Abstract
Introduction
Carotid body paragangliomas (PGLs) are highly vascularized lesions that arise from the paraganglia located at the carotid bifurcation.
Purpose
To evaluate the usefulness of gray-scale ultrasound (US) and color Doppler ultrasound (CDUS) in the detection and follow-up of carotid PGLs of the neck.
Materials and methods
The authors retrospectively reviewed US and CDUS examinations of the neck performed in 40 patients with PGL syndrome type 1 and single or bilateral neck PGLs confirmed by CT or MRI; the patients had a total of 60 PGLs of the neck. US and CDUS outcome was compared to the outcome of second-line imaging techniques such as magnetic resonance imaging (MRI) or computed tomography (CT). The following findings were considered: presence/absence of focal lesions at US imaging and difference in maximum diameter of the lesion measured at US and MRI/CT. Results were compared using the Student's t-test.
Results
Of the 60 PGLs of the neck only 5 (8.3%) were not visualized at US or CDUS examination. The difference in maximum diameter of these lesions measured at CT/MRI and US/CDUS ranged between −5 mm and +16 mm (mean difference 2.2 ± 6.0). This difference was statistically significant (p = 0.008).
Conclusions
US and CDUS are useful methods for identifying carotid PGLs also measuring less than 10 mm in diameter. However, diagnostic accuracy of US and CDUS is reduced in the measurement of the exact dimensions of the lesions.
Keywords: Carotid body paraganglioma, Carotid-body tumor, Ultrasound, Color Doppler ultrasound
Sommario
Introduzione
I paragangliomi (PGLs) carotidei sono lesioni altamente vascolarizzate che originano dai paragangli localizzati a livello della biforcazione carotidea.
Scopo
Valutare l’utilità dell’ecografia (US) e dell’Eco color Doppler (USD) del collo nella diagnosi e nel follow-up dei PGLs carotidei.
Materiali e metodi
Abbiamo visionato retrospettivamente tutte le US e gli USD del collo, eseguiti nell’Ospedale Santa Chiara di Trento tra il 2007 e il 2011, di soggetti affetti da sindrome paraganglioma di tipo 1 con sicuri paragangliomi del collo singoli o bilaterali. Abbiamo quindi confrontato i risultati con quelli di metodiche di imaging di secondo livello, Risonanza Magnetica (RM) o Tomografia Computerizzata (TC). Sono stati calcolati i casi discordanti in termini di presenza/assenza della lesione focale alle immagini ecografiche e in termini di differenze del diametro maggiore rilevate tra le due metodiche.
Risultati
Sono stati revisionati i dati di imaging (US e/o USD, RM o TC) eseguiti tra il 2007 e il 2011 di 40 pazienti aventi 60 sicuri paragangliomi del collo. Di questi solo 5/60 (8,3%) non sono stati visualizzati mediante US e/o USD. La differenza nella misura del diametro maggiore di tali lesioni rilevate dalle due tecniche di imaging, è risultata compresa tra -5 e +16 mm (media di 2,2 ± 6,0). Tale differenza è risultata statisticamente significativa (p = 0,008) (test-Student).
Conclusioni
L’US e l’USD del collo risultano metodiche di imaging utili nell’identificazione dei PGL carotidei anche in caso di dimensioni inferiori al centimetro. La loro accuratezza diagnostica si riduce se consideriamo le misure esatte delle lesioni.
Introduction
Carotid body paragangliomas (PGLs) are highly vascularized lesions of the parasympathetic nervous system, which derive from the embryonic neural crest cells [1,2]. Numerous terms have been used to identify these rare tumors arising from the paraganglia. One name formerly used was “glomus tumor” which reflected the rich vascularization that characterizes these tumors; another name “chemodectoma” refers to the function as chemoreceptor. Currently the lesion is identified as a “paraganglioma” followed by the anatomical site of origin [3].
Carotid PGLs are the most common PGLs of the head and neck, generally referred to as head and neck PGLs. Their incidence is still unknown due to the rarity of this disease which often remains undiagnosed, but it seems to be approximately 0.012% [4]. The lesions can occur at any age, but onset is most frequently observed between the third and sixth decade of life (mean age 55 years) [5,6]. They are usually non-secreting, benign and slow-growing tumors located in the lateral portion of the neck and they are usually asymptomatic [7].
Symptoms, if any, are usually caused by compression exerted by the mass (compression of the cranial nerves and the sympathetic chain thus resulting in neuropathy or paralysis of the vagus and hypoglossal nerves). A carotid PGL may occur as a painless cervical pulsatile mass with or without dysphagia and dysphonia. The presence of vascular murmur near the mass is rare, but it may be a sign of severe carotid artery compression [8]. Carotid PGLs are hereditary in more than 35% of cases (PGL syndrome) [9]. They are associated with germline mutations in genes encoding subunits of the succinate dehydrogenase (SDH) enzyme complex or with an assembly factor. SDH is part of the Krebs cycle and is also involved in the respiratory chain representing the mitochondrial complex II. PGL syndrome is classified in different subtypes with different clinical features depending on the gene involved:
-
1.
Type 1 (PGL1): SDHD gene mutation [2,10];
-
2.
Type 2 (PGL2): SDHAF2 gene mutation [11];
-
3.
Type 3 (PGL3): SDHC gene mutation [12];
-
4.
Type 4 (PGL4): SDHB gene mutation [13];
-
5.
Type 5 (PGL5): SDHA gene mutation [14].
PGL1 syndrome is associated with pheochromocytoma and with a high prevalence of head and neck PGLs, often bilateral and/or multifocal; PGL3 syndrome is associated with head and neck PGLs and rarely with pheochromocytoma; PGL4 syndrome is particularly associated with pheochromocytoma, often extra-adrenal pheochromocytoma, and with a high risk of malignancy. In the literature PGL2 syndrome has only been associated with multifocal head and neck PGLs, and rare cases of extra-adrenal pheochromocytoma have been reported in connection with PGL5 syndrome.
The syndrome follows an autosomal dominant pattern of transmission, but in PGL1 and PGL2 the phenotype is manifested only in carriers of a paternally inherited mutation. When the mutated maternal allele is transferred to the children, they become asymptomatic carriers thereby suggesting the presence of genomic imprinting [15].
In a population living in the Trentino Region (a geographical area in Italy including the Mocheni valley, Pinè Plateau and Cembra valley) the authors identified a founder effect for SDHD mutation c.341A > G [2]. A population-based study conducted in the Trentino area on a sample of 4000 people living in the valleys and on the plateau showed a prevalence of 1.5% of the mutation in the general population. The penetrance of this founder mutation is particularly high in this area, about 80%, while the phenotype resulted variable with a high prevalence of glomus carotid PGL, but a low prevalence of pheochromocytoma and malignant variants [2,10,16].
The purpose of the present study was to compare US and/or CDUS examinations with magnetic resonance imaging (MRI) or computed tomography (CT) of the neck in the detection of PGL of the neck in a population affected by PGL1 syndrome and a high frequency of PGL of the carotid glomus.
Materials and methods
The authors retrospectively reviewed the outcome of US and CDUS examinations of the neck carried out between 2007 and 2011 in their hospital in 290 patients who were carriers of mutations of the SDHD gene, belonging to 99 families of which 205 with paternal inheritance. A total of 40 patients were included in the study (14 males and 26 females; mean age 52 ± 18 years (range 25–84 years)) with 60 single or bilateral carotid PGLs of the neck confirmed by CT or MRI. All patients were carriers of a paternally inherited SDHD mutation. US and CDUS outcome was compared to the outcome of second-line imaging techniques, MRI or CT. US examinations were performed with the equipment in use in the Department of Radiology and the Outpatient Clinic of Endocrinology of the Santa Chiara Hospital using multifrequency linear probes (10–14 MHz). The examinations were performed in different locations and at different times by different US operators. It was not possible to recheck the dimensions of the lesions as many patients had in the meantime undergone surgery and/or the dimensions of the PGLs had increased during the follow-up.
Analysis of the data
Results of the US and CDUS examinations were retrospectively reviewed and compared to the outcome of second-line imaging techniques, MRI or CT. The following findings were considered: presence/absence of focal lesions at US imaging and difference in maximum diameter of the lesion measured at US imaging and MRI/CT. Statistical analysis was carried out using the Student's t-test; a difference of 5% (p < 0.05) was considered statistically significant. Sensitivity and specificity of US imaging were also calculated.
Results
The 40 patients had a total of 60 lesions (Table 1). At US and CDUS imaging all detected lesions appeared hypoechoic, well-defined, inhomogeneous, hypervascular and located at the carotid bifurcation (Figs. 1 and 2). In all the studied patients (100%) at least one carotid PGL was detected both at first-line imaging (US and/or CDUS) and at second-line imaging (MRI or CT). Of the 60 PGLs detected at CT or MR imaging, only 5/60 (8.3%) were not detected at US and/or CDUS (Table 2).
Table 1.
Characteristics of the examined patients (n = 40).
| Age | Sex | Carotid PGL | US (mm) | CT/MRI (mm) | Δ (mm) | Carotid PGL | US (mm) | CT/MRI (mm) | Δ (mm) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 54 | F | Right | 21 | 17 | −4 | Left | 40 | 40 | |
| Case 2 | 84 | M | Right | 31 | 43 | 12 | Left | 0 | ||
| Case 3 | 68 | M | Right | 0 | Left | 18.6 | 17 | −1.6 | ||
| Case 4 | 37 | M | Right | 25 | 20 | −5 | Left | 13 | 14 | 1 |
| Case 5 | 59 | F | Right | 14 | 25 | 11 | Left | 13 | 13 | |
| Case 6 | 51 | M | Right | 0 | Left | 5 | 5 | 0 | ||
| Case 7 | 26 | F | Right | 15 | 15 | Left | 35 | 30 | −5 | |
| Case 8 | 39 | M | Right | 11 | 11 | 0 | Left | 0 | ||
| Case 9 | 63 | F | Right | 6 | 6 | Left | 29 | 30 | 1 | |
| Case 10 | 54 | F | Right | 45 | 45 | 0 | Left | 17 | 17 | 0 |
| Case 11 | 68 | F | Right | 15 | 10 | −5 | Left | 28 | 40 | 12 |
| Case 12 | 74 | F | Right | 50 | 50 | 0 | Left | 90 | 90 | 0 |
| Case 13 | 63 | M | Right | 0 | Left | 22 | 20 | −2 | ||
| Case 14 | 41 | F | Right | 13 | 13 | 0 | Left | 15 | 15 | 0 |
| Case 15 | 55 | F | Right | 13 | 20 | 7 | Left | 0 | ||
| Case 16 | 80 | F | Right | 19 | 20 | 1 | Left | 35 | 30 | −5 |
| Case 17 | 76 | F | Right | 35 | 50 | 15 | Left | 38 | 45 | 7 |
| Case 18 | 56 | M | Right | 27 | 38 | 11 | Left | 10 | 10 | |
| Case 19 | 29 | F | Right | 14 | 14 | 0 | Left | 25 | 36 | 11 |
| Case 20 | 28 | F | Right | 10 | 10 | 0 | Left | 10 | 7 | −3 |
| Case 21 | 42 | F | Right | 0 | Left | 45 | 60 | 15 | ||
| Case 22 | 78 | F | Right | 5 | 6 | 1 | Left | 16 | 12 | −4 |
| Case 23 | 55 | F | Right | 0 | Left | 26 | 35 | 9 | ||
| Case 24 | 28 | M | Right | 9 | 10 | 1 | Left | 10 | 20 | 10 |
| Case 25 | 62 | M | Right | 28 | 40 | 12 | Left | 24 | 40 | 16 |
| Case 26 | 25 | F | Right | 30 | 30 | 0 | Left | 0 | ||
| Case 27 | 39 | F | Right | 6.8 | 4 | −2.8 | Left | 0 | ||
| Case 28 | 30 | F | Right | 0 | Left | 35 | 40 | 5 | ||
| Case 29 | 63 | F | Right | 15 | 15 | 0 | Left | 0 | ||
| Case 30 | 10 | F | Right | 0 | Left | 11 | 6.5 | −4.5 | ||
| Case 31 | 67 | M | Right | 0 | Left | 30 | 25 | −5 | ||
| Case 32 | 43 | M | Right | 0 | Left | 5 | 5 | 0 | ||
| Case 33 | 41 | F | Right | 15 | 20 | 5 | Left | 0 | ||
| Case 34 | 71 | F | Right | 35 | 30 | −5 | Left | 0 | ||
| Case 35 | 27 | F | Right | 17 | 18 | 1 | Left | 26 | 25 | −1 |
| Case 36 | 64 | F | Right | 15 | 27 | 12 | Left | 0 | ||
| Case 37 | 73 | M | Right | 18 | 21 | 3 | Left | 14 | 14 | 0 |
| Case 38 | 56 | M | Right | 15 | 10 | −5 | Left | 40 | 40 | 0 |
| Case 39 | 70 | F | Right | 8.5 | 10 | 1.5 | Left | 0 | ||
| Case 40 | 66 | F | Right | 37 | 38 | 1 | Left | 0 |
PGL = paraganglioma; US = ultrasound; CT/MRI = computed tomography/magnetic resonance imaging Δ = delta.
Figure 1.
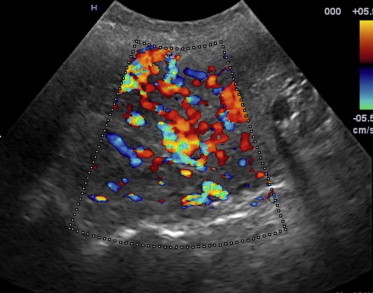
Right-side carotid paraganglioma with a transverse axis diameter of 50 mm; at CDUS the mass appears markedly hypervascular.
Figure 2.
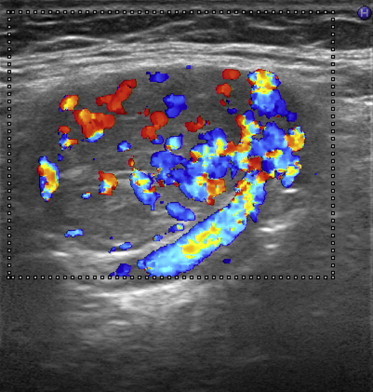
US imaging of a nodular, oval, solid and hypoechoic mass with a transverse axis diameter of 31 mm located near the right carotid bifurcation; at CDUS the mass appears richly vascularized.
Table 2.
Comparison of the number of paragangliomas visualized (+) and not visualized (−) at US/CDUS and CT/MRI.
| US− | US+ | Total PGLs | |
|---|---|---|---|
| CT/MRI+ | 5 | 55 | 60 |
| CT/MRI− | 20 | 0 | 20 |
| Total | 25 | 55 | 80 |
US = ultrasound and/or color Doppler US; PGL = paraganglioma; CT/MRI = computed tomography/magnetic resonance imaging.
In identifying these lesions, US combined with CDUS thus showed a sensitivity of 92% and a specificity of 100%. US imaging furthermore depicted 86% of lesions measuring a maximum diameter of 5–10 mm. However, CT/MRI and US/CDUS results were conflicting in the measurement of the maximum diameter of the lesions. The difference in maximum diameter of the lesions measured at CT/MRI and US/CDUS ranged between −5 mm and +16 mm (mean difference 2.2 ± 6.0) (Table 1). Differences in recorded measurements were analyzed for significance using the Student's t-test and found statistically significant (p = 0.008).
Discussion
The discovery that the PGL 1 syndrome is endemic in a particular area of the Trentino Region allowed the study of a large, homogeneous group of individuals mainly affected by neck paraganglioma. The US/CDUS study of some of the patients of the present series affected by carotid PGLs makes it clear that the most important feature and the first thing to study is the location of the lesion: a PGL of the neck arises at the carotid bifurcation between the external and internal carotid artery widening the bifurcation without infiltrating it (Fig. 3). A PGL appears as a hypoechoic, inhomogeneous, well-defined and highly vascularized mass [18,19]. In the present series some longstanding PGLs appeared highly inhomogeneous (Fig. 3) probably due to the presence of an internal degenerative process, and they were all fed by the external carotid artery (Fig. 4). US/CDUS identified nearly all the PGLs (92%) presented by the study subjects, although measurement of the dimensions of the lesions was not accurate. Although CT and particularly MRI are considered the gold standard in the diagnosis of carotid PGLs [20,21], US and CDUS may be considered useful first-line methods in the identification of these lesions even in masses measuring less than 10 mm in diameter. The present study shows a high sensitivity and specificity of the US methods despite a reduced diagnostic accuracy related to the exact dimensions of the lesions, and it might therefore be appropriate to submit the patients to CT or MRI for a morphological (Fig. 5) and/or functional assessment (111 In-OctreoScan) (Fig. 6) to avoid invasive procedures such as needle aspiration.
Figure 3.
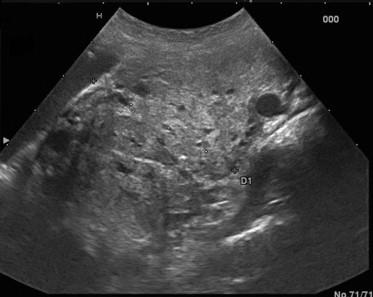
US imaging detects a PGL localized at the right carotid bifurcation between the external and internal carotid artery widening the bifurcation without infiltrating it.
Figure 4.
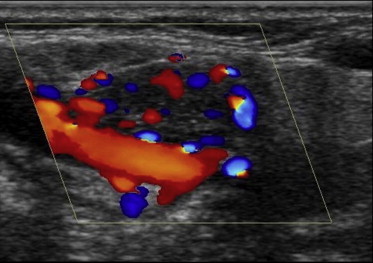
CDUS of a clearly hypervascularised right-side carotid PGL fed by the external carotid artery.
Figure 5.
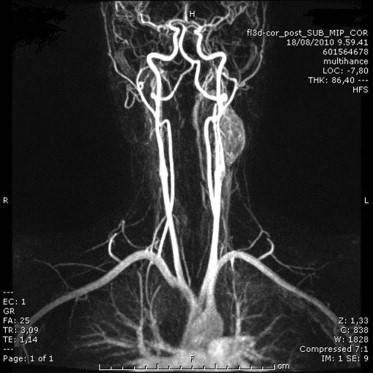
Contrast enhanced MRI reveals a large solid hypervascular mass in the left lateral-cervical region, on the postero-lateral side of the internal carotid artery immediately under the bifurcation; there is intense and moderately inhomogeneous impregnation in the arterial phase; cranio-caudal axis diameter is about 40 mm and axial plane dimensions are 30 × 18 mm.
Figure 6.
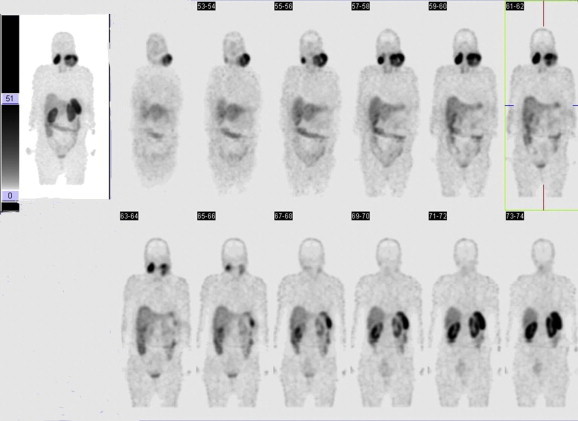
111 Octreotide scintigraphy: total body CT scans 4 and 24 h after somatostatin analog administration show two large areas of intense and inhomogeneous tracer uptake localized bilaterally in the latero-cervical region; the lesion on the left is larger than the one on the right and has a maximum diameter of about 90 mm.
However, in the follow-up, US and CDUS can be particularly valid first-line tools, thereby reserving second-line techniques such as MRI and CT for selected cases, where it is important to know the accurate dimensions of the lesions. In general, the combination of these morphological imaging methods provides sufficient information about the nature and extension of the lesion allowing a correct diagnosis of neck PGL [17] without submitting the patient to cytologic analysis which does often not provide additional information, may lead to vascular complications and diagnostic errors [22]. The role of contrast enhanced US (CEUS) in the initial diagnosis and classification of malignant masses requires further testing [23].
Conflict of interests
The authors have no conflict of interest to disclose.
Appendix A. Supplementary material
The following is the Supplementary data related to this article:
References
- 1.Najibi S., Terramani T.T., Brinkman W., Thourani V.H., Smith R.B., 3rdm, Lumsden A.B. Carotid body tumors. J Am Coll Surg. 2002;194:538–539. doi: 10.1016/s1072-7515(02)01131-6. [DOI] [PubMed] [Google Scholar]
- 2.Schiavi F., Demattè S., Cecchini M.E., Taschin E., Bobisse S., Del Piano A. The endemic paraganglioma syndrome type 1: origin, spread, and clinical expression. J Clin Endocrinol Metab. 2012;97(4):E637–E641. doi: 10.1210/jc.2011-2597. [DOI] [PubMed] [Google Scholar]
- 3.Martin T.P. What we call them: the nomenclature of head and neck paragangliomas. Clin Otolaryngol. 2006;31:185–186. doi: 10.1111/j.1365-2273.2006.01214.x. [DOI] [PubMed] [Google Scholar]
- 4.Grotemeyer D., Loghmanieh S.M., Pourhassan S., Sagban T.A., Iskandar F., Reinecke P. Dignity of carotid body tumors. Review of the literature and clinical experiences. Chirurg. 2009;80:854–863. doi: 10.1007/s00104-009-1724-x. [DOI] [PubMed] [Google Scholar]
- 5.Lack E.E., Cubilla A.L., Woodruff J.M., Farr H.W. Paragangliomas of the head and neck region. A clinical study of 69 patients. Cancer. 1977;39:397–409. doi: 10.1002/1097-0142(197702)39:2<397::aid-cncr2820390205>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Sajid M.S., Hamilton G., Baker D.M. A multicenter review of carotid body tumour management. Joint vascular research group. Eur J Vasc Endovasc Surg. 2007;34:127–130. doi: 10.1016/j.ejvs.2007.01.015. Epub 2007 Apr 2. [DOI] [PubMed] [Google Scholar]
- 7.Myssiorek D., Ferlito A., Silver C.E., Rodrigo J.P., Baysal B.E., Fagan J.J. Screening for familial paragangliomas. Oral Oncol. 2008;44:532–537. doi: 10.1016/j.oraloncology.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Batsakis J.G. 2nd ed. Williams & Wilkins; Baltimore, MD: 1979. Tumors of the head and neck. Clinical and pathological considerations. [Google Scholar]
- 9.Raygada M., Pasini B., Stratakis C.A. Hereditary paragangliomas. Adv Otorhinolaryngol. 2011;70:99–106. doi: 10.1159/000322484. Epub 2011 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baysal B.E., Ferrell R.E., Willett-Brozick J.E., Lawrence E.C., Myssiorek D., Bosch A. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 11.Hao H.X., Khalimonchuk O., Schraders M., Dephoure N., Bayley J.P., Kunst H. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. Epub 2009 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemann S., Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 13.Astuti D., Latif F., Dallol A., Dahia P.L., Douglas F., George E. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. Epub 2001 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnichon N., Brière J.J., Libé R., Vescovo L., Rivière J., Tissier F. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benn D.E., Robinson B.G. Genetic basis of phaeochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2006 Sep;20(3):435–450. doi: 10.1016/j.beem.2006.07.005. [Review] [DOI] [PubMed] [Google Scholar]
- 16.Opocher G., Boaretto F., Pignataro V., Demattè S., Cecchini M.E., Erlic Z. The pheocromocytoma and paraganglioma syndrome: founder effects and PGL 1 syndrome. Ann Endocrinol. 2009;70:157–160. doi: 10.1016/j.ando.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berg R. Imaging and management of head and neck paragangliomas. Eur Radiol. 2005;15:1310–1318. doi: 10.1007/s00330-005-2743-8. [DOI] [PubMed] [Google Scholar]
- 18.Stoeckli S.J., Schuknecht B., Alkadhi H., Fish U. Evaluation of paragangliomas presenting as a cervical mass on color-coded Doppler sonography. Laryngoscope. 2002;112:143–146. doi: 10.1097/00005537-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Giannoni M.F., Irace L., Vicenzini E., Massa R., Gossetti B., Benedetti-Valentini F. Carotid body tumors: advantages of contrast ultrasound investigation. J Neuroimaging. 2009;19:388–390. doi: 10.1111/j.1552-6569.2008.00323.x. Epub 2008 Oct 21. [DOI] [PubMed] [Google Scholar]
- 20.Casagranda G., Demattè S., Donner D., Sammartano S., Rozzanigo U., Peterlongo P. Paragangliomas in an endemic area: from genetics to morphofunctional imaging. A pictorial essay. Radiol Med. Feb 2011 doi: 10.1007/s11547-011-0739-9. [DOI] [PubMed] [Google Scholar]
- 21.Rao A.B., Koeller K.K., Adair C.F. From the archives of the AFIP. Paragangliomas of the head and neck: radiologic-pathologic correlation. Armed forces institute of pathology paraganglioma of head and neck: radiologic-pathologic correlation. Radiographics. 1999;19:1605–1632. doi: 10.1148/radiographics.19.6.g99no251605. [DOI] [PubMed] [Google Scholar]
- 22.Camci C., Sari R., Buyukberber S., Kutlu R., Sevinc A., Cokkeser Y. Non-invasive imaging methods before fine-needle aspiration in the diagnosis of cervical masses. Int J Clin Pract. 2002;56:147–148. [PubMed] [Google Scholar]
- 23.Friedrich-Rust M., Glasemann T., Polta A., Eichler K., Holzer K., Kriener S. Differentiation between benign and malignant adrenal mass using contrast-enhanced ultrasound. Ultraschall Med. 2011;32:460–471. doi: 10.1055/s-0031-1273408. Epub 2011 Jun 10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


