Abstract
Objective
To evaluate autoimmune disease progression in asymptomatic and pauci-symptomatic mothers of children with neonatal lupus (NL).
Methods
Clinical information on mothers enrolled in the Research Registry for NL (RRNL) was obtained from medical records. Genotyping was performed for −308A/G tumour necrosis factor (TNF)α, 869T/C transforming growth factor (TGF)β and −889C/T interleukin (IL)1α.
Results
Of the 321 mothers enrolled, 229 had at least 6 months of follow-up. Of the 51 mothers who were asymptomatic at the NL child’s birth, 26 progressed: 12 developed pauci-undifferentiated autoimmune syndrome (pauci-UAS), 2 poly-UAS, 7 SS, 4 SLE and 1 SLE/SS. The median time to develop any symptom was 3.15 years. Of the 37 mothers classified as pauci-UAS at the NL child’s birth, 16 progressed: 5 developed poly-UAS, 6 Sjögren syndrome (SS), 4 systemic lupus erythematosus (SLE) and 1 SLE/SS. Of the pauci-UAS mothers enrolled within 1 year, the median time to progression was 6.7 years. Four mothers developed lupus nephritis (two asymptomatic, two pauci-UAS). The probability of an asymptomatic mother developing SLE by 10 years was 18.6%, and developing probable/definite SS was 27.9%. NL manifestations did not predict disease progression in an asymptomatic mother. Mothers with anti-Sjögren syndrome A antigen (SSA/)Ro and anti-Sjögren syndrome B antigen (SSB)/La were nearly twice as likely to develop an autoimmune disease as mothers with anti-SSA/Ro only. Only TGFβT/T was significantly higher in SLE mothers compared to asymptomatic mothers (p = 0.03).
Conclusions
Continued follow-up of asymptomatic NL mothers is warranted since nearly half progress, albeit few develop SLE. While the anti-SSB/La antibodies may be a risk factor for progression, further work is needed to determine reliable biomarkers in otherwise healthy women with anti-SSA/Ro antibodies identified solely because of an NL child.
Neonatal lupus (NL) provides a unique opportunity to study disease evolution because asymptomatic mothers are often found to have anti-Sjögren syndrome A antigen (SSA)/Ro and anti-Sjögren syndrome B antigen (SSB)/La antibodies based solely on identification of heart block/rash in a child.1 Thus, a mother may have no clinical manifestations of disease despite the fact that the antibody confers a pathological effect on the fetus. Counselling these families includes discussion regarding risk of another affected pregnancy; data support a 16% to 24% recurrence rate of NL in subsequent pregnancies.1–4 However, maternal health is a relevant concern, as it is increasingly evident that autoantibodies can predate clinical disease by years, although predicting who will progress remains enigmatic. In the US Department of Defense Serum Repository, 115 of 130 patients with systemic lupus erythematosus (SLE) were found to have had at least 1 autoantibody years before diagnosis.5 Relevant to mothers of NL children, in 40% of the military recruits eventually diagnosed with SLE, anti-SSA/Ro and anti-SSB/La anteceded the diagnosis.
This study leveraged the Research Registry for Neonatal Lupus (RRNL)2 to address the extent and timing of maternal disease evolution. Accordingly, emphasis was on evaluating mothers who were asymptomatic or had a paucity of symptoms at enrolment. In addition to questionnaires and review of medical records, antibody profiles, and the frequencies of select polymorphisms in the tumour necrosis factor (TNF)α (−308A/G), interleukin (IL)1α (−889C/T) and transforming growth factor (TGF)β1 (869T/C) genes were analysed. These polymorphisms relate to the production of proinflammatory (TNFα, IL1α) and anti-inflammatory/profibrosing cytokines (TGFβ1) and may be candidates conferring risk for SLE and NL.6–9
PATIENTS AND METHODS
Study subjects
RRNL mothers have antibodies to SSA/Ro and/or SSB/La and a child with NL and are enrolled at the time of identification of NL or years later.2 To acknowledge recall bias, mothers were stratified into two groups based on enrolment time relative to the child’s birth: mothers enrolled within 1 year of the birth of the NL child (group I) and those enrolled after 1 year (group II). For study inclusion, follow-up for at least 6 months was required. All mothers provided informed consent, approved by the Institutional Review Board of New York University (NYU) School of Medicine.
Disease definitions
A mother was classified into one of six categories based on enrolment questionnaire and medical record: asymptomatic, no clinical symptoms of rheumatic illness; probable Sjögren syndrome (SS), at least two of the following: dry eyes, dry mouth or parotid enlargement but no objective evidence of keratoconjunctivitis sicca, xerostomia, or lymphocytic foci on salivary gland biopsy; definite SS, the revised European criteria for SS;10 SLE, four or more revised American College of Rheumatology (ACR) criteria;11,12 SLE/SS (secondary SS), criteria for SLE and at least two of the following: dry eyes, dry mouth, parotid enlargement, objective evidence of keratoconjunctivits sicca, xerostomia, or lymphocytic foci on biopsy. For mothers not classified into one of these categories, undifferentiated autoimmune syndrome (UAS) was assigned. UAS was subdivided. Pauci-UAS, up to two of the following: arthralgias; oral or nasal ulcers; photosensitivity; lymphopenia; Raynaud phenomenon; dry eyes or dry mouth or parotid enlargement (if women presented two of the last three only, they would be considered probable SS). Poly-UAS, more than two of the above symptoms, or serositis or arthritis without meeting the criteria for SLE11,12 or SS.10
Disease progression was considered if a mother developed new symptoms or signs of autoimmunity (eg, new occurrences of dry eyes, dry mouth, photosensitivity, palatal ulcers, pain on deep breathing, joint pain and swelling, Raynaud phenomenon, serositis, kidney disease) suggesting a change in classification. This was monitored with semiannual questionnaires. If a questionnaire indicated new symptoms the mother was contacted by telephone, and doctor notes and laboratory data were obtained.
A mother was considered to be a “congenital heart block (CHB) mother” if she had ever had an affected child with CHB, in isolation or in association with an NL rash based on photographs and/or medical records; and a “rash mother” if she had any child with an NL rash and never had a child with CHB.
Detection of antibodies to SSA/Ro and SSB/La protein
Determination of antibodies to SSA/Ro and SSB/La was performed by the clinical immunology laboratory at the NYU Hospital for Joint Diseases using a commercial ELISA kit (Diamedix, Miami, Florida, USA). Reactivity to the 52-kDa SSA/Ro, 60-kDa SSA/Ro, or 48-kDa SSB/La ribonucleoproteins was determined by ELISA using recombinant proteins and/or sodium dodecyl sulfate (SDS) immunoblot (MOLT4) as described previously.13,14
Genotyping
Available blood specimens were used to obtain DNA which was genotyped for −308A/G TNFα, 869T/C TGFβ and −889C/T IL1α, by allelic restriction fragment length polymorphisms (RFLP) as described previously.6–9 Several mothers were previously genotyped,6,7 but maternal health was not addressed.
Statistical analysis
To consider the information collectively, data from the asymptomatic mothers of group II were combined but controlled for the fact that the mothers in group II enrolled >1 year after the NL child’s birth, resulting in delayed entry into the study and left-truncated data. Disease-free survival based on time since delivery and relative risk estimates were obtained by fitting Cox Proportional Hazards models allowing for left-truncated data using the SAS PROC PHREG software program (SAS, Cary, North Carolina, USA). The analysis of time to progression for pauci-UAS mothers at entry was based only on data from group I because of the inability to accurately estimate time of initiation of pauci-symptoms in group II subjects. These analyses were based on the Kaplan–Meier approach and Cox model. Multivariable Cox models were also fitted to the data to identify risk factors for disease progression for mothers who were asymptomatic at delivery. The following potential risk factors were considered: race (coded dichotomously as Caucasian vs other, due to insufficient sample sizes of other races), age of the mother, incidence of rash and CHB and whether the mother had anti-SSA/Ro in the presence or absence of anti-SSB/La antibodies.
RESULTS
Maternal characteristics at entry
By December 2006, 387 NL children and their 321 anti-SSA/Ro+ mothers were enrolled in the RRNL; 138 mothers enrolled within 1 year of the NL child’s birth (group I) and 103 had follow-up between 6 months and 12 years (tables 1 and 2). In all, 32 were asymptomatic, 20 pauci-UAS, 12 poly-UAS, 12 probable SS, 6 definite SS, 10 SLE and 11 enrolled with SLE/SS. Of the 183 mothers in group II, follow-up data were available for 126 mothers (6 months to 11.5 years), of which 19 were asymptomatic. The mean time from NL child’s birth to enrolment of these mothers was 7 years (range 1 to 29 years).
Table 1.
Mothers by initial diagnosis, and their progression (n=229), group I: mothers enrolled within 1 year of birth of a neonatal lupus (NL)-affected child (n=103)
| Initial diagnosis at enrolment |
n1* | n2† | Diagnosis after follow-up |
n | Mean time to diagnosis |
Mean follow-up (years) |
CHB‡ | Rash1 |
|---|---|---|---|---|---|---|---|---|
| Asymptomatic | 44 | 32 | Asymptomatic | 14 | – | 3.1 | 10 | 4 |
| Pauci-UAS | 11 | 2 | 4.7 | 6 | 5 | |||
| Poly-UAS | 2 | 1 | 1 | 2 | 0 | |||
| Probable SS | 1 | 5 | 7.4 | 1 | 0 | |||
| Definite SS | 2 | 3 | 6.3 | 2 | 0 | |||
| SLE | 2 | 4 | 7.1 | 1 | 1 | |||
| Pauci-UAS | 26 | 20 | Pauci-UAS | 11 | – | 3.3 | 9 | 2 |
| Poly-UAS | 2 | 2 | 5.0 | 0 | 2 | |||
| Probable SS | 3 | 3 | 7.6 | 3 | 0 | |||
| Definite SS | 2 | 2 | 4.3 | 2 | 0 | |||
| SLE | 2 | 2 | 4.8 | 2 | 0 | |||
| Poly-UAS | 17 | 12 | Poly-UAS | 7 | – | 5.4 | 6 | 1 |
| Probable SS | 1 | 3 | 7.3 | 1 | 0 | |||
| SLE | 3 | 4 | 6.4 | 1 | 2 | |||
| SLE/SS | 1 | 4 | 7.5 | 1 | 0 | |||
| Probable SS | 16 | 12 | Probable SS | 9 | – | 3.2 | 8 | 1 |
| Definite SS | 2 | 4 | 4.8 | 0 | 2 | |||
| RA | 1 | 1 | 7 | 1 | 0 | |||
| Definite SS | 6 | 6 | Definite SS | 5 | – | 4.7 | 4 | 1 |
| SLE/SS | 1 | 3 | 9.5 | 0 | 1 | |||
| SLE | 15 | 10 | SLE | 8 | – | 4.9 | 2 | 6¶ |
| SLE/SS | 2 | 6 | 9.2 | 2 | 0 | |||
| SLE/SS | 14 | 11 | SLE/SS | – | 3.1 | 7 | 4 |
The maternal mean age at the birth of the NL child was 30 years in both groups (16–44 years). The ethnic distribution was: 74.1% Caucasian, 9.7% African–American, 8.4% Hispanic, 5.0% Asian and 2.8% unknown/unanswered. Of 229 mothers studied, 171 had at least 1 NL child with CHB and 58 only had an NL rash child.
n1, Total number of mothers who enrolled with specific diagnoses
n2, mothers who were followed for at least 6 months
CHB, mothers who had a child with congenital heart block (CHB), or a child with CHB+rash
rash, mothers who had a child with NL rash only
one mother had a child with liver involvement only but for brevity is included with rash mothers. RA, rheumatoid arthritis
SLE, systemic lupus erythematosus
SS, Sjögren syndrome
UAS, undifferentiated autoimmune syndrome.
Table 2.
Mothers by initial diagnosis, and their progression (n=229), group II: mothers enrolled >1 year after birth of a neonatal lupus (NL)-affected child (n=126)
| Initial diagnosis at enrolment |
n1* | n2† | Diagnosis after follow-up |
n | Mean time to diagnosis |
Mean follow-up (years) |
CHB‡ | Rash§ | Time from birth to latest follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | 29 | 19 | Asymptomatic | 11 | – | 4.9 | 10 | 1 | 9 |
| Pauci-UAS | 1 | 1 | 1.5 | 1 | 0 | 3.2 | |||
| Probable SS | 2 | 4 | 8.1 | 2 | 0 | 11 | |||
| Definite SS | 2 | 3 | 7.5 | 2 | 0 | 10 | |||
| SLE | 2 | 8 | 10.6 | 2 | 0 | 12 | |||
| SLE/SS | 1 | 5 | 10.5 | 1 | 0 | 14 | |||
| Pauci-UAS | 23 | 17 | Pauci-UAS | 10 | – | 4.5 | 8 | 2 | 9 |
| Poly-UAS | 3 | 1 | 4.5 | 3 | 0 | 7 | |||
| Probable SS | 1 | 4 | 5.7 | 1 | 0 | 20.2 | |||
| SLE | 2 | 6 | 9.7 | 2 | 0 | 12 | |||
| SLE/SS | 1 | 2 | 3.8 | 1 | 0 | 10.9 | |||
| Poly-UAS | 12 | 7 | Poly-UAS | 4 | – | 3.6 | 3 | 1 | 13 |
| Probable SS | 1 | 3 | 8.3 | 0 | 1 | 10.2 | |||
| SLE/SS | 2 | 3 | 10.2 | 2 | 0 | 12 | |||
| Probable SS | 35 | 21 | Probable SS | 16 | – | 5.5 | 11 | 5 | 13 |
| Definite SS | 1 | 2 | 7 | 1 | 0 | 12 | |||
| SLE/SS | 4 | 3 | 4.5 | 2 | 2 | 9 | |||
| Definite SS | 13 | 13 | Definite SS | 11 | – | 4.7 | 9 | 2 | 15 |
| SLE/SS | 2 | 7 | 8.1 | 2 | 0 | 12 | |||
| SLE | 32 | 22 | SLE | 22 | – | 6.2 | 16 | 6 | 13 |
| SLE/SS | 39 | 27 | SLE/SS | – | 4.4 | 21 | 6 | 15 |
The maternal mean age at the birth of the NL child was 30 years in both groups (16–44 years). The ethnic distribution was: 74.1% Caucasian, 9.7% African–American, 8.4% Hispanic, 5.0% Asian and 2.8% unknown/unanswered. Of 229 mothers studied, 171 had at least 1 NL child with CHB and 58 only had an NL rash child.
n1, Total number of mothers who enrolled with specific diagnoses
n2, mothers who were followed for at least 6 months
CHB, mothers who had a child with congenital heart block (CHB), or a child with CHB+rash
rash, mothers who had a child with NL rash only. SLE, systemic lupus erythematosus
SS, Sjögren syndrome
UAS, undifferentiated autoimmune syndrome. Extended rep
Maternal disease progression
Asymptomatic mothers
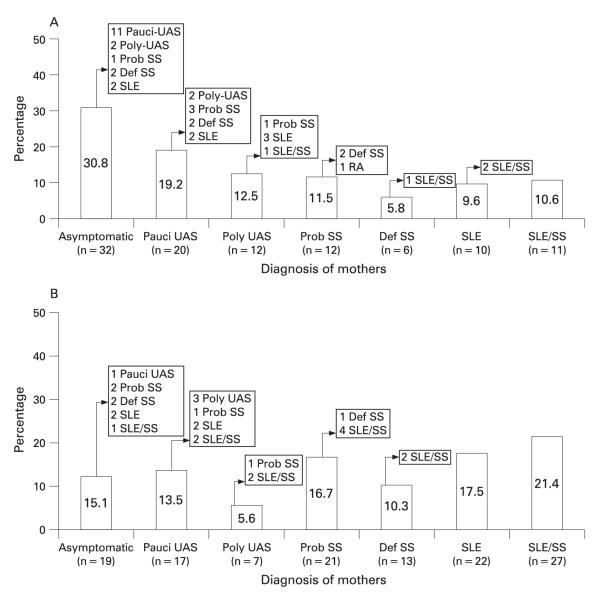
Of the 51 mothers (32 group I, 19 group II) asymptomatic at enrolment, 25 (49%) remained asymptomatic with mean follow-up of 4.1 (0.8) years (range 0.5 to 9) (tables 1 and 2, fig 1). Mothers in group I who remained asymptomatic were followed for a mean time of 3.1 years (range 0.5 to 9), whereas group II was followed for 9 years since birth.3–15 Of these 26 mothers, 12 (46%) developed pauci-UAS after 1.6 (0.6) years (range 0.5 to 6.5). Two (7.7%) developed poly-UAS after 0.9 (0.1) years. Three (11.5%) developed probable SS (4.3 (0.5) years). Four (15.4%) developed definite SS after 2.9 (2) years. Four (15.4%) developed SLE after 5.6 (1.5) years. One (3.8%) developed SLE/SS after 5 years.
Figure 1.
Assignment of rheumatological classification of mothers at enrolment and follow-up (n = 229). A. Group I: mothers enrolled in the Research Registry for Neonatal Lupus (RRNL) within 1 year of birth of affected child (n = 103). B. Group II: mothers enrolled in the RRNL more than 1 year after birth of affected child (n = 126). SLE, systemic lupus erythematosus; SS, Sjögren syndrome; UAS, undifferentiated autoimmune syndrome.
Of the five mothers developing systemic lupus erythematosus (SLE), one had malar rash, serositis, arthritis and was positive for anti-nuclear antibodies (ANA) 6 years after enrolment. A second, who had negative anti-double-stranded (ds)DNA and anti-Sm at entry, developed class IV nephritis 5 years later but remains negative for anti-dsDNA and Sm. A third mother developed a rash and photosensitivity 2.5 years after enrolment; at that time she had anti-dsDNA. A fourth developed photosensitivity, arthritis, class II/III nephritis and anti-dsDNA after 9 years. A fifth mother (systemic lupus erythematosus (SLE)/Sjögren syndrome (SS)) developed sicca symptoms, rash, serositis and arthritis 5 years after entry. At enrolment, she was positive for anti-nuclear antibodies (ANA), but negative for anti-dsDNA and anti-Sm.
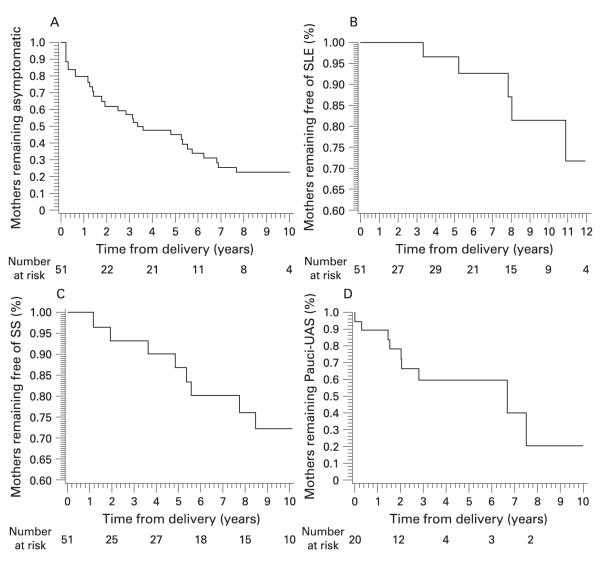
Figure 2A shows the estimated distribution of time from delivery to disease progression (UAS/SS/SLE) for the asymptomatic mothers. The estimated median time to develop any symptom was 3.15 years and the estimated disease-free survival probability at 10 years was 22.3%. Figures 2B,C show the estimated distributions of the time to development of SLE and probable/definite SS, respectively. The probability of an asymptomatic mother developing SLE by 10 years was 18.6% and developing probable/definite SS was 27.9%. The number of subjects at risk shown at different time points may actually increase because of the “delayed entry” into the study of the group II mothers who enrolled a year or more after the child’s birth.
Figure 2.
Time to disease progression for mothers initially asymptomatic or diagnosed with pauci-undifferentiated autoimmune syndrome (pauci-UAS). A. Estimated distribution of time to disease progression (development of any symptom for initially asymptomatic mothers). B. Estimated distribution of time to development of systemic lupus erythematosus (SLE) in initially asymptomatic mothers. C. Estimated distribution of time to development of probable or definite Sjögren syndrome (SS) in initially asymptomatic mothers. D. Kaplan–Meier disease-free survival curve for time to progression in initially pauci-UAS mothers.
Pauci-UAS mothers
Of 37 pauci-UAS mothers at enrolment, 16 (43%) progressed; 5 developed poly-UAS, 4 probable SS, 2 definite SS, 4 SLE and 1 SLE/SS. All five mothers who developed SLE had a history of photosensitivity. One developed class IV nephritis and anti-dsDNA 9 years after enrolment. One developed a malar rash, discoid rash and oral ulcers 2 years after entry/birth. One developed arthritis, oral ulcers, anti-dsDNA and ACL 1 year after entry. One mother had arthritis and Raynaud phenomenon 2 years after entry, and developed oral ulcers and lymphopenia 4 years later. Another mother had photosensitivity, arthralgias, positive anti-dsDNA at entry, and developed a malar rash and nephritis 6 years later.
Poly-UAS mothers
A total of 19 mothers entered with poly-UAS. Two developed probable SS after 2.8 years, and six SLE (three with secondary SS) after 4 years. None developed nephritis.
SS and SLE mothers
Three mothers with definite SS developed SLE after a mean time of 4 years. One had photosensitivity, dry eyes, dry mouth, parotid enlargement and a lip biopsy, and 3.4 years later developed class II nephritis, serositis and oral ulcers. The second mother, after 10 years of sicca symptoms and a positive Schirmer test, developed a deep vein thrombosis, a pulmonary embolism and nephrotic range proteinuria. She had negative anti-dsDNA and anti-Sm at enrolment. The third mother developed arthritis and pleuritis 3 years after enrolment. Of the 70 mothers with SLE, 38 (54%) had secondary SS at enrolment.
NL manifestation and maternal diagnosis
NL manifestations did not predict disease progression in an asymptomatic mother (tables 1 and 2). Combining groups I and II, 40 (23.4%) of the 171 CHB mothers were asymptomatic at enrolment compared to 11 (19%) of the 58 rash mothers, p = 0.6. In group I, 45.5% of the CHB mothers (10/22) and 40% of the rash mothers (4/10) remained asymptomatic at follow-up (mean 3.1 years). In group I, 55 (92%) of the 60 CHB mothers who did not have SLE at enrolment did not progress to SLE during follow-up compared to 18 out of 22 (82%) of rash mothers (p = 0.24).
Reactivity to SSA/Ro-SSB/La and maternal diagnosis
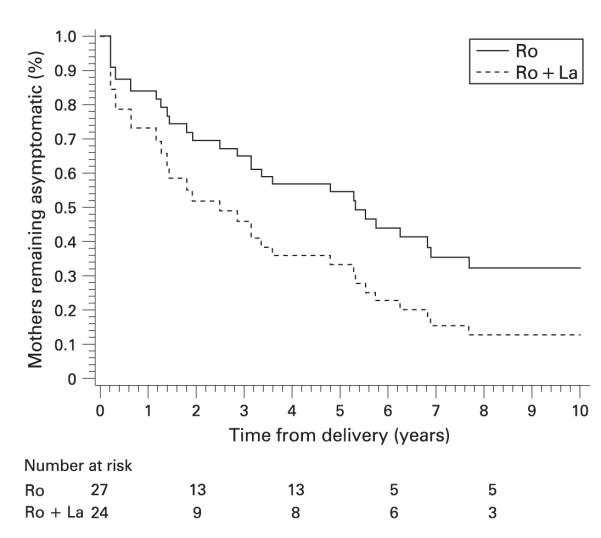
With regard to antibody profiles, 27 of the 51 asymptomatic mothers (53%) had only anti-SSA/Ro, compared to 24 (47%) with anti-SSA/Ro and anti-SSB/La (table 3). Significantly more mothers with only anti-SSA/Ro remained asymptomatic or did not progress beyond pauci-UAS, compared to mothers with both antibodies (24/27 vs 13/24, p = 0.011). Asymptomatic mothers with both antibodies were approximately 1.8 times more likely to develop any rheumatic disease than those with only anti-SSA/Ro (95% CI 0.81 to 4.0; p = 0.14). The estimated median time to progression for mothers with anti-SSA/Ro and SSB/La was 1.9 years compared to 5.3 years for mothers with anti-SSA/Ro alone (fig 3). By 8 years after delivery, the probability of remaining disease-free was 12.8% for women with both antibodies compared to 32.1% for those with only anti-SSA/Ro. In pauci-UAS mothers the same covariates were tested using the Cox model for the asymptomatic women, but none were significant.
Table 3.
Antibody frequencies of initially asymptomatic mothers(n=51)
| Initial diagnosis | Final diagnosis asymptomatic/pauci UAS (%) |
Final diagnosis poly/SS/SLE (%) |
p Value |
|---|---|---|---|
| Asymptomatic (n=51): |
|||
| +Ro/+La (n=24) | 13 (54) | 11 (46) | 0.011 |
| +Ro (n=27) | 24 (89) | 3 (11) |
SLE, systemic lupus erythematosus
SS, Sjögren syndrome
UAS, undifferentiated
autoimmune syndrome.
Figure 3.
Estimates of the distribution of time to disease progression by antibody status.
From the 51 asymptomatic mothers followed for at least 3 years, 27 samples were available from enrolment to determine antibodies to the 52Ro component. The rationale was based on the mean time to progression, which was 3 years. There was no difference in the 52Ro positivity of the mothers who progressed (9/9) compared with those who did not (16/18). The 52Ro component was not a predictor for SLE or SS, since 90% of the 143 mothers analysed were positive.
Genetic polymorphisms and maternal disease
Genotypic analysis for selected polymorphisms of TGFβ, TNFα and IL1α was performed in available samples from Caucasian mothers followed >3 years (table 4). A total of 55 samples were analysed for the 869T/C TGFβ. The T/T genotype was less frequently represented in asymptomatic mothers who remained asymptomatic or progressed to pauci-UAS or SS, compared to those who enrolled with or progressed to SLE (2/14 vs 20/41, p = 0.03). An increased frequency of the −308A allele of TNFα (AA+GA vs GG) was observed in SLE compared to historical controls (77% vs 24%, respectively; p<0.001) (table 4).7 IL1α −889 (C/C) was also significantly higher in SLE compared to historical controls (55% vs 32%, respectively, p = 0.017) (table 4).9 However, TNFα −308A/G and IL1α −889C/T did not distinguish SLE from asymptomatic mothers who remained asymptomatic or progressed to pauci UAS or SS (table 4).
Table 4.
Frequency of genotypes in Research Registry for Neonatal Lupus (RRNL) mothers studied followed for at least 3 years*
| Allelic frequency | p Value† | Genotypic frequency | p Value† | ||||
|---|---|---|---|---|---|---|---|
| IL1a –889C/T (n=43): | |||||||
| Diagnosis | C | T | C/C | C/T | T/T | ||
| Asymptomatic to asymptomatic, pauci-UAS and SS (n=10) |
16 | 4 | 0.75 | 6 | 4 | 0 | 1.0 |
| Initial or progression to SLE (n=33) |
49 | 17 | 18 | 13 | 2 | ||
| TGFβ 869T/C (n=55): | |||||||
| Diagnosis | C | T | C/C | T/C | T/T | ||
| Asymptomatic to asymptomatic, pauci-UAS and SS (n=14) |
16 | 12 | 0.03 | 4 | 8 | 2 | 0.03 |
| Initial or progression to SLE (n=41) |
27 | 55 | 6 | 15 | 20 | ||
| TNFα –308G/A (n=58): | |||||||
| Diagnosis | G | A | G/G | G/A | A/A | ||
| Asymptomatic to asymptomatic, pauci-UAS and SS (n=15) |
20 | 10 | 0.4 | 5 | 10 | 0 | 0.5 |
| Initial or progression to SLE (n=43) |
49 | 37 | 10 | 29 | 4 | ||
The genotype frequencies calculated for interleukin (IL)1α –889C/T, transforming growth factor (TGF)β 869T/C and tumour necrosis factor (TNF)α –308G/A were CC+CT vs TT, CC+TC vs TT, AA+GA vs GG, respectively. In addition, these three polymorphisms were compared to historical controls, there was an increased frequency of the IL1α –889(C/C) in systemic lupus erythematosus (SLE) (32% vs 55%, respectively, p=0.017), TGFβ 869(T/T) in SLE (49% vs 35% to 40%, respectively, p=not significant) and –308A allele of TNFα (AA+GA vs GG) in SLE (24% vs 77%, respectively; p<0.001).6,9,30–32 Comparisons between the groups were performed by Fisher exact test. p<0.05 was considered significant.
Only Caucasian mothers were included in this analysis
p values for comparison of mothers with SLE vs asymptomatic mothers.
SS, Sjögren syndrome, UAS, undifferentiated autoimmune syndrome.
DISCUSSION
Since the inception of the RRNL in 1994, mothers of children with manifestations of NL have been followed yearly.15–18 At enrolment and/or birth of the NL child, many mothers were either asymptomatic or had insufficient criteria for the diagnosis of a classifiable rheumatic disease. The child’s manifestation of NL did not influence the frequency or severity of progression of asymptomatic mothers, although it had been reported that mothers whose children had rash were more likely to progress than those whose children had CHB.19 Despite up to 9 years of follow-up from enrolment and 15 years from an affected birth, mothers do not invariably progress to a rheumatic disease. Half of the enrolled asymptomatic women developed symptoms of an autoimmune disease after a median of 3 years. However, many mothers had <2 symptoms, the most common being photosensitivity and arthralgias. SLE and SS progression were relatively uncommon: 10% and 14%, respectively. Moreover, only two asymptomatic mothers developed nephritis.
The earliest study of 11 asymptomatic NL mothers followed for a mean of 4.5 years reported 8 that developed a rheumatological disease.4 Closely paralleling the RRNL experience, in a Finnish study of 31 mothers, 7 of 15 (47%) initially asymptomatic women remained symptom-free after a mean time of 8 years.20 In another report from Finland, 10 of 23 asymptomatic mothers with CHB remained disease-free after a mean of 9.6 years.21 In another cohort of 64 CHB mothers in Toronto, only 14% of 42 asymptomatic mothers progressed during 10 years of follow-up.22 This very low progression rate is difficult to interpret since only half of the mothers had documented anti-SSA/Ro/SSB/La antibodies.
In accordance with several of the previous studies cited, the presence of anti-SSA/Ro-SSB/La is associated with disease progression. In one limited study, immunoblot analysis of the fine specificity of anti-SSA-Ro/SSB-La reactivity in six asymptomatic NL mothers did not predict the three mothers who developed clinical symptoms.23 Consistent with previous literature,2,24 more than 90% of the mothers in the RRNL had antibodies to 52Ro which did not predict disease progression.
Separate from NL, other investigators have focused on the temporal relationship of antibody detection to more advanced autoimmune disease, Arbuckle et al studied autoantibody profiles in 130 patients with SLE whose sera were obtained 4.4 years before diagnosis.5 The mean interval between the detection of anti-SSA/Ro/SSB/La and diagnosis was 3.7 years. In a study of 87 patients with incomplete lupus erythematosus (ILE), defined as ≥1 but <4 ACR criteria for SLE, 8 progressed to SLE 4.4 years from the onset of the first symptom.25 Given the overall very low prevalence of anti-SSA-Ro antibodies in that report, no inference regarding the risk of anti-SSB/La in disease progression can be made. In the European study of 122 patients with ILE, 25 developed SLE within 3 years of enrolment, 22 in the first year of follow-up.26 The antibody profile was not reported. In 148 Italian patients who were anti-SSA/Ro positive diagnosed with undifferentiated connective tissue disease (UCTD) followed for a mean time of 4.8 years, 24.3% developed a rheumatic disease.27 Anti-SSB/La was detected in 48 and unlike our RRNL study, did not predict disease progression. Another European study followed 28 patients with ILE for 13 years; 16 (57%) developed SLE within 5 years.28 Only two patients with ILE had anti-SSA/Ro antibodies at enrolment, neither progressed to SLE.
While the data presented on genetic polymorphisms are limited, the role of genes in conferring risk for disease progression in patients who have already crossed a threshold in immune imbalance by virtue of established autoantibodies is intriguing. The frequency of the TGFβ genotype, TT, (associated with fibrosis)29 in the asymptomatic mothers was lower than in those with or who progressed to SLE and lower than the observed 35% to 40% frequency in healthy Caucasians.30–33 The TT genotype has been reported to be associated with anti-SSA/Ro antibody production in a Japanese cohort of patients with SLE.34 TGFβ has pleotropic effects with regard to immunomodulation and fibrosis making the net effect of increased or decreased levels of this cytokine difficult to predict. A higher production of TGFβ might be favourable with regard to forestalling SLE given its pivotal role to maintain tolerance via regulation of lymphatic proliferation.35 Thus, the results herein are unexpected. However, the profibrosing potential could promote disease progression in affected children of these mothers, as implicated in CHB pathogenesis.7 Perhaps a decrease in TGFβ conferred by the absence of TT (presence of C) protects the asymptomatic mothers from more advanced organ disease. The association of polymorphisms for TNFα (−308A/G) and IL1α (−889C/T) in SLE9 prompted the study of these cytokines. While there was a significant association with SLE of TNFα −308 A allele and IL1α −889 C/C compared to historical controls, neither was significantly higher in SLE compared to asymptomatic mothers.
Several limitations of the study are acknowledged. At the inception of the RRNL, blood samples were not requested to confirm the presence of the antibodies in cases where positive tests for anti-SSA/Ro-SSB/La were confirmed by a commercial laboratory. As the RRNL matured, blood was requested but not required. While there were semiannual follow-up questionnaires, mothers were not routinely seen by doctors unless symptoms prompted medical visits. When patients developed symptoms, the laboratory results generally reflected antibody testing as part of a rheumatological evaluation. This absence of complete information on antibody testing other than anti-SSA/ Ro-SSB/La is acknowledged as an unavoidable limitation. Albeit this is the largest study to date on follow-up of mothers of children with neonatal lupus, the number of individuals comprising the data for the disease-free survival curve plot and accompanying disease-free survival estimates is limited.
In conclusion, reassuringly, only half of the asymptomatic mothers of NL children progressed, while the majority developed only mild symptoms. Life-threatening diseases such as lupus nephritis were rarely seen. In considering progression of “silent” autoimmunity, identification of a biomarker that predicts the risk of disease progression would be a major advance in family counselling and would be expected to provide insight into the pathogenesis of autoimmune disease. Larger studies are needed to confirm the presence of SSA/Ro/SSB/La antibodies and select TGFβ polymorphisms as risk factors for disease progression.
Acknowledgements
We thank Ann Rupel and Amy Lawless for assistance in editing the manuscript.
Funding: This work was funded by NIH-NIAMS Grant No. NIH, RO1 AR42455-01 (Maternal Autoantibodies: Pathogenesis of Neonatal Lupus) to JPB, NIH Contract NO1-AR-4-2220 (Research Registry for Neonatal Lupus) to JPB, March of Dimes Birth Defects Foundation Grant No. 6-FY05-142 to JPB, AHA Heritage Affiliate Grant No. 0655938T to RMC, and an SLE Lupus Foundation grant to PMI.
Footnotes
Competing interests: None declared.
Ethics approval: Ethics approval was obtained.
REFERENCES
- 1.Izmirly PM, Rivera TL, Buyon JP. Neonatal lupus syndromes. Rheum Dis Clin North Am. 2007;33:267–85. doi: 10.1016/j.rdc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 3.Julkunen H, Eronen M. The rate of recurrence of isolated congenital heart block: a population-based study. Arthritis Rheum. 2001;44:487–8. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.McCune AB, Weston WL, Lee LA. Maternal and fetal outcome in neonatal lupus erythematosus. Ann Intern Med. 1987;106:518–23. doi: 10.7326/0003-4819-106-4-518. [DOI] [PubMed] [Google Scholar]
- 5.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 6.Clancy RM, Backer CB, Yin X, Chang MW, Cohen SR, Lee LA, et al. Genetic association of cutaneous neonatal lupus with HLA class II and tumor necrosis factor alpha: implications for pathogenesis. Arthritis Rheum. 2004;50:2598–603. doi: 10.1002/art.20442. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RM, Backer CB, Kapur RP, Yin X, Molad Y, Buyon JP. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of tumor necrosis factor-α and transforming growth factor-β1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol. 2003;171:3253–61. doi: 10.4049/jimmunol.171.6.3253. [DOI] [PubMed] [Google Scholar]
- 8.Parks CG, Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS, et al. Systemic lupus erythematosus and genetic variation in the interleukin 1 gene cluster: a population based study in the southeastern United States. Ann Rheum Dis. 2004;63:91–4. doi: 10.1136/ard.2003.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parks CG, Pandey JP, Dooley MA, Treadwell EL, St Clair EW, Gilkenson GS, et al. Genetic polymorphisms in tumor necrosis factor (TNF)-α and TNF-β in a population-based study of systemic lupus erythematosus: associations and intereaction with the interleukin-1 α-889 C/T polymorphism. Hum Immunol. 2004;65:622–31. doi: 10.1016/j.humimm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CE, Chan EK, Miranda E, Gross M, Di Donato F, Buyon JP. The 52-kd protein as a target of intermolecular spreading of the immune response to components of the SS-A/Ro-SS-B/La complex. Arthritis Rheum. 1997;40:936–44. doi: 10.1002/art.1780400523. [DOI] [PubMed] [Google Scholar]
- 14.Clancy RM, Buyon JP, Ikeda K, Nozawa K, Argyle DA, Friedman DM, et al. Maternal antibody responses to the 52-kd SSA/Ro p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 15.Neiman AR, Lee LA, Weston WL, Buyon JP. Cutaneous manifestations of neonatal lupus without heart block: characteristics of mothers and children enrolled in a national registry. J Pediatr. 2000;137:674–80. doi: 10.1067/mpd.2000.109108. [DOI] [PubMed] [Google Scholar]
- 16.Waltuck J, Buyon JP. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544–51. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Martin V, Lee LA, Askanase AD, Katholi M, Buyon JP. Long-term followup of children with neonatal lupus and their unaffected siblings. Arthritis Rheum. 2002;46:2377–83. doi: 10.1002/art.10638. [DOI] [PubMed] [Google Scholar]
- 18.Buyon JP. The heart and skin of neonatal lupus: does maternal health matter? Am J Med. 2000;108:741–3. doi: 10.1016/s0002-9343(00)00447-2. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence S, Luy L, Laxer R, Krafchik B, Silverman E. The health of mothers of children with cutaneous neonatal lupus erythematosus differs from that of mothers of children with congenital heart block. Am J Med. 2000;108:705–9. doi: 10.1016/s0002-9343(00)00408-3. [DOI] [PubMed] [Google Scholar]
- 20.Julkunen H, Kurki P, Kaaja R, Heikkila R, Immonen I, Chan EK, et al. Isolated congenital heart block. Long-term outcome of mothers and characterization of the immune response to SS-A/Ro and to SS-B/La. Arthritis Rheum. 1993;36:1588–98. doi: 10.1002/art.1780361114. [DOI] [PubMed] [Google Scholar]
- 21.Julkunen H, Eronen M. Long-term outcome of mothers of children with isolated heart block in Finland. Arthritis Rheum. 2001;44:647–52. doi: 10.1002/1529-0131(200103)44:3<647::AID-ANR113>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Press J, Uziel Y, Laxer RM, Luy L, Hamilton RM, Silverman ED. Long-term outcome of mothers of children with complete congenital heart block. Am J Med. 1996;100:328–32. doi: 10.1016/S0002-9343(97)89492-2. [DOI] [PubMed] [Google Scholar]
- 23.Tseng C, Di Donato F, Buyon JP. Stability of immunoblot profile of anti-SSA/Ro-SSB/La antibodies over time in mothers whose children have neonatal lupus. Lupus. 1996;5:212–5. doi: 10.1177/096120339600500308. [DOI] [PubMed] [Google Scholar]
- 24.Salmonsson S, Dörner T, Theander E, Bremme K, Larsson P, Wahren-Herlenius M. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum. 2002;46:1233–41. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 25.Vilá LM, Mayor AM, Valentín AH, García-Soberal M, Vilá S. Clinical outcome and predictors of disease evolution in patients with incomplete lupus erythematosus. Lupus. 2000;9:110–5. doi: 10.1191/096120300678828073. [DOI] [PubMed] [Google Scholar]
- 26.Swaak AJ, van de Brink H, Smeenk RJ, Manger K, Kalden JR, Tosi S, et al. Incomplete lupus erythematosus: results of a multicenter study under the supervision of the EULAR Standing Committee on International Clinical Studies Including Therapeutic Trials (ESCISIT) Rheumatology (Oxford) 2001;40:89–94. doi: 10.1093/rheumatology/40.1.89. [DOI] [PubMed] [Google Scholar]
- 27.Cavazzana I, Franceschini F, Belfiore N, Quinzanini M, Caporali R, Calzavara-Pinton P, et al. Undifferentiated connective tissue disease with antibodies to Ro/SSa: clinical features and follow-up of 148 patients. Clin Exp Rheumatol. 2001;19:403–9. [PubMed] [Google Scholar]
- 28.Stahl Hallengren C, Nived O, Sturfelt G. Outcome of incomplete systemic lupus erythematosus after 10 years. Lupus. 2004;13:85–8. doi: 10.1191/0961203304lu477oa. [DOI] [PubMed] [Google Scholar]
- 29.Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV. Genotypic variation in the transforming growth factor-β1 gene: association with transforming growth factor-β1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation. 1998;66:1014–20. doi: 10.1097/00007890-199810270-00009. [DOI] [PubMed] [Google Scholar]
- 30.Hinke V, Seck T, Clanget C, Scheidt-Nave C, Ziegler R, Pfeilschifter J. Association of transforming growth factor-β1 (TGFβ1) T29 → C gene polymorphism with bone mineral density (BMD), changes in BMD, and serum concentrations of TGF-β1 in a population-based sample of postmenopausal German women. Calcif Tissue Int. 2001;69:315–20. doi: 10.1007/s002230020024. [DOI] [PubMed] [Google Scholar]
- 31.Hubacek JA, Weichetova M, Bohuslavova R, Skodova Z, Adamkova V, Stepan JJ. Genetic polymorphisms of TGF-β, PAI-1, and COL1A-1, and determination of bone mineral density in Caucasian females. Endocr Regul. 2006;40:77–81. [PubMed] [Google Scholar]
- 32.Brazova J, Sismova K, Vavrova V, Bartosova J, Macek M, Jr, Lauschman H, et al. Polymorphisms of TGF-β1 in cystic fibrosis patients. Clin Immunol. 2006;121:350–7. doi: 10.1016/j.clim.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Ziv E, Kahn A, Cauley J, Morin P, Saiz R, Browner W. No association between the TGF-β 1 Leu10Pro polymorphism and osteoporosis among white women in the United States. Am J Med. 2003;114:227–31. doi: 10.1016/s0002-9343(02)01393-1. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Morinobu A, Kanagawa S, Koshiba M, Hashimoto H, Kumagai S. Transforming growth factor beta 1 gene polymorphism in Japanese patients with systemic lupus erythematosus. Kobe J Med Sci. 2007;53:15–23. [PubMed] [Google Scholar]
- 35.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]