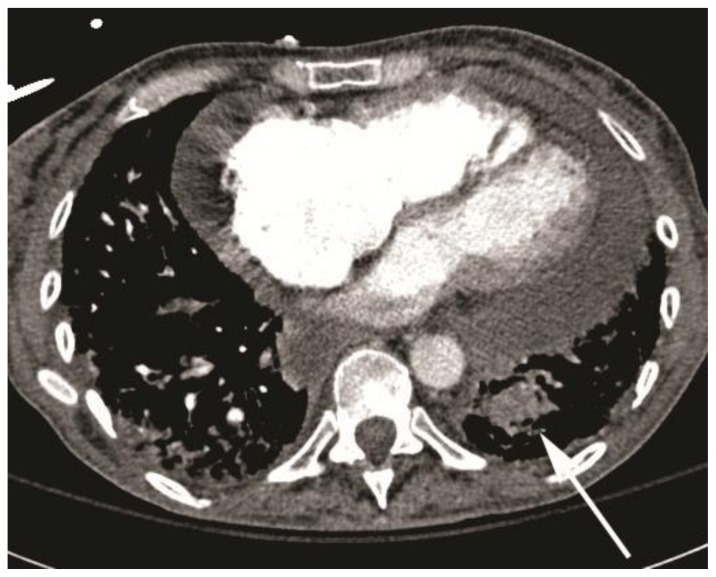
Follow-up CT scan in 4 hours and 4 days showed no evidence for intraluminal contrast extravasation, and continued thrombosis of a posterior subsegmental branch of the left lower lobe pulmonary artery (Fig 6). The patient was put back on transplant list and successfully received a bilateral lung transplant. Explant pathology showed evidence of a thrombosed pulmonary aneurysm.
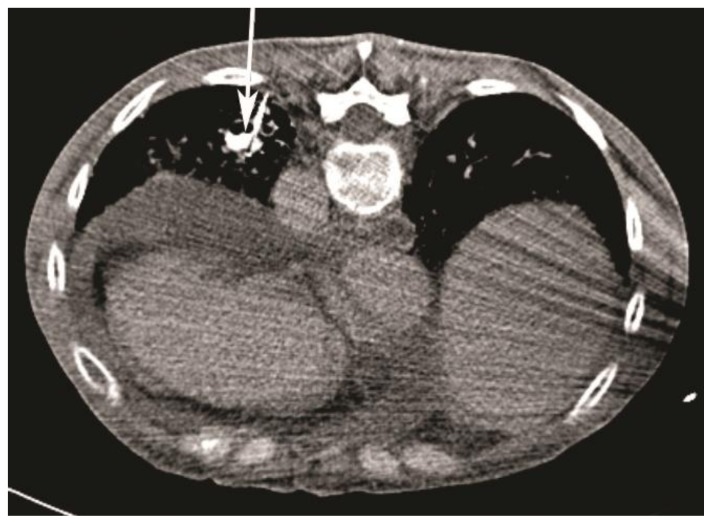
Figure 6.
59-year-old woman with scleroderma and a solitary lung nodule, which turned out to be an aneurysm after biopsy. Follow-up CT angiogram (64-Channel CT scanner. Sensation 64, Siemens Medical Solutions, 120 kV, 250 mAs, 3 mm reformation, 100 c.c. of 300 mg/ml iodine concentration non-ionic contrast) in 4 days showing no evidence for intraluminal contrast extravasation with a thrombosed posterior sub-segmental branch of left lower lobe pulmonary artery (white arrow, patient is in supine position).
DISCUSSION
Scleroderma is an idiopathic, autoimmune multisystem disorder characterized by the abnormal deposition of collagen and matrix proteins within cutaneous and visceral tissues [1]. Pulmonary involvement is now the leading cause of death in scleroderma patients. Patients with involvement of the lung parenchyma are also at an increased risk for pulmonary malignancies, approximately 5 times the rate in the general population [2]. The two main types of clinically significant pulmonary involvement are interstitial lung disease (16–43% of patients) and pulmonary hypertension (PHTN) (12–25% of patients). Many patients develop some degree of both complications. Due to vascular involvement in these patients, up to 60% of patients have PHTN as a late consequence [3]. PHTN is a risk factor for the development of a pulmonary artery aneurysm (PAA) [1]. The incidence of PAA is 1:14000 and the gender ratio is 1:1. Other risk factors and causes for developing PAA are iatrogenic, infections, congenital heart diseases, connective tissue disease, vasculitis, arteriovenous fistula, age above 60, and trauma. Early diagnosis is crucial since the mortality rate for patients with a ruptured PAA is 100% (Table 1). While MRI is less useful in assessing the lung parenchyma because of poorer spatial resolution, in CT we would find saccular or fusiform areas of dilatation, with homogeneous contrast material filling that occurs simultaneously with that of the pulmonary artery. Here we present a case of PAA secondary to pulmonary hypertension in a patient with scleroderma. The PAA, which was initially thrombosed and did not opacify with contrast, and because of that was misdiagnosed with a potential malignant nodule, became patent after the insertion of the needle tip. Although the exact cause for the aneurysm could not be determined, a known history of right heart catheterization and possible over-enthusiastic catheterization of pulmonary artery and pulmonary capillary wedge pressure measurements may have been the culprit. In patients with scleroderma there is evidence of generalized endothelial inflammation, which could predispose for aneurysm formation. Another possible explanation may be microangiopathy of the vasa vasorum causing vascular wall ischemia [4]. In our case the patient had severe pulmonary hypertension, which would have increased her risk for developing PAA.
Table 1.
| Etiology | Iatrogenic Causes Pulmonary hypertension Infections Congenital heart diseases Connective tissue disease Vasculitis Trauma Arteriovenous fistula |
|
| Incidence | 1 in 14000 | |
| Gender ratio | 1:1 | |
| Age predilection | Age older than 60 years | |
| Risk factors | All conditions involving: pulmonary hypertension, degenerative changes of elastic media, long-term steroid use, age older than 60 years | |
| Treatment | Surgical resection (lobectomy) Local resection (aneurysmectomy) Selective transcatheter angioembolization Percutaneous embolization |
|
| Prognosis | Early diagnosis is crucial. The mortality rate for patients with a ruptured pulmonary artery aneurysm is 100%. Clear guidelines for treatment do not exist, but treatment is recommended in the presence of a progressive increase in size. | |
| Findings on imaging of the lung | X-Ray | A pulmonary nodule |
| CT | Saccular or fusiform areas of dilatation, with homogeneous contrast material filling that occurs simultaneously with that of the pulmonary artery. | |
| MRI | MRI is less useful in assessing the lung parenchyma (especially in assessing pulmonary nodules) because of poorer spatial resolution. | |
| PET | Uniform 18F-FDG distribution within the vessel. | |
PAA can also be associated with or without arteriovenous communication. This is clinically relevant in our case as arteriovenous communication would allow systemic delivery of pro-thrombotic agent (in our case microfibrillar collagen) and presenting a high risk for embolic stroke. PAAs with arteriovenous communication are more likely in congenital malformations and not in PAA secondary to pulmonary hypertension [5]; however in our case CTA was also performed to rule out arteriorvenous communication.
Corrective procedures for PAAs have included resection, lobectomy, coil embolization, aneurysmorrhaphy and banding [6]. In our case the patient had severe underlying pulmonary parenchymal disease and pulmonary hypertension which would have made her a poor candidate for surgical intervention. Thus after discussion it was decided thrombotic therapy would be most appropriate to avoid aneurysmal bleeding, and the aneurysm was injected with MFC. MFC is prepared from purified bovine corium collagen. It serves as matrix for fibrin deposition and provides a mechanical and chemical stimulus for platelet activation and aggregation leading to embolus formation [7]. The reason we didn’t coil was at least two folds: (a) we did not have a stable access to the aneurysm, as the needle was held in place only by the aneurysms wall. To deploy the coils we were concerned that it could push the needle out of the aneurysm and we would loose access; (b) The needle was a 19G and the coils fitting through this needle were small and thus many coils were required to be deployed. This in turn would increase the chances of dislodging the needle and loosing access. In our case, once the diagnosis of PAA was made by CTA, the point of entry to aneurysm and the access was not removed. It was thought possible to have continuous leakage of the aneurysm if the needle were removed, particularly given our patient’s pulmonary artery systolic pressures of 105 mmHg. MFC was selected as thrombotic agent because it was readily available, easily mixed and injected through the needle, and once forced into the aneurysm, it tracks back into the feeding vessels to block them.
In conclusion, the most important factor in making the diagnosis of aneurysm is a high degree of suspicion. Although pulmonary artery aneurysm is a rare disorder, it should be considered in the differential of a lung nodule with rapid growth, especially in the setting of pulmonary hypertension.
TEACHING POINT
In the case of diagnostic biopsy of a lung nodule, diagnostic surveillance should be performed to confirm absence of aneurysm before diagnostic biopsy. However, if punctured, measures should be taken to avoid proceeding with biopsy and to embolize the aneurysm with the needle in place.
Table 2.
Differential diagnosis of solitary lung nodule [8–10]. Because there is considerable overlap in the internal characteristics (e.g. attenuation, cavitation, wall thickness) of benign and malignant nodules, the radiologic findings are only suggestive and a tissue sample is required for a definite diagnosis.
| Differential diagnosis | Plain Radiograph | CT | PET |
|---|---|---|---|
| Aneurysm | Hilar enlargement or a lung nodule. | Saccular or fusiform areas of dilatation, with homogeneous contrast material filling that occurs simultaneously with that in the pulmonary artery. | Uniform 18F-FDG distribution within the vessel. |
| Malignancy(pulmonary carcinoma, lymphoma, carcinoid tumor, solitary metastasis) | Malignant nodules typically have irregular, lobulated, or spiculated borders. | The enhancement of malignant neoplasms was significantly greater than that of granulomas and benign neoplasms. | Increased glucose metabolism in tumors results in increased uptake, trapping, and accumulation of FDG, permitting differentiation of benign and malignant nodules. |
| Infection(granuloma, nocardia, abscess, round pneumonia) | An intracavitary protrusion, a replacement of a cavity by a nodule, or a rapidly growing mass. | An enhancing round lung mass with attenuation similar to that of central arteries. | Increased 18F-FDG uptake in a vessel wall occurs in early lesions due to the accelerated localization of inflammatory and immune cells in the vessel wall. |
| Hamartoma | A popcorn pattern of calcification. | Presence of intranodular fat and a popcorn pattern of calcification. | Uptake appears less than or equal to normal lung parenchyma, |
ABBREVIATIONS
- CT
Computed-Tomography
- CTA
CT-Angiography
- HU
Hounsfield Units
- MFC
Microfibrillar Collagen
- PAA
Pulmonary Artery Aneurysm
- PHTN
Pulmonary Hypertension
REFERENCES
- 1.Abraham D, Varga J. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal AK, McLaughlin JK, Gridley G, Nyrén O. Incidence of cancer among patients with systemic sclerosis. Cancer. 1995;76:910. doi: 10.1002/1097-0142(19950901)76:5<910::aid-cncr2820760528>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Battle R, Davitt M. Prevalence of Pulmonary Hypertension in Limited and Diffuse Scleroderma. Chest. 1996;110:1515–19. doi: 10.1378/chest.110.6.1515. [DOI] [PubMed] [Google Scholar]
- 4.Attaran R, Guarrai D. Ascending aortic aneurysm in a man with scleroderma. Clin Rheumatol. 2007;26:1027–8. doi: 10.1007/s10067-006-0267-5. [DOI] [PubMed] [Google Scholar]
- 5.Chung CW, Doherty JU, Kotler R, Finkelstein A, Dresdale A. Pulmonary Artery Aneurysm Presenting as a Lung Mass. Chest. 1995;108:1164–6. doi: 10.1378/chest.108.4.1164. [DOI] [PubMed] [Google Scholar]
- 6.Arnaoutakis G, Nwakanma L, Conte J. Idiopathic pulmonary artery aneurysm treated with surgical correction and concomitant coronary artery bypass grafting. Ann Thorac Surg. 2009;88(1):273–5. doi: 10.1016/j.athoracsur.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251:217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 8.Erasmus Jeremy J, Connolly John E, Page McAdams H, Roggli Victor L. Solitary Pulmonary Nodules: Part I Morphologic Evaluation for Differentiation of Benign and Malignant Lesions. Radiographics. 2000;20:43–58. doi: 10.1148/radiographics.20.1.g00ja0343. [DOI] [PubMed] [Google Scholar]
- 9.Erasmus Jeremy J, Page McAdams H, Connolly John E. Solitary Pulmonary Nodules: Part II. Evaluation of the Indeterminate Nodule. Radiographics. 2000;20:59–66. doi: 10.1148/radiographics.20.1.g00ja0259. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen ET, Silva CI, Seely JM, Chong S, Lee KS, Müller NL. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. Am J Roentgenol. 2007;188(2):W126–34. doi: 10.2214/AJR.05.1652. [DOI] [PubMed] [Google Scholar]