Abstract
The supraspinatus tendon is composed of 5 different layers consisting of intertwining bundles. On a front portion of the tendon, the layers become coated bundles which insert on the trochanter. At the insertion, the superficial or bursal surface of the tendon corresponding to the tendon fibers in contact with the subacromial bursa can be distinguished from the deep surface corresponding to the fibers in contact with the glenohumeral joint. A tendon tear may involve partial or total disruption of the tendon fibers and is called full-thickness tear if it affects the entire tendon, and partial-thickness tear if it involves only part of the tendon. Partial-thickness tears of the supraspinatus tendon include lesions of the superficial, deep and central surface or tendon delamination.
A contrast enhanced examination requires injection of contrast agent into the joint (arthrography followed by computed tomography (CT) or magnetic resonance imaging (MRI)) to study the deep surface, and injection into the subacromial bursa (bursography followed by CT) to study the superficial surface. MRI and ultrasound (US) examination allow the study of these different tendon layers without the use of contrast agent (which is not possible at CT).
Keywords: Sonography, Shoulder, Tendons, Lesions
Sommario
Il tendine del sovraspinato ha una struttura lamellare composta da 5 diversi strati, costituiti da fasci intrecciati. Essi, su una sezione frontale del tendine, costituiscono dei veri e propri fasci che rivestono e si inseriscono sul trochite.
A livello dell’inserzione distinguiamo il lato superficiale o bursale del tendine, corrispondente alle fibre tendinee in contatto con la borsa sottoacromiale, dal lato profondo, corrispondente alle fibre in contatto con l’articolazione gleno-omerale.
La rottura tendinea è una soluzione di continuità, parziale o totale, delle fibre di un tendine; è definita a tutto spessore se interessa l’intero tendine, è definita non a tutto spessore se riguarda solo parte del tendine.
Tra le lesioni non a tutto spessore del sovraspinato, si distinguono lesioni del versante superficiale, del versante profondo e lesioni centrali o slaminamenti.
Se si realizza un esame con mezzo di contrasto, servirà iniettarlo nell’articolazione (artrografia seguita da TC o RM) per osservare la superficie profonda e iniettarlo nella borsa sottoacromiale (bursografia seguita da TC) per studiare la superficiale. La RM, come l’ecografia, consente di studiare senza mezzo di contrasto questi differenti strati tendinei (cosa non possibile mediante TC) e le loro rotture.
Introduction
In recent years, thanks to the technological development [1,2], ultrasound (US) has assumed a key role in the study of tendons and has perhaps become even more accurate than magnetic resonance imaging (MRI) [3]. The role of US in the study of full-thickness tears of the supraspinatus tendon [4–6] is well-known, but numerous authors [7–9] have also published studies carried out on articular surface and bursal surface partial-thickness tears [10,11] with five differents layers (Fig. 1).
Figure 1.
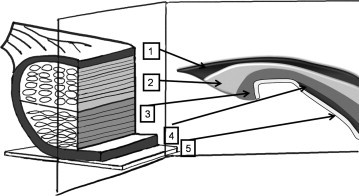
Diagram inspired by Clark (11) showing the 5 layers, which constitute the supraspinatus tendon, in 3 dimensions (a) and along an anterior surface of the tendon (b). Layer 1, superficial surface, is thin (1 mm thick) and consists of fibers of the coracohumeral ligament. Layer 2 (3–5 mm thick) is composed of tendon fibers parallel to the tendon, grouped in bundles. These bundles, 1–2 mm in diameter, extend directly from the supraspinatus muscle to the humerus. Layer 3 (3 mm thick) has a tendon structure of smaller bundles which are less uniformly oriented than Layer 2. Layer 4 consists of loose connective tissue containing large collagen fibers. Most of these fibers are located on the capsular surface (extra-articular). However, along the anterior edge of the supraspinatus tendon, these fibers join those of the coracohumeral ligament forming a ligament coating at the anterior surface of the supraspinatus. Layer 5 (from 1.5 to 2 mm thick) is composed of intertwined collagen fibers. This layer corresponds to the joint capsule and extends medially to the glenoid labrum, externally to the humerus, where Sharpey’s fibers insert directly on the bone. Copyright © 2010 Published by Elsevier Masson SAS for Société française de rhumatologie.
The supraspinatus tendon inserts on the trochanter. The superficial surface of the tendon which is convex and situated in contact with the subacromial bursa can be distinguished from the deep surface which is situated on the articular surface of the tendon (Fig. 2). The deep surface is in close contact with the cephalic cartilage. These two layers (superficial and deep) have a different mechanic effect on the supraspinatus tendon [12–14].
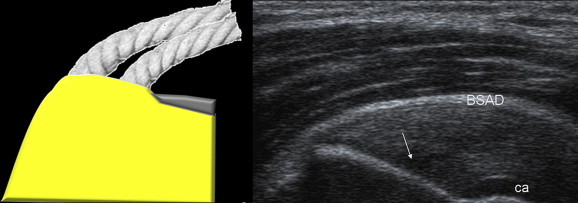
Figure 2.
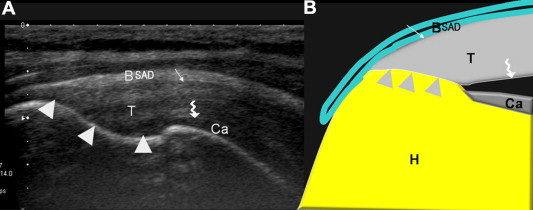
Normal US anatomy of the supraspinatus tendon, anterior surface (A: US; B: diagram). The supraspinatus tendon (T) inserts on the trochanter (arrowhead). The superficial surface (simple arrow) of the tendon is convex and in contact with the subacromial bursa (subacromial-subdeltoid bursa) and can be distinguished from the deep surface (curved arrow) located on the articular side of the tendon. The deep surface is in close contact with the cephalic cartilage (Ca). Note the fibrillar appearance of the supraspinatus tendon (Fig. 2A).
The supraspinatus tendon can be illustrated by two strings representing, in a frontal section, a set of tendon bundles of the superficial layer and of the deep layer. Rupture of the two strings is considered as a full-thickness tear (Fig. 3).
Figure 3.
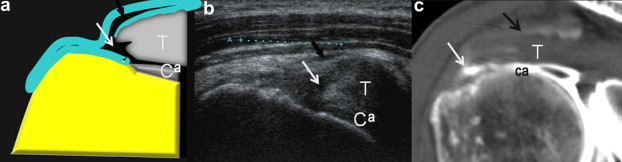
US appearance of a small full-thickness tear. Diagram of full-thickness tear (white arrow) of the supraspinatus tendon (T), anterior surface. Note the interruption of the deep surface at the cephalic cartilage (Ca) and the communication with the subacromial-subdeltoid bursa (black arrow). The retracted tendon appears lax. US coronal view shows a deep surface tear starting in the cartilage and extending throughout the entire thickness of the tendon. The tendon is retracted and has lost its tense and fibrillar appearance.
Rupture of one of the two strings is considered a partial-thickness tear (superficial if the superficial string is torn and deep if the deep string is torn). The thickness of the two strings depends on the degree of tendon tear and can be classified according to Ellman [15] and Snyder [16]. Interstitial tear is located between the two strings at the insertion. A cleavage tear causes a gap between the two strings which runs between the tendon fibers (Fig. 4).
Figure 4.
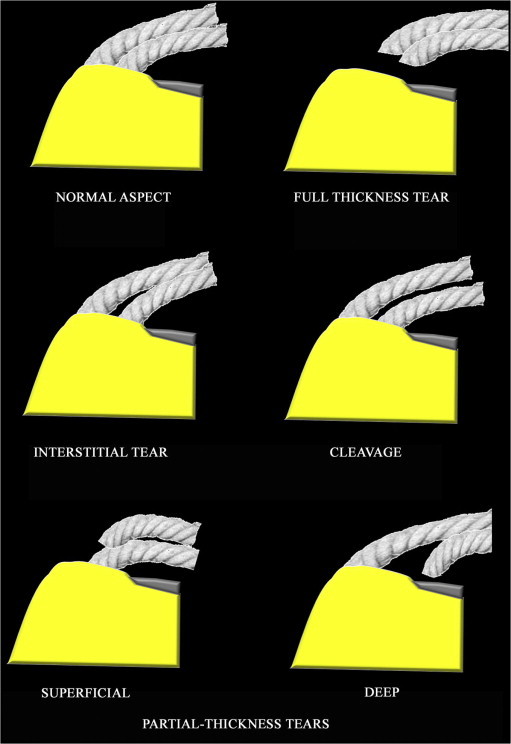
String theory. The supraspinatus tendon is presented as two strings representing the surface layer and the deep layer bundles of one surface. Rupture of both strings is a full-thickness tear; rupture of one of the two strings is a partial-thickness tear (superficial if the superficial string is torn and deep if the deep string is torn). An interstitial lesion is located between the two strings at the insertion. A cleavage is a gap between the two strings running between the tendon fibers. Copyright © 2010 Published by Elsevier Masson SAS for Société française de rhumatologie.
Superficial surface tear of the supraspinatus tendon
These tears are called superficial [17] and they frequently affect the bursal surface of the supraspinatus tendon and can therefore not be examined at arthro-CT, which permits opacification only of the joint surface i.e. the deep layer. They can only be studied by US and MRI (or arthro-MRI) (Figs. 5–9) using T2-weighted sequences, or by bursography combined with CT due to the opacification of the subacromial bursa [18]. In the authors’ opinion, these lesions are underestimated due to the role of arthro-CT in preoperative evaluation in France. They occur with a close frequency than the deep tears found by Osaki in a series of cadavers [17], a finding which is in agreement with the authors’ experience in US examinations.
Figure 5.
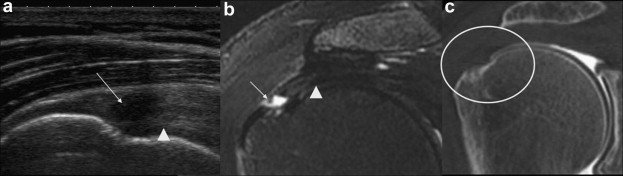
US and MRI evidencing superficial surface tear (false-negative arthro-CT). US coronal view shows tear with retraction of the superficial fibers of the supraspinatus tendon (arrow). The image evidences a tense deep string which has maintained its fibrillar appearance (arrowhead) which permits exclusion of full-thickness tear. Coronal T2-weighted MRI with fat saturation shows tear with retraction of the superficial fibers of the supraspinatus tendon (arrow). Note the deep string visible also in the US image (arrowhead). Copyright © 2010 Published by Elsevier Masson SAS for Société française de rhumatologie.
Figure 6.
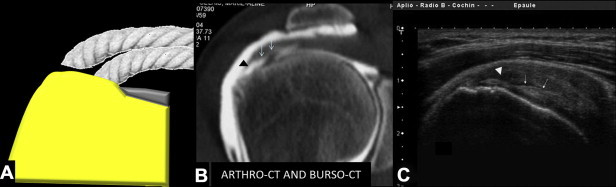
Superficial tear of the supraspinatus tendon with no serious retraction of the fibers. (A) diagram; (B) arthro-CT coronal view. (C) US coronal view shows avulsion of the superficial fibers of the supraspinatus tendon (arrowheads) without retraction, but with cleavage (arrows). Note that the most superficial fibers are not retracted (not flat); there is evidence of lax fibers.
Figure 7.
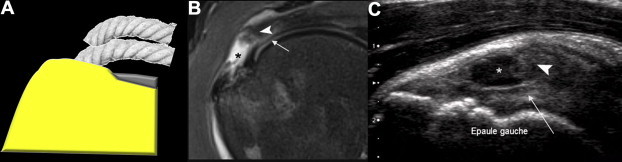
Surface tear of the supraspinatus tendon. (A) diagram; (B) coronal T2-weighted MRI. (C) US coronal view of the supraspinatus tendon shows avulsion of the superficial fibers of the supraspinatus tendon (*) with retraction of the superficial string (arrowheads). The deep string (arrows) appears tense at US examination suggesting that full-thickness tear may be excluded. Note the lax appearance of the fibers in the superficial portion and no retraction of the deep string.
Figure 8.
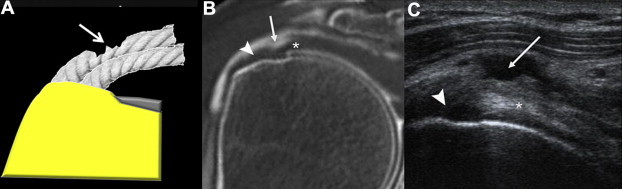
Abrasion of the superficial fibers of the supraspinatus tendon: A) diagram; B) arthro-CT coronal view of the supraspinatus tendon; C) coronal US scan of the supraspinatus tendon. The tear has spared the fibers of the distal tendon (arrow) which inserts on the greater tuberosity (arrowhead). Note the depth of the abrasion and the persisting tense deep string (*) suggesting lack of communication with the joint and therefore absence of full-thickness tear.
Figure 9.
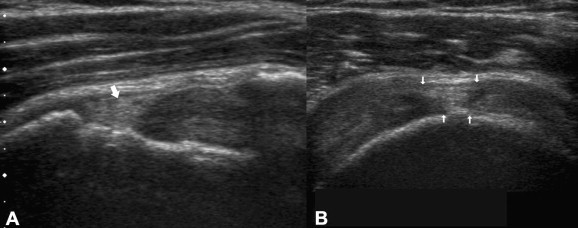
US image shows tear of the superficial portion of the supraspinatus filled with echoic material (arrows). A) Coronal scan. B) Sagittal scan.
The lesions can be divided into two types:
-
-
Avulsion of the distal fibers at the trochanter (Fig. 5) sometimes causing only cleavage if the fibers remain in place (Fig. 6), otherwise retraction of the fibers or the superficial string (Fig. 7), sometimes causing thinning of the tendon.
-
-
Surface abrasion which has not involved the distal fibers inserting on the trochanter (Fig. 8).
These lesions are usually hypoechoic and this appearance permits distinction from the healthy tendon. However, they may appear hyperechoic or isoechoic compared to the tendon (Fig. 9), particularly if the subacromial bursa is very thick and the lesions are old [19]. These "echoic" lesions are pitfalls of US imaging due to lack of sufficient contrast with the normal tendon.
One of the elements these two types of lesions have in common, independently of the type, is the persistence of a tightly stretched "deep fibrillar string" that makes it possible to predict the integrity of the joint arthrogram without bursal communication (Figs. 6 and 8). Another common feature is the presence of a bursal disorder, i.e. a thickened subacromial bursa with or without fluid collection. Identification of a disorder affecting the subacromial-subdeltoid bursa without intra-articular fluid collection should lead the physician to look carefully for a lesion affecting the superficial portion of the tendon. The search for a conflict with the coracoacromial ligament or the acromion is therefore particularly important (dynamic maneuvers).
The presence of a trochanteric surface irregularity at the disinsertion should make the physician suspect a lesion. This finding is sensitive but not specific because it is present in all deep surface tears, in all full-thickness tears and also in chronic enthesopathies.
Deep surface tears of the supraspinatus tendon
In young subjects, posterior superior glenoid impingement (or a Walch impingement which occurs when the arm is lifted) between the deep surface of the supraspinatus tendon and the posterior superior glenoid edge may cause a tendon tear. In young subjects, lesions may also be caused by chronic anterior instability, which can sometimes modify the deep surface cuff where the supra- and infraspinatus tendons cross or by a mechanism caused by a direct impact. These mechanisms occur both in young patients and in adults, but in the latter the lesions occur more often without any undue stress or trauma. These degenerative tears occur mainly in the “critical zone” as described by Codman [20] about 1 cm medial to the insertion of the deep surface of the supraspinatus. However, in the authors’ experience, lesions of the deep surface are often located at the site of the insertion on the trochanter. They occur mainly as small nonspecific cortical erosions which allow detection of the corresponding tendon anomaly. Lesions of the deep surface of the tendon are slightly more frequent than those of the superficial surface [17]. The pathophysiology of these lesions does normally not include subacromial impingement, so the bursal surface of the tendon and the subacromial-subdeltoid bursa are generally normal (but the association of multiple lesions is possible).
In order to study these lesions of the deep surface, it is necessary to opacify the glenohumeral joint and perform arthrography using arthro-CT or arthro-MRI.
To make a US diagnosis it is necessary to show:
-
-
That the tear is located at the articular surface of the tendon and therefore in frequent contact with the cephalic cartilage;
-
-
That the tear does not involve the entire thickness of the tendon;
-
-
That the bursal surface is not involved.
With regard also to the string theory, the appearance of the strings changes according to the degree of retraction of the deep fibers of the torn tendon:
-
-
The image can be simple and linear, usually hypoechoic, if the fibers or deep lying strings are not retracted (Fig. 10). This image can extend into an intratendinous cleavage between the two strings.
-
-
If the "deep string" is slightly retracted, the linear image will become triangular, square or rectangular (Fig. 11) depending on the degree of retraction.
-
-
If the deep fibers are too retracted under the acromion, the lesion will be difficult or impossible to visualize on the US image (Fig. 12).
Figure 10.
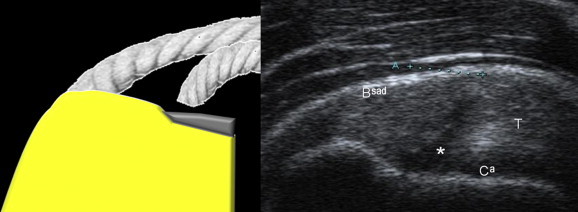
Coronal US scan of a partial-thickness tear of the deep surface of the supraspinatus tendon. The deep fibers of the tendon are disinserted, but not retracted, and this explains the linear appearance of the tear (*). This lesion communicates with the joint as it continues with the cephalic cartilage without affecting the entire thickness of the tendon.
Figure 11.
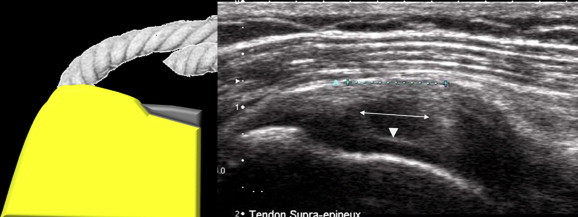
Coronal US scan of a partial-thickness tear of the deep surface of the supraspinatus tendon. The deep fibers of the tendon are disinserted and clearly retracted (arrows), and this explains the rectangular appearance of tendon injury zone. This lesion communicates with the joint cavity as it continues with the cephalic cartilage (arrowhead) which is highly visible (a hyperechoic line above the cartilage showing the cartilage interface sign also known as the double cortex sign). This injury does not involve the entire thickness of the tendon and is therefore a partial-thickness tear.
Figure 12.
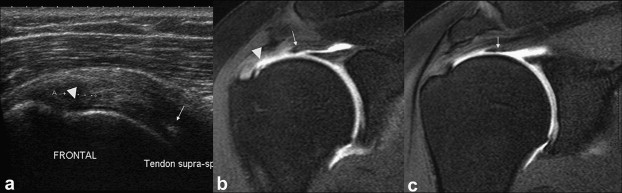
Partial-thickness tear of the deep surface of the supraspinatus tendon. Coronal US scan (a) and arthro-MRI (b, c). The deep fibers of the tendon are disinserted and considerably retracted (arrows) causing a US pitfall. The US image shows some anomalies of the deep surface (arrowheads) at the insertion of the tendon, but does not clearly show the retracted string almost at the base of the humeral head (arrows).
The echotexture of deep surface tears is theoretically hypoechoic; however, like superficial tears, also deep tears can contain hyperechoic or echoic material [10] and become a US imaging pitfall.
Central tears (interstitial)
Interstitial tears are difficult to assess because they are not in communication with the joint surface or with the bursal surface of the tendons. They are therefore not visible at contrast enhanced examinations or at arthroscopy. According to Yamanaka [21] they account for 55% of partial-thickness tears. As they are centrally located at the insertion of the tendon, they can only be studied by MRI and US. Their appearance is sometimes difficult to differentiate from that of deep surface lesions because of their proximity to the cephalic cartilage. They generally appear as linear or triangular hypoechoic images (Fig. 13) with irregularities related to the cortical bone of the trochanteric region. They may be extended through an intratendinous cleavage and they can sometimes be filled with an echoic material and mimic the pitfall lesions described under deep surface tears [10]. It is difficult to evaluate the symptoms, and power Doppler may help to determine the "hyperemic" character of the enthesopathy of the region. Differential diagnosis between enthesopathy and these interstitial lesions is very difficult. The hypoechoic band (Fig. 14) should be evaluated in two planes (frontal and sagittal); there may be a hyperechoic posterior reinforcement. Some authors recommend abduction and external rotation of the arm to enhance cleavages of the infraspinatus tendon. High frequency probes and tissue harmonic imaging are required to improve US ability to identify these cleavages. Cystic images may be identified along the extension of the cleavages.
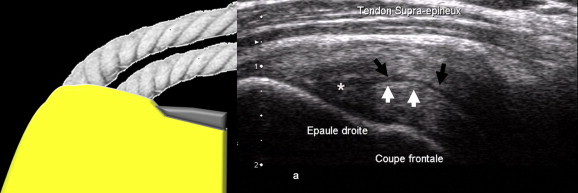
Figure 13.
Interstitial tendon tear (arrow) at the insertion; there is no contact with the bursal surface or the joint surface of the tendon (coronal US image of the supraspinatus tendon).
Figure 14.
US image of a cleavage. A cleavage is defined as a hypoechoic line (black arrow) within the tendon; its starting point is a tendon tear (*). It is sometimes underlined by a hyperechoic band (white arrow) and must be visible in both planes.
Conclusions
US imaging is acquiring an increasingly important role in the examination of painful rotator cuffs. The advantages of US imaging are the low cost, the possibility to compare the affected and the contralateral shoulder and the possibility to perform a dynamic study, which can in some cases provide information indicating a tendinous conflict.
The ability of US imaging in detecting full-thickness tears is well-known and has proved to be equivalent to that of MRI [4–6]. With regard to partial-thickness tears, the literature confirms the accuracy of US in the identification of deep surface tears of the of the rotator cuff tendons. These partial-thickness tears are more frequent (18.5%) than full-thickness tears (11.7%) [7] and they can be painful. Some authors have reported sensitivity and specificity of US imaging in the diagnosis of these lesions as 94% and 93%, respectively [8] while others have reported 54% and 91%, respectively [9]. In addition to providing the diagnosis of these lesions, US plays an important role in presurgical planning [22,23].
The limitation of US examination [24,25] is linked to the instrumentation; the US machine must be new and of good quality and must be equipped with a high frequency probe. The physician must furthermore understand that this examination is difficult and that it requires years of experience integrating US technique with a good knowledge of the anatomy of the shoulder and its pathologies. Obesity is a considerable drawback as the US beams are absorbed by the adipose tissues; obesity furthermore increases the distance between the probe and the anatomic structures under examination thus imposing a lower frequency and therefore a lower resolution of the probe. Also decreased mobility of the shoulder, regardless of the cause (pain, capsular contracture, arthropathy) makes it difficult to study the cuff, if retropulsion of the shoulder and internal rotation of the arm is limited.
Conflict of interest
The authors have no conflict of interest to declare.
Appendix. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Guerini H., Feydy A., Campagna R. Harmonic sonography of rotator cuff tendons: are cleavage tears visible at last? J Radiol. 2008;89:333–338. doi: 10.1016/s0221-0363(08)93008-0. [DOI] [PubMed] [Google Scholar]
- 2.Strobel K., Zanetti M., Nagy L., Hodler J. Suspected rotator cuff lesions: tissue harmonic imaging versus conventional US of the shoulder. Radiology. 2004;230:243–249. doi: 10.1148/radiol.2301021517. [DOI] [PubMed] [Google Scholar]
- 3.Rutten M.J., Spaargaren G.J., van Loon T., de Waal Malefijt M.C., Kiemeney L.A., Jager G.J. Detection of rotator cuff tears: the value of MRI following ultrasound. Eur Radiol. 2010 Feb;20(2):450–457. doi: 10.1007/s00330-009-1561-9. Epub 2009 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teefey S.A., Middleton W.D., Payne W.T., Yamaguchi K. Detection and measurement of rotator cuff tears with sonography: analysis of diagnostic errors. AJR Am J Roentgenol. 2005;184:1768–1773. doi: 10.2214/ajr.184.6.01841768. [DOI] [PubMed] [Google Scholar]
- 5.Teefey S.A., Rubin D.A., Middleton W.D., Hildebolt C.F., Leibold R.A., Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A:708–716. [PubMed] [Google Scholar]
- 6.de Jesus J.O., Parker L., Frangos A.J., Nazarian L.N. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR Am J Roentgenol. 2009;192:1701–1707. doi: 10.2214/AJR.08.1241. [DOI] [PubMed] [Google Scholar]
- 7.Reilly P., Macleod I., Macfarlane R., Windley J., Emery R.J. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88:116–121. doi: 10.1308/003588406X94968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiener SN, Seitz WH Jr. Sonography of the shoulder in patients with tears of the rotator cuff: accuracy and value for selecting surgical options. AJR Am J Roentgenol 1993; 160:103-7; discussion 109–110. [DOI] [PubMed]
- 9.Brenneke S.L., Morgan C.J. Evaluation of ultrasonography as a diagnostic technique in the assessment of rotator cuff tendon tears. Am J Sports Med. 1992;20:287–289. doi: 10.1177/036354659202000309. [DOI] [PubMed] [Google Scholar]
- 10.van Holsbeeck M.T., Kolowich P.A., Eyler W.R., Eyler W.R., Craig J.G., Shirazi K.K. US depiction of partial-thickness tear of the rotator cuff. Radiology. 1995;197:443–446. doi: 10.1148/radiology.197.2.7480690. [DOI] [PubMed] [Google Scholar]
- 11.Clark J.M., Harryman D.T., 2nd Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J Bone Jt Surg Am. 1992;74:713–725. [PubMed] [Google Scholar]
- 12.Fukuda H., Hamada K., Nakajima T., Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;304:60–67. [PubMed] [Google Scholar]
- 13.Sano H., Ishii H., Trudel G., Uhthoff H.K. Histologic evidence of degeneration at the insertion of 3 rotator cuff tendons: a comparative study with human cadaveric shoulders. J Shoulder Elbow Surg. 1999;8:574–579. doi: 10.1016/s1058-2746(99)90092-7. [DOI] [PubMed] [Google Scholar]
- 14.Sano H., Wakabayashi I., Itoi E. Stress distribution in the supraspinatus tendon with partial-thickness tears: an analysis using two-dimensional finite element model. J Shoulder Elbow Surg. 2006;15:100–105. doi: 10.1016/j.jse.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;254:64–74. [PubMed] [Google Scholar]
- 16.Snyder S.J., Pachelli A.F., Del Pizzo W., Friedman M.J., Ferkel R.D., Pattee G. Partial thickness rotator cuff tears: results of arthroscopic treatment. Arthroscopy. 1991;7:1–7. doi: 10.1016/0749-8063(91)90070-e. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki J., Fujimoto S., Nakagawa Y., Masuhara K., Tamai S. Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Jt Surg Am. 1988;70:1224–1230. [PubMed] [Google Scholar]
- 18.Fermand M., Blanchard J.P., Vergeron H., Goldberg D. Rotator cuff imaging using bursography coupled to helical computed arthrotomography. Rev Rhum Engl Ed. 1999;66:131–135. [PubMed] [Google Scholar]
- 19.Fermand M., Sihassen C., Mauget D., Sarazin L., Chevrot A., Drapè J.L. Hyperechoic rotator cuff tendon tear. J Radiol. 2005;86:159–163. doi: 10.1016/s0221-0363(05)81336-8. [DOI] [PubMed] [Google Scholar]
- 20.Codman E.A. Rupture of the supraspinatus-1834 to 1934. J Bone Jt Surg Am. 1937;19:643–652. [Google Scholar]
- 21.Yamanaka K., Fukuda H. Pathological studies of the supraspinatus tendon with reference to incomplete thickness tear. In: Takagishi N., editor. The shoulder. 1987. pp. 220–224. [Google Scholar]
- 22.Lehman R.C., Perry C.R. Arthroscopic surgery for partial rotator cuff tears. Arthroscopy. 2003;19:E81–E84. doi: 10.1016/s0749-8063(03)00692-3. [DOI] [PubMed] [Google Scholar]
- 23.Nové-Josserand L., Labrique J.F. Traitement arthroscopique des lésions non transfixiantes de la coiffe des rotateurs: Symposium. Revue de Chirurgie Orthopédique et Réparatrice de l’Appareil Moteur. 2004;90:35–48. [PubMed] [Google Scholar]
- 24.Morvan G., Wybier M., Mathieu P., Vuillemin-Bodaghi V., Busson J., Guerini H., editors. Echographie de l’épaule: bilan, attente et limites. Elsevier Masson; 2006. [Google Scholar]
- 25.Guérini H., Fermand M., Godefroy D., Feydy A., Chevrot A., Morvan G. Ultrasound of partial-thickness tears of the supra-spinatus tendon. Rev Rhum Mono. 2010;77(3):213–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


