Abstract
The scope of this study was to determine whether contrast-enhanced ultrasonography (CEUS), compared with basic US, can increase diagnostic confidence and provide relevant information on blunt scrotal trauma. Over a period of 75 months we examined 40 patients seen consecutively for blunt scrotal trauma using high-resolution US, color-power Doppler, low mechanical index CEUS, and power Doppler after IV administration of contrast medium (SonoVue®). In the 24 cases that were positive, concordance between basal US and CEUS findings was grade 0 (absent) in 4 cases, grade 1 (low) in 3, grade 2 (moderate) in 8, and grade 3 (high) in 9. The relevance of the additional information provided by CEUS was classified as follows: high in 4/40 (10%), moderate 7/40 (17,5%), low 13/40 (32,5%), none in 14/40 (35%). Our findings demonstrate that CEUS is appreciably more sensitive in detecting damage caused by blunt scrotal trauma, particularly small lesions. It is also useful for differential diagnosis and marginalization of corpuscular fluid collections, fractures, and above all ruptures, which require immediate surgery. In our series 2 out of 3 (67%) patients with testicular rupture were diagnosed only by CEUS. We feel that the use of CEUS can significantly improve diagnostic confidence in cases of closed scrotal trauma although these conclusions need to be confirmed in larger case series.
Keywords: Sonography, Contrast media, Acute scrotum, Contrast-enhanced sonography, Scrotal trauma
Sommario
Scopo del nostro lavoro è stato di valutare l’eventuale maggiore confidenza diagnostica e contenuto informativo dell’ecografia con mezzo di contrasto e.v. (CEUS) nel trauma scrotale chiuso rispetto all’indagine ecografica (US) di base. Nell’arco di 75 mesi abbiamo esaminato 40 pazienti consecutivi con trauma scrotale chiuso, utilizzando US ad alta risoluzione, color-power-Doppler basale, ecocontrastografia a basso indice meccanico, power Doppler dopo mdc e.v. Il mdc usato è stato il SonoVue®. Nei 24 casi positivi, la concordanza tra US basale e CEUS è stata di grado 0 (assente) in 4 casi, di grado 1 (bassa) in 3, di grado 2 (medio) in 8, di grado 3 (elevato) in 9. La rilevanza del contenuto informativo aggiuntivo della CEUS veniva ritenuta: elevata in 4/40 (10%), media 7/40 (17,5%), bassa 13/40 (32,5%), assente in 14/40 (35%). I nostri risultati mostrano che la CEUS migliora sensibilmente la detezione dei segni di trauma rispetto l’US basale, specialmente nelle piccole lesioni. Essa è inoltre importante nella diagnosi differenziale e marginalizzazione delle raccolte fluide corpuscolate, nelle fratture e, di grande evidenza, nelle rotture, che impongono l’immediato intervento chirurgico: nella nostra casistica 2 casi su 3 di rotture (67%) si sono resi evidenti soltanto alla CEUS. Riteniamo che l’uso della CEUS possa aumentare significativamente la confidenza nella diagnostica del trauma scrotale chiuso, ma sono necessarie conferme da casistiche più sostanziose.
Introduction
Ultrasonography with color Doppler is the imaging modality most widely used to study traumatic and nontraumatic pathology of the scrotum [1]. Traumatic causes include blunt (or closed) trauma (related to sports injuries in 50% of all cases and to automobile accidents in 9–17%)—the most common—followed by penetrating injuries and rare forms like electrical or thermal injuries. In general, the right testis is injured more frequently, because it is usually larger and also because it also lies cranial to the left testis and therefore more likely to be trapped against the pubis or inner thigh [2]. Testicular trauma can produce contusions/hematomas, hematoceles, lacerocontusions/fractures, and even testicular rupture. Sonographic diagnosis of testicular rupture is based on the presence of tunica albuginea discontinuity, which generally results in hemorrhage and extrusion of the testicular contents into the scrotal sac. If surgery is performed promptly, the testis can be saved in 80–90% of all cases [3–5]. Rapid, accurate diagnosis is fundamental for treatment planning and preservation of the organ. Diagnostic delays or errors can delay orchiectomy, increasing the risk of reduced fertility, infections, ischemia, infarction, and even atrophy [5–7].
Contrast-enhanced ultrasonography (CEUS) is currently considered a reliable imaging technique for the study of tissue perfusion, and its use in the diagnosis of lesions involving various organs has been standardized by European practice guidelines [8]. There are very few studies on the use of CEUS for the assessment of testicular perfusion. Many of those that have been published involve experiments performed on animals, which indicate that the method offers clear advantages in the detection of ischemia (focal or diffuse) [9–18]. Current knowledge on the use of CEUS in the assessment of scrotal trauma is based on a few anecdotal reports [14,19,20].
The aim of the present study (the preliminary results of which were reported in 2008 [21]) was to evaluate the possible diagnostic gains associated with systematic use of CEUS in patients who have suffered blunt trauma to the scrotum.
Materials and methods
The study was approved by the local ethics committee, and all patients undergoing investigation provided written informed consent. When patients were under 18 years of age, the radiologist informed the patient and his parents that SonoVue has not been approved for use in this age group.
Over 72 months (January 2004–March 2010), we examined the scrotal contents of 40 patients (age range: 14–59 years, mean 29 years) seen consecutively for closed scrotal trauma. Examinations consisted of high-resolution US, color-power Doppler at baseline, low-mechanical index CEUS, and contrast-enhanced power Doppler. Patients were studied in the supine position with a rolled towel between their legs to support the scrotum and the penis displaced superiorly or superolaterally and immobilized with another towel.
Examinations were performed with an Esatune scanner and (later in the study) with a Technos MPX (both from Esaote, Genoa, Italy), both equipped with Contrast Tuned Imaging (CnTI) software. The scans were done with a linear-array transducer (4–13 MHz - LA 532) with a derated pressure of 20–30 Kpa. (The latter setting could be modified by the examiner independently of the depth of the beam’s focal point). Scans were made in the transverse and sagittal planes (and other planes if necessary) with appropriate adjustment of focus, depth, and gain settings. Additional scans were done with a convex 3.5 MHz probe (CA 430) with derated pressure of 40–50 KPa and a mechanical index of 0.06–0.08 (depending on the focal point) in cases with extensive funiculoscrotal damage and when the assessment was extended to include the abdominal organs in patients with multiple trauma (depending on the clinical conditions of the patient and in agreement with the Emergency Department physician caring for the patient). The testes were examined at baseline with side-by-side images on the monitor to compare their size, echotexture, and vascular features on color-power Doppler. The examination was then extended to the epididymidal and funicular structures, which were examined along their long axes. The acoustic contrast agent used was SonoVue® (Bracco, Milan, Italy), an aqueous solution of microbubbles consisting of sulfur hexafluoride enclosed in elastic phospholipid shells. The agent was administered as a rapid IV bolus (2.4–4.8 mL), and the line was flushed with 5–10 mL of normal saline. Images were acquired in real time for 3–5 min, with occasional flashes at a high MI to promote recirculation of the contrast medium. The affected testis was scanned longitudinally, transversely, and obliquely in real time, and findings were compared with those of the contralateral gonad when indicated. Care was taken to exert minimal pressure on the testicles to avoid provoking mechanical reductions in perfusion. When indicated, we also examined the upper abdominal organs during the later stages of CEUS (after the first 3 min—or later—through the seventh minute after the Sonovue injection).
Video recordings of the examinations were edited to eliminate nonessential phases and transferred to a personal computer in .AVI format. The recordings were later examined by three operators who had not been involved in the examinations themselves (O.C., C.C. and A.R.), and findings were interpreted by consensus opinion.
The degree of concordance between the baseline sonogram and the CEUS examination was rated with an arbitrary scoring system in which 0 indicated lack of concordance and scores of 1, 2, and 3 reflected low, moderate, and high concordance, respectively. Next, the relevance of the additional information obtained with CEUS was rated none (no additional information); low (additional information obtained that was not important for diagnosis or treatment); moderate (additional information that was somewhat relevant but not enough to radically modify case management); or high (relevant additional information that radically modified management). Rupture was diagnosed when, in addition to possible US/CEUS evidence of intratesticular alterations involving the echostructure or perfusion, there was damage extending to the surface of the gonad, all the way to the tunica albuginea. When the scan revealed hypoechoic/hypoperfused bands with more or less well-defined margins that involved the entire testis, from one margin to the other, without discontinuity of the tunica albuginea, the diagnosis of testicular fracture was made. The presence of a hypoechoic/hypoperfused area or band that was located within a larger area of hyperechogenicity and did not extend to the borders of the testis was defined as a lacerocontusion and classified as <50% or >50%.
The definitive diagnosis was based on surgical findings (when surgery was necessary) and on follow-up findings (clinical, sonography, CEUS) in the other cases.
Results
In all of the cases we examined, CEUS was completed with no adverse effects, and the results were regarded as qualitatively acceptable for inclusion in the study. Sixteen of the 40 patients were classified as “true negatives” on the basis of the basal US examination and CEUS (grade 3 concordance). The final diagnoses for the other 24 patients were as follows: testicular rupture reflected by interruption of the tunica albuginea (n = 3); testicular fracture (n = 3); lacerocontusion >50% (n = 3); lacerocontusion <50% (n = 4); total testicular ischemia secondary to funicular trauma (n = 1); incomplete ischemic trauma (n = 4); hamartomatosis (n = 1), arteriovenous malformation (n = 1); and hematocele with no evidence of testicular injury (n = 4). A total of 14 patients had hematoceles: the 4 with no evidence of testicular damage (just noted) and 10 others with testicular damage.
In the 24 patients with positive findings, concordance between US and CEUS findings was classified as grade 0 (absent) in 4 cases; grade 1 (low) in 3; grade 2 (moderate) in 8; and grade 3 (high) in 9 (Figs. 1 and 2). The 4 cases with no concordance (grade 0) included 1 patient whose baseline examination was indicative of a testicular fracture. Traumatic damage was excluded by CEUS, which revealed instead an arteriovenous malformation (Fig. 3). The second and third patients in this subgroup presented baseline US evidence of local lacerocontusive damage, but CEUS revealed complete rupture of the testicles. In the fourth case, the baseline examination was suggestive of focal intraparenchymal hemorrhage, but CEUS was negative for traumatic injury, revealing instead pre-existing, nontraumatic lesions (multiple hamartomas probably associated with the Cowden syndrome, according to the surgical pathology report) (Table 1, Fig. 4).
Figure 1.
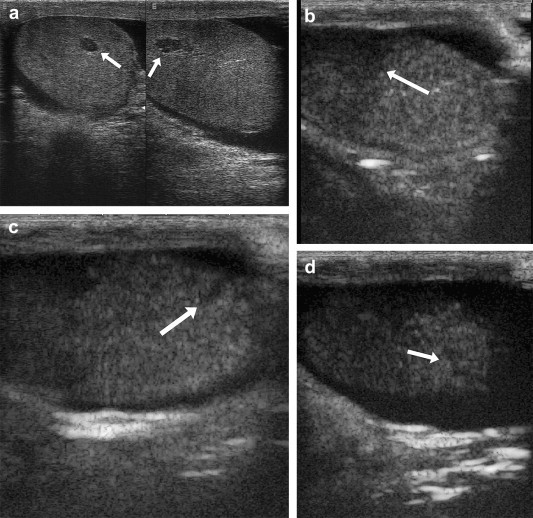
False negative US finding in a case of testicular rupture. US (a) reveals only a single intratesticular lesion (arrow). CEUS documents the presence of hypoperfusion caused by contusion injury to the upper mid-polar region, which was not seen on US (b, arrow), interruptions of the tunica albuginea (c and d arrows), and concomitant hematocele.
Figure 2.
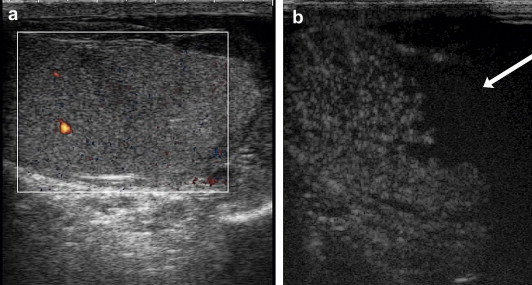
False negative US finding in a case of testicular rupture. (a) CEUS shows evidence of substantial loss of tunica albuginea (arrow). There is also evidence of nonperfusion related to extensive lacerocontusive injuries to the lower anterior mid-polar region at 35″ (b) and minimal perfusion at 106″, which were not seen on the US study, and concomitant hematocele.
Figure 3.
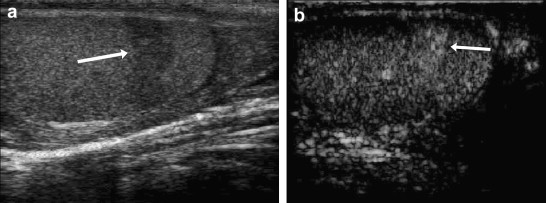
The basic US examination reveals a hypoechoic band extending from the anterior to the posterior margin of the lower peripolar region (arrow), which is suggestive of fracture (a). CEUS reveals accentuated locoregional perfusion (arrow) that is already evident in the early phase (b) and decreases progressively without disappearing in the later phases, findings indicative of an arteriovenous malformation.
Table 1.
US and CEUS findings in our case series.
| Number cases | Grade concordance US/CEUS | |
|---|---|---|
| True negatives | 16 | 3 |
| True positives | 24 (tot.) | |
| 4 | 0 | |
| 3 | 1 | |
| 8 | 2 | |
| 9 | 3 | |
| Tunica albuginea discontinuity | 3 | |
| Testicular fracture | 3 | |
| Lacerocontusions >50% | 3 | |
| Lacerocontusions <50% | 4 | |
| Testicular ischemia (funicular trauma) | 1 | |
| Incomplete ischemic trauma | 4 | |
| Hamartomatosis | 1 | |
| Arteriovenous malformation | 1 | |
| Hematocele without testicular damage | 4 |
Figure 4.
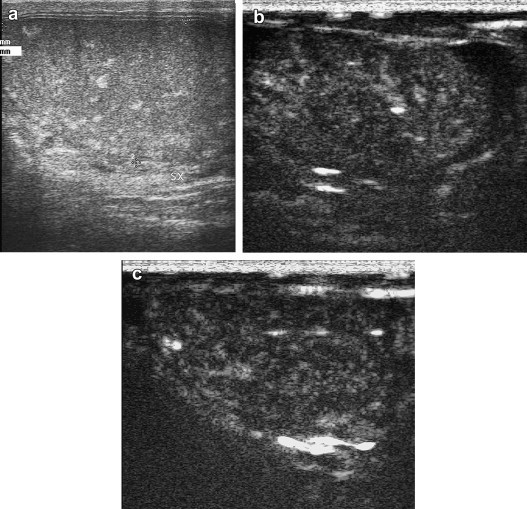
The basic US examination reveals (a) areas of spontaneous non-calcific hyperechogenicity that were suggestive of focal intratesticular hemorrhages. The absence on CEUS of hypovascular areas or bands within the testis (b,c) together with inhomogeneous enhancement, in some cases focal, excluded the possibility that the lesions seen on the basic examination were traumatic. Indeed they were ultimately diagnosed as hamartomas related to the Cowden syndrome.
The relevance of the information added by the CEUS examination was rated high in 4/40 (10%) cases, moderate in 9/40 (22.5%), low in 13/40 (32.5%), and none in the remaining 14/40 (35%) (Fig. 5). In only one case of rupture (with multiple fragments that were highly visible on both the baseline and CEUS examinations), the use of power Doppler after the injection of contrast medium proved to be superior to gray-scale CEUS for pinpointing the site of active hemorrhage (Fig. 6). Examination of the upper abdomen during the later phases of the CEUS examination revealed no lesions involving the parenchymal organs of this region. In most cases, the alterations seen on the baseline examination were more conspicuous on CEUS, with higher echogenicity between lesions/fluid collections and the normal parenchyma, and the contrast-enhanced examination also furnished more information on the morphology and size of the lesions (especially those that were small). It is important to note, however, that contrast enhancement was also associated with some loss of resolution, and this explains why the baseline US examination sometimes provided better visualization of small-volume intrascrotal fluid collections that were not frankly corpuscular. In any case, none of the patients with negative findings on CEUS presented significant functional alterations (involving hormone secretion or spermatogenesis) during follow-up, which ranged from 6 months to around 5 years. In patients who did not undergo surgery, follow-up included both US and CEUS (capable of revealing even small lesions that could not be visualized with other means) until healing was complete (intervals that ranged from 5 days to approximately 3 months with a mean of 4 weeks).
Figure 5.
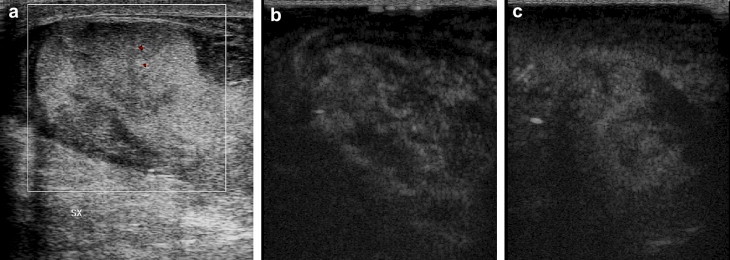
Basal US assessment with color Doppler is suggestive of lacerocontusive foci with interruption of the tunica albuginea (a). CEUS reveals focal contusions and lacerocontusions with no evidence of tunica albuginea discontinuity (b,c).
Figure 6.
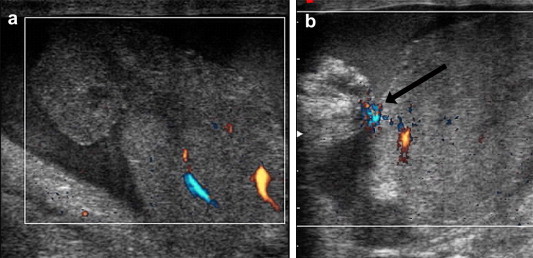
Multifragmented rupture (a). Power Doppler after administration of contrast agent reveals extravasation (arrow) caused by active hemorrhage (b).
Discussion
Early reports on the use of CEUS in the work-up of scrotal pathology indicated that it could be used to identify abscesses and areas of necrosis, which appear as nonenhanced areas [13,14]; to document increases in the mean contrast-medium transit time in the presence of a varicocele [15]; to evaluate tumor vascularization [16]; to facilitate extraction of sperm from infertile patients (by pinpointing better perfused areas of the gonad) [17]; and to characterize and follow-up ambiguous cases of segmental ischemia [18]. Testicular trauma could thus represent a new field of application for CEUS.
Severe trauma generally produces hematoceles, hematomas, fractures, or complete rupture of the testis. The most common causes are sports-related injuries, motor vehicle accidents, and straddle injuries, which are associated in many cases with pelvic fractures. If the tunica albuginea appears intact on US, the injury can be treated conservatively. However, surgery must be performed immediately when the continuity of the capsule appears interrupted (an unequivocal sign of testicular rupture); when complete vessel transections are present; when the testis is not being perfused; or when there are ambiguous baseline US findings in a patient with clinical findings that are highly suggestive of testicular rupture [4,22,23]. Surgical treatment involves debridement and suturing of the tunica albuginea [1,3] and re-anastomosis of any microvessels that have been completely transected.
Identifying the testicular fracture plane on gray-scale US can be very difficult. In a study conducted some time ago by Jeffrey et al. the fracture plane was detected in only 17% of the patients examined [24]. Gray-scale US is also poorly suited for differentiating hematomas from extruded testicular contents and—even more fundamental—for verifying the integrity of the tunica albuginea. This structure can be difficult to identify even when it is intact, and it is extremely difficult to pinpoint small areas of discontinuity [2]. Sonographic diagnosis of testicular rupture is based on findings of poorly defined testicular margins, echotexture heterogeneity, and above all discontinuities in the tunica albuginea. When surgery is performed promptly (within 72 h), 80%–90% of ruptured testes can be saved, whereas delayed surgical intervention is associated with salvage rates of only 45%–55% [1,24–26].
Reported figures on the diagnostic accuracy of this approach range from 56% to 94% [2]. Rapid, accurate diagnosis is fundamental for planning treatment that can save the gonad. Our experience includes two cases of testicular rupture that were diagnosed with CEUS after the gray-scale examination yielded false negative findings. Without CEUS, the injured testes would have been at high risk for serious complications such as infection, ischemia, infarction, and even atrophy of the injured testis (probably caused by ischemia and reabsorption of the nonviable tissue, which extrudes through the breach in the tunica as a result of intratesticular pressure increases related to the presence of a hematoma), and later immune-mediated damage to the contralateral gonad, which would have severely compromised the patients’ fertility.
In the few cases studied with CEUS uniform enhancement of the entire testicle seems to exclude the presence of major traumatic lesions. Parenchymal lesions appear as markedly hypoperfused areas (focal contusions or laceration-contusions) or bands (fractures, ruptures), which are easy to distinguish from the background enhancement of the surrounding parenchyma (in accordance with the well-known axiom “no signal, no blood flow”). Based on our preliminary findings, CEUS seems to provide additional information in cases of blunt scrotal trauma that is not furnished by gray-scale US or color-power Doppler studies. It provides more complete information on testicular vascularization, which is essential for excluding infarctions (which—especially when complete--can already be suspected on the basis of complementary color-power-Doppler), and useful information on lesion features such as visibility, size, and margin characteristics, which are not easy to evaluate on the basal examination [19]. This is important in patients with minor traumatic lesions, which are sometimes misdiagnosed on the basic US examination but appear hypoechoic/hypovascularized on CEUS. It is also valuable, however, when the testis is ruptured. In these cases, sonographers can document interruption of the tunica albuginea (which is an indication for immediate surgery) by demonstrating the presence of peripheral hypoechoic/hypovascularized bands that extend to some degree into the parenchyma of the testis. Compared with the basic US examination, CEUS also seems to offer higher resolution and improved diagnostic certainty in the identification of intrascrotal corpuscular fluid collections, which are frequently present in the early stages of scrotal trauma. In these cases, the use of acoustic contrast enhancement allows better definition of the margins of the effusion from those of the adjacent parenchyma, which are often irregular and ragged in cases of rupture. This can be difficult during the basal US examination if the two components appear more or less isoechoic.
The difference in frequency between commonly used transducers and those designed specifically for use with acoustic contrast media (which have much lower frequencies) explains the relatively low quality of the CEUS (compared with that of the basal image). Technological improvements will soon be able to reduce the occurrence of false negative findings related to the low spatial resolution that can be achieved with low-frequency insonation (2.0–2.5 MHz), which is necessary when specific harmonic-based software is used (as it was in our study). The introduction of contrast media based on microbubbles that resonate at higher frequencies than those of SonoVue will undoubtedly improve the system’s resolution.
In the present case series, the use of CEUS allowed us to correctly stage 3 cases in which the basal sonographic study produced false positive results indicative of testicular trauma. In 1, where rupture was suspected, CEUS revealed only focal contusions/lacerocontusions with no evidence of tunica albuginea discontinuity, and the next 2 months’ follow-up revealed that these lesions healed completely. In another case, the basal examination revealed a hypoechoic band, which raised the suspicion of fracture. In contrast, CEUS showed intense locoregional perfusion in the early phases that decreased progressively but was still evident in later phases, which was compatible with a diagnosis of an arterovenous malformation (which could be suspected on the basis of basal US findings). The third case (later treated with elective surgery) was characterized by the presence of intratesticular areas of spontaneous, non-calcific hyperechogenicity that were suggestive of focal hemorrhages. The absence on CEUS of areas or bands of hypo- or avascularity indicated that the basal US findings were not related to trauma, and the patient was referred to a urologist. The postoperative pathology report indicated the presence of hamartomatosis (Cowden syndrome). Power Doppler studies of the scrotum after IV administration of contrast medium (more likely to be associated with artifacts than CEUS [27]) and complementary studies of the parenchymal organs of the upper abdomen during the late or early phases can provide additional information (at no added cost) on the vascular architecture of the testes and/or other sites of traumatic injury, and this is especially useful when iodinated contrast media are contraindicated [19,28–31].
Ultrasonography is clearly indicated in cases of blunt scrotal trauma since testicular rupture (reflected by discontinuity of the tunica albuginea) requires prompt surgical intervention. It can also be used for noninvasive evaluation of the scrotal contents, testicular integrity and vascularization, hematomas, fluid collections, and foreign bodies, and it is a useful follow-up tool for confirming the resolution of these lesions. (It is interesting to note that 10%–15% of all testicular tumors develop after trauma.) [32]. To our knowledge, there are no reports in the literature on the use of CEUS to evaluate blunt scrotal trauma (with the exception of a few anecdotal reports, including some by our group [14,18,20]. Valentino et al. [20] reported on the use of CEUS for emergency work-up of scrotal pathology. Their series included 7 cases of trauma, in which CEUS provided accurate definition of fractures, their relation to the tunica albuginea, and the presence of intra- or extratesticular hematomas. Although the data we have collected thus far are related to a fairly small number of patients, they indicate that CEUS is superior to gray-scale US in detecting traumatic injuries, especially when the lesions are small (which appear as areas that are clearly hypoechoic/hypoperfused); in defining the extension and nature of corpuscular fluid collections; in the diagnosis of testicular fracture (hypoechoic/hypoperfused bands that extend to but do not involve the tunica) and rupture with interruption of the tunica albuginea (more or less well-demarcated, hypoechoic/hypoperfused bands that extend to and involve the periphery). In our series 2 out of 3 cases (66.6%) of rupture were seen only on CEUS, which made it possible to provide prompt surgical intervention that would otherwise have been delayed. In 1/3 of our patients the additional information provided by the contrast-enhanced examination (classified as highly relevant in 10% and moderately relevant in 17.5%) substantially altered case management by furnishing better documentation/staging of the traumatic lesions and by excluding the traumatic nature of lesions observed on the baseline study. Another advantage of CEUS (compared with US) is that it can detect ongoing hemorrhages [20,33]. The use of CEUS to study scrotal disorders has been limited thus far, but evidence seems to be emerging [18,20] that one of its most interesting applications may be in the work-up of the acute scrotum, including both traumatic (as demonstrated by the present report) and nontraumatic lesions. In these circumstances, CEUS has proved capable of resolving cases that were ambiguous on combined Doppler/US studies, thereby making a concrete contribution to the therapeutic management of the case.
Conclusions
Our findings indicate that the use of CEUS can substantially increase radiologists’ diagnostic confidence in the face of blunt scrotal trauma. Further studies are obviously needed before this method can be recommended as a first-line study in cases of this type.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Footnotes
Award for the best oral presentation at the XXII SIUMB National Congress.
Appendix. Supplementary data
References
- 1.Bhatt S., Dogra V.S. Role of US in testicular and scrotal trauma. RadioGraphics. 2008;28:1617–1629. doi: 10.1148/rg.286085507. [DOI] [PubMed] [Google Scholar]
- 2.Deurdulian C., Mittelstaedt C.A., Chong W.K., Fielding J.R. US of acute scrotal trauma: optimal technique, imaging findings, and management. Radiographics. 2007;27:357–369. doi: 10.1148/rg.272065117. [DOI] [PubMed] [Google Scholar]
- 3.Buckley J.C., McAninch J.W. Use of ultrasonography for the diagnosis of testicular injuries in blunt scrotal trauma. J Urol. 2006;175:175–178. doi: 10.1016/S0022-5347(05)00048-0. [DOI] [PubMed] [Google Scholar]
- 4.Cass A.S., Luxenberg M. Testicular injuries. Urology. 1991;37:528–530. doi: 10.1016/0090-4295(91)80317-z. [DOI] [PubMed] [Google Scholar]
- 5.Gross M. Rupture of the testicle: the importance of early surgical treatment. J Urol. 1969;101:196–197. doi: 10.1016/s0022-5347(17)62310-3. [DOI] [PubMed] [Google Scholar]
- 6.Herbener T.E. Ultrasound in the assessment of the acute scrotum. J Clin Ultrasound. 1996;24:405–421. doi: 10.1002/(SICI)1097-0096(199610)24:8<405::AID-JCU2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Howlett D.C., Marchbank N.D., Sallomi D.F. Pictorial Review. Ultrasound of the testis. Clin Radiol. 2000;55:595–601. doi: 10.1053/crad.2000.0499. [DOI] [PubMed] [Google Scholar]
- 8.Claudon M., Cosgrove D., Albrecht T., Bolondi L., Bosio M., Calliada F. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 9.Metzger-Rose C., Krupinski E.A., Wright W.H., Baker M.R., McCreery T.P., Barrette T.R. Ultrasonographic detection of testicular ischemia in a canine model using phospholipid coated microbubbles (MRX-115) J Ultrasound Med. 1997;16:317–324. [PubMed] [Google Scholar]
- 10.Yamaguchi A., Hayashi Y., Kojima Y., Miyagawa H., Ito M., Kohri K. Testicular torsion: usefulness of contrast-enhanced power Doppler sonography. Int J Urol. 2005;12:849–851. doi: 10.1111/j.1442-2042.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 11.Paltiel H.J., Kalish L.A., Susaeta R.A., Frauscher F., O’Kane P.L., Freitas-Filho L.G. Pulse-inversion US imaging of testicular ischemia: quantitative and qualitative analyses in a rabbit model. Radiology. 2006;239:718–729. doi: 10.1148/radiol.2393050210. [DOI] [PubMed] [Google Scholar]
- 12.Liang R.X., Xue E.S., Lin L.W., Yu L., Chen S., Yu L.Y. Correlation between sonographic appearance of experimental testicular ischemia and histological changes of the testis after reperfusion. Zhonghua Nan Ke Xue. 2009;15:115–121. [PubMed] [Google Scholar]
- 13.Puttemans T. BMUS and EFSUMB Proceedings of the 21th Euroson Congress. 6th–8th Dec. 2009. CEUS of the testis. [Google Scholar]
- 14.Moschouris H., Stamatiou K., Lampropoulou E., Kalikis D., Matsaidonis D. Imaging of the acute scrotum: is there a place for contrast-enhanced ultrasonography? Int Braz J Urol. 2009;35:692–705. doi: 10.1590/s1677-55382009000600008. [DOI] [PubMed] [Google Scholar]
- 15.Caretta N., Palego P., Schipilliti M., Torino M., Pati M., Ferlin A. Testicular contrast harmonic imaging to evaluate intratesticular perfusion alterations in patients with varicocele. J Urol. 2010;183:263–269. doi: 10.1016/j.juro.2009.08.140. [DOI] [PubMed] [Google Scholar]
- 16.Lung P.F.C., Sellars M.E.K., Sidhu P.S. BMUS and EFSUMB Proceedings of the 21th Euroson Congress. 6th–8th Dec. 2009. Use of contrast enhanced ultrasound in the investigation of indeterminate focal testicular lesions: evidence of vascularity is an important diagnostic parameter. [Google Scholar]
- 17.Schurich M., Aigner F., Frauscher F., Pallwein L. The role of ultrasound in assessment of male fertility. Rev Eur J Obstet Gynecol Reprod Biol. 2009;144:S192–S198. doi: 10.1016/j.ejogrb.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Bertolotto M., Derchi L.E., Sidhu P.S., Serafini G., Valentino M., Grenier N. Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up. AJR Am J Roentgenol. 2011;196:834–841. doi: 10.2214/AJR.10.4821. [DOI] [PubMed] [Google Scholar]
- 19.Catalano O., Lobianco R., Sandomenico F., Mattace Raso M., Siani A. Real-time, contrast-enhanced sonographic imaging in emergency radiology. Radiol Med. 2004;108:454–469. [PubMed] [Google Scholar]
- 20.Valentino M., Bertolotto M., Derchi L., Bertaccini A., Pavlica P., Martorana G. Role of contrast enhanced ultrasound in acute scrotal diseases. Eur Radiol. 2011;21:1831–1840. doi: 10.1007/s00330-010-2039-5. [DOI] [PubMed] [Google Scholar]
- 21.Lobianco R., Caiazzo C., Catalano O., Regine R., Ragozzino A., Siani A. Contrast-enhanced ultrasound (CE-US) in the evaluation of scrotal trauma: preliminary results. Ultraschall Med. 2008;29(S1):S35. [Google Scholar]
- 22.Mulhall J.P., Gabram S.G., Jacobs L.M. Emergency management of blunt testicular trauma. Acad Emerg Med. 1995;2:639–643. doi: 10.1111/j.1553-2712.1995.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 23.Fournier G.R., Laing F.C., McAninch J.W. Scrotal ultrasonography in the management of testicular trauma. Urol Clin North Am. 1989;16:377–385. [PubMed] [Google Scholar]
- 24.Jeffrey R.B., Laing F.C., Hricak H., McAninch J.W. Sonography of testicular trauma. AJR Am J Roentgenol. 1983;141:993–995. doi: 10.2214/ajr.141.5.993. [DOI] [PubMed] [Google Scholar]
- 25.Krone K.D., Carroll B.A. Scrotal ultrasound. Radiol Clin North Am. 1985;23:121–139. [PubMed] [Google Scholar]
- 26.Gorman B., Carroll B.A. The scrotum. In: Rumack C.M., Wilson S.R., Charboneau J.W., editors. Diagnostic ultrasound. 3rd ed. Elsevier Mosby; St Louis, Mo: 2005. pp. 849–888. [Google Scholar]
- 27.Pepe P., Panella P., Pennisi M., Aragona F. Does color Doppler sonography improve the clinical assessment of patients with acute scrotum? Eur J Radiol. 2006;60:120–124. doi: 10.1016/j.ejrad.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Catalano O., Lobianco R., Sandomenico F., Siani A. Splenic trauma: evaluation with contrast-specific sonography and a second-generation contrast medium: preliminary experience. J Ultrasound Med. 2003;22:467–477. doi: 10.7863/jum.2003.22.5.467. [DOI] [PubMed] [Google Scholar]
- 29.Catalano O., Lobianco R., Mattace Raso M., Siani A. Blunt hepatic trauma: evaluation with contrast-enhanced sonography: sonographic findings and clinical application. J Ultrasound Med. 2005;24:299–310. doi: 10.7863/jum.2005.24.3.299. [DOI] [PubMed] [Google Scholar]
- 30.Catalano O., Aiani L., Barozzi L., Bokor D., De Marchi A., Faletti C. CEUS in abdominal trauma: multi-center study. Abdom Imaging. 2009;34:225–234. doi: 10.1007/s00261-008-9452-0. [DOI] [PubMed] [Google Scholar]
- 31.Valentino M., Ansaloni L., Catena F., Pavlica P., Pinna A.D., Barozzi L. Contrast-enhanced ultrasonography in blunt abdominal trauma: considerations after 5 years of experience. Radiol Med. 2009 Oct;114(7):1080–1093. doi: 10.1007/s11547-009-0444-0. [DOI] [PubMed] [Google Scholar]
- 32.Wittenberg A.F., Tobias T., Rzeszotarski M., Minotti A.J. Sonography of the acute scrotum: the four T’s of testicular imaging. Curr Probl Diagn Radiol. 2006;35:12–21. doi: 10.1067/j.cpradiol.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Catalano O., Cusati B., Nunziata A., Siani A. Active abdominal bleeding: contrast-enhancedsonography. Abdom Imaging. 2006;31:9–16. doi: 10.1007/s00261-005-0369-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


