Abstract
Purpose
To retrospectively assess the diagnostic accuracy of immediate post-procedural CEUS, 24-h CEUS, and 24-h CT in verifying the effectiveness of thermal ablation of liver tumors ablation, using the combined results of 3-month post-procedure CEUS and MDCT as the reference standard.
Materials and methods
From our database, we selected patients who had immediate post-procedural CEUS and 24-h CEUS and MDCT examinations after undergoing thermal ablation of a liver tumor between January 2009 and March 2010. The study population consisted of 53 subjects and 55 tumors (44 HCC and 11 metastasis) were evaluated. Thirty-seven tumors were treated with radiofrequency and 18 with microwave ablation.
Post-procedural CEUS, 24-h CEUS and MDCT, and 3-month follow-up CEUS and MDCT images were blindly reviewed by two radiologists, who measured the size of the ablation area on the post-procedural and 24-h studies. They also evaluated the ability of each of these three index tests to predict the outcome (residual tumor vs. no residual tumor) using imaging studies done at the 3-month follow-up as the reference standard.
Results
Mean tumor diameter on preablation CEUS (the day before treatment) was 20 ± 9 mm. Mean diameter of the necrotic area was 29 ± 9 mm on post-procedural CEUS, 34 ± 11 mm on 24-h CEUS, and 36 ± 11 mm on 24-h MDCT. Diameters of the necrotic area (mean and maximum) on post-procedural CEUS were significantly smaller than those measured on 24-h CEUS or 24-h MDCT, which were not significantly different. For predicting the presence of residual tumor at the 3-month follow-up, post-procedural CEUS, 24-h CEUS, and 24-h MDCT displayed sensitivity of 33%, 33%, and 42%; specificity of 92%, 97%, and 97%; negative predictive value of 84%, 85%, and 83%. The accuracy parameters of these three imaging modalities were not significantly different from one another.
Conclusions
In patients undergoing thermal ablation for liver tumors, the immediate post-procedural CEUS seems comparable to 24-h CEUS and MDCT in terms of detecting residual disease.
Keywords: Contrast-enhanced ultrasound, Ultrasonography, Interventional, Ablation techniques, Sensitivity and specificity
Sommario
Scopo
Determinare retrospettivamente l’accuratezza diagnostica nella valutazione di efficacia delle ablazioni di tumori epatici della CEUS eseguita al termine della procedura ablativa, della CEUS e della la tomografia computerizzata multi-detettore (TCMD) eseguite a 24 ore, utilizzando la CEUS e TCMD a 3 mesi di follow-up come standard di riferimento.
Materiali e metodi
Abbiamo selezionato dal nostro data base i pazienti sottoposti a CEUS subito dopo una procedura di ablazione e a CEUS e TCMD dopo 24 ore tra gennaio 2009 e marzo 2010. Il campione era composto da 53 soggetti in cui abbiamo valutato 55 lesioni (44 HCC e 11 metastasi). Trentasette lesioni sono state trattate con ablazione a radiofrequenza, 18 mediante microonde. La CEUS post-trattamento, la CEUS e la TCMD eseguite a 24 ore, e la CEUS e la TCMD eseguite a tre mesi di follow-up sono state rivalutate in cieco da due radiologi. Abbiamo confrontato tra loro le dimensioni della termoablazione misurate alla CEUS post-procedura, alla CEUS e alla TCMD eseguite a 24 ore. Abbiamo calcolato l’accuratezza diagnostica della CEUS post-procedura, della CEUS e della TCMD a 24 ore valutando la capacità di ogni metodica nel rilevare il tessuto vitale residuo utilizzando come standard di riferimento il follow-up a 3 mesi (TCMD e CEUS combinate).
Risultati
Il diametro medio del tumore alla CEUS il giorno prima del trattamento era di 20 ± 9 mm. Il diametro medio della necrosi ottenuta è di: 29 ± 9 mm alla CEUS post-trattamento, 34 ± 11 mm alla CEUS a 24 ore e 36 ± 11 mm alla TCMD a 24 ore. Il diametro medio ed il diametro massimo dell’area di necrosi misurati alla CEUS post-trattamento rispetto alla CEUS o alla TCMD eseguite a 24 ore sono risultati significativamente inferiori. La differenza tra le dimensioni dell’area di necrosi misurate alla CEUS e alla TCMD a 24 ore non è significativa. La CEUS post-trattamento, la CEUS a 24 ore e la TCMD a 24 ore hanno dimostrato i seguenti parametri di accuratezza diagnostica nell’individuare il tumore residuo rispetto al follow-up: sensibilità, 33%, 33%, 42%; specificità, 92%, 97%, 97%; valore predittivo negativo 84%, 85% and 83%. La differenza tra i parametri di accuratezza diagnostica delle tre metodiche non è risultata significativa.
Conclusioni
L’accuratezza diagnostica della CEUS post-trattamento nell'individuare tessuto tumorale residuo dopo ablazione di tumori epatici è comparabile a quella della CEUS e della TCMD a 24 ore.
Introduction
The past decade has witnessed a rapid increase in the popularity of thermal ablation as a nonsurgical option for the management of localized primary and secondary liver tumors. It is considered a curative treatment for Barcelona Clinic Liver Cancer Stage I hepatocellular carcinomas (HCC), and reported rates of local tumor control in this setting exceed 80% [1,2]. In addition, local tumor control and survival rates comparable with those obtained with surgical resection have recently been reported in selected patients with liver metastases from colorectal and breast cancers [3,4].
The aim of thermal ablation is to destroy by heat the entire tumor mass together with a safety margin consisting of a 5–10 mm rim of normal tissue. An applicator is inserted directly into the tumor under imaging guidance, and the local temperature is increased until it exceeds a threshold of about 50 °C, thereby inducing coagulation necrosis [5]. Different types of energy (e.g., radiofrequency, microwave, laser) can be used to generate locally the heat necessary to obtain thermal ablation of the tumor tissues [6–8].
To ensure good local tumor control, the completeness of the ablation must be verified immediately after treatment so that any residual viable tissue can be retreated during the same session. All currently available imaging modalities used for this purpose have limitations related to the reactive, hyperemic halo that develops around the ablated tissue and remains visible for several days. This halo impairs detection of viable residual tissue and is a possible source of both false positive and false negative findings [9,10].
In institutions where ablation is performed under multidetectorCT (MDCT) guidance, this imaging modality is also used frequently to assess the completeness of the ablation at the end of the procedure [11]. Promising preclinical experience has also been reported with magnetic resonance imaging for early post-ablation evaluation [12]. However, throughout the world, ablations are usually performed under ultrasound (US) guidance and monitoring, so it would be ideal to have an ultrasound-based modality for the immediate post-procedure assessment. Unfortunately, gray-scale US is not a very precise tool for assessing the completeness of an ablation. During the procedure, a hyperechoic patch appears on the screen, which reflects steam generated within the ablated tissue. However, the dimensions of this area appear to correlate poorly with the final dimensions of the necrotic area [13–15]. In theory, contrast-enhanced ultrasound (CEUS) might provide a simple solution to this problem, but its use in the immediate post-ablation phase has been investigated only in a few preliminary studies [16,17].
The aim of the present retrospective study was to define the diagnostic accuracies of CEUS assessment of liver tumor ablation, immediately after and 24 h after the procedure, and of MDCT in the 24 h assessment. Combined findings of CEUS and MDCT performed at 3-months were used as reference standards.
Materials and methods
IRB approval was obtained for this retrospective study.
Patients and tumors
Between January 2009 and February 2010, 105 patients underwent percutaneous ablation of liver tumors at our institution. In 53 of these cases (median patient age, 72 years; interquartile range 65–79 years) CEUS was performed 5–10 min after the thermal ablation procedure. CEUS and CT were performed 24 h after the procedure and repeated at the 3-month follow-up visit. All examinations were available in the hospital database. Two of these patients were treated twice (for different tumors) during the study period, so we evaluated a total of 55 tumors. Forty-four tumors were HCCs, and the other 11 were liver metastases. Thirty-seven tumors were treated with radiofrequency ablation and 18 with microwave ablation. The characteristics of the tumors are reported in Table 1.
Table 1.
Tumor characteristics.
| Parameter | Frequence | Percentage |
|---|---|---|
| Number of tumors | 55 | |
| Type of tumor | ||
| HCC | 44 | 80% |
| Metastasis | 11 | 20% |
| Colorectal | 5 | 46% |
| Breast | 2 | 18% |
| Lung | 2 | 18% |
| Others | 2 | 18% |
| Arterial phase enhancement | ||
| Present | 49 | 89% |
| Absent | 6 | 11% |
| Segment | ||
| II | 5 | 9.1% |
| III | 3 | 5.5% |
| IV | 7 | 12.7% |
| V | 14 | 25.4% |
| VI | 9 | 16.4% |
| VII | 8 | 14.5% |
| VIII | 9 | 16.4% |
| Value (mm) | ±SD (mm) | |
|---|---|---|
| Lesion diameters on preablation CEUSa | ||
| Minimum diameter | 24 | 8 |
| Maximum diameter | 34 | 10 |
Performed a few hours before ablation.
Imaging
Informed consent was obtained from all patients before each imaging study. None of the patients was allergic to iodine-based or sulfur hexafluoride-based contrast agents, and no adverse effects were reported.
All patients had undergone 1) MDCT (at our institution or at the referring institution) within the month before the ablation procedure and 2) CEUS (at our institution) a few hours before the procedure. CEUS was repeated 5–10 min after the ablation procedure (immediate post-procedural examination), the day after the procedure, and at the 3-month follow-up assessment. During the study period, the immediate post-procedural CEUS was performed mainly to obtain better measurements of the ablation area than those possible with gray-scale ultrasound, and none of the patients underwent additional ablation on the basis of the results of this examination. MDCT was performed the day after the procedure and at the 3-month follow-up assessment.
For the CEUS examinations, sulfur hexafluoride-based contrast medium (SonoVue, Bracco, Milan, Italy – 2.4–4.8 ml) was injected intravenously through a 20-gauge cannula, and the line was flushed with 10 ml normal saline. The examination was then performed with sonographic units (Alpha 10 [Aloka, Tokyo, Japan]; Sequoia, [Siemens, Munich, Germany]; Aplio-XG, [Toshiba, Tokyo, Japan]) equipped with contrast-specific software that operates at a low mechanical index (range: 0.04–0.1). The index tumor was imaged continuously for up to 5 min. Video recordings were made of each phase of the examination (arterial phase 10–30 s, portal phase 50–90 s, late phase 120–360 s) and stored on an external hard disk.
MDCT was performed the day after the procedure and at the 3-month follow-up with a 16-slice scanner (Brilliance, Philips, Amsterdam, The Netherlands). First the upper abdomen was imaged without contrast. Iodine-based contrast medium (Xenetix) was then administered (350 mg/ml, flow-rate 3.5–4 ml/s, 2 ml/kg) and the upper abdomen rescanned during the arterial phase (bolus tracking, threshold 120 HU, delay 13 s), the portal phase (70–90 s), and the late phase (180 s). Scanning parameters were as follows: slice thickness 2 mm, increment 1 mm, pitch 0.688, collimation 1.5 mm. The examination was recorded on the PACS system.
Imaging evaluation and measurements
Two radiologists blindly reviewed all images. When multiple tumors had been ablated during a single session, we evaluated only the lesion that had been imaged in all vascular phases during both the immediate post-procedural and 24-h CEUS examinations. All ablation area measurements were made during the portal phase; the lesions were evaluated in all vascular phases to determine whether there was any residual viable tumor tissue.
To ensure comparability, post-procedural and 24-h CEUS measurements of the ablation zone were made at the same level (with the aid of anatomical landmarks) and in the same scanning plan. We measured the maximum diameter and the diameter perpendicular to the maximum diameter of the ablation area and calculated their mean. During the pre-ablation CEUS, we evaluated the vascularity of each tumor and divided the lesions into two subgroups characterized by the presence/absence of enhancement during the arterial phase (Table 1).
Imaging evidence of persistent disease/incomplete treatment was defined as follows: 1) for tumors with arterial enhancement: nodular enhancement at the periphery of the ablation area, with or without wash-out in portal/late phases; 2) for tumors without arterial enhancement: presence of irregular portal/late phase wash-out at the periphery of the ablation area; 3) an ablation area smaller than the original lesion. Consensus between the two radiologists was obtained when judgments were discordant.
We also evaluated the quality of the immediate post-procedural CEUS examination using the following 3-point scale: 1 = not diagnostic, 2 = difficult to read, 3 = good quality.
Ablation procedure
Inclusion criteria for thermal ablation procedures at our institution are as follows: Patients with HCC must be ineligible for surgical resection; have no more than three tumors, none measuring more than 3 cm in diameter, or a single lesion measuring no more than 5 cm; absence of portal thrombosis; platelet count > 50,000; PT > 50%; and serum bilirubin < 2 mg/dl. Those with liver metastases must fulfill these criteria: ineligibility for surgery; no extrahepatic metastases or stable extrahepatic metastases; no more than 5 liver metastases, none measuring more than 5 cm; platelet count > 50,000; and PT > 50%.
Radiofrequency ablation was performed with an internally cooled 17-gauge needle-like electrode (Cool-Tip, Valleylab, Boulder CO, US). For microwave ablations, we used a 2.45 GHz generator, and power was delivered through a 14-gauge mini-choked antenna (HS Amica, HospitalService, Aprilia, Italy). All equipment was used in accordance with the manufacturers' instructions. The ablations were performed with the patient under general anesthesia. Ultrasound guidance and monitoring was obtained with an Alpha 10 sonographic unit (Aloka, Tokyo, Japan) equipped with a 5 MHz interventional probe.
Statistical analysis
ANOVA for repeated measures and paired t-test with Holm's correction for multiple comparisons were used to compare ablation-zone diameters measured on the post-procedural and 24-h CEUS images and the 24-h MDCT images.
For each of the three imaging modalities we calculated diagnostic accuracy parameters (AUC and diagnostic odds ratios) in assessing the presence of residual tumor and compared them using Fisher's exact test. We used 3-month follow-up images as the reference standard, defining persistence of disease as 3-month MDCT and/or 3-month CEUS evidence of residual viable tissue at the ablation site. R Software (v 2.14.0, R Development Core Team 2011) was used for all calculations.
Results
Ablation size
The mean tumor diameter measured on CEUS the day before treatment was 20 ± 9 mm, and the mean diameters of the necrotic zone were 29 ± 9 mm on the post-procedural CEUS, 34 ± 11 mm on the 24-h CEUS, and 36 ± 11 mm on the 24-h MDCT examinations. The mean and maximum diameters of the ablated zone on the post-procedural CEUS were significantly smaller than those observed at 24-h with either CEUS or MDCT (p < 0.001 for all) Fig. 1. The diameters measured on 24-h CEUS and MDCT images were not significantly different (p > 0.1 for means and maximums).
Figure 1.
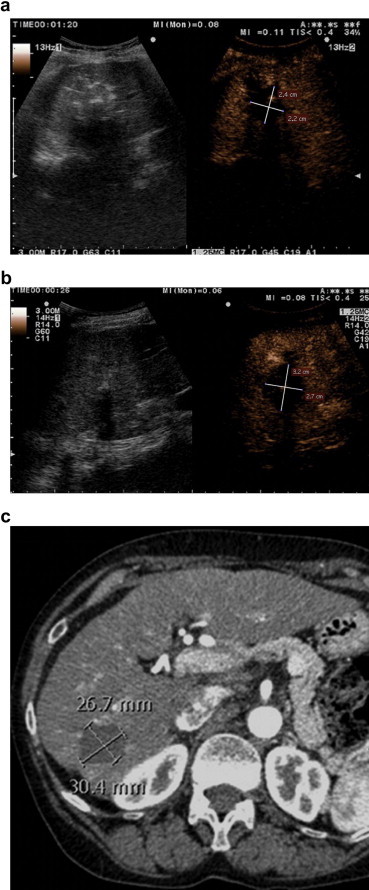
Microwave ablation of a 15-mm HCC in a 78-year-old woman. Ablation area measured on (a) post-procedural CEUS, (b) 24-h CEUS, and (c) 24-h CT. Note that the ablation zone seems smaller on the immediate post-procedural CEUS compared with 24-h imaging studies.
Image quality
The immediate post-procedural CEUS examination was considered nondiagnostic in 4 cases (7.3%); difficult to read in 20 (36.4%); and of good quality in 31 (56.3%).
Diagnostic accuracy
One patient was lost to follow-up. The 3-month follow-up assessment revealed viable tissue at the ablation site of 12/54 (22%) tumors. At 10 (83%) of these 12 tumors, the viable tissue was detected with CEUS and MDCT; at the other 2 tumors (17%), it was seen only on MDCT (Fig. 2). The immediate post-procedural CEUS results correctly predicted the 3-month follow-up imaging findings for 43 (79%) of the 54 tumors (sensitivity 33%, specificity 93%) (Table 2). The diagnostic odds-ratio was 6.2 (95% CI, 0.69–33.31).
Figure 2.
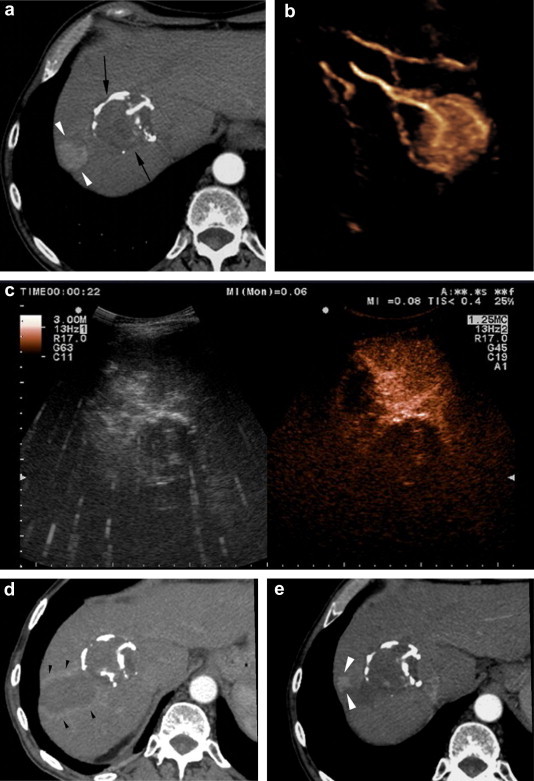
Radiofrequency ablation of a new HCC nodule in a 78-year old man with known HCC. (a) Follow-up CT image 10 years after successful percutaneous ethanol injection ablation of an HCC nodule (black arrows) reveals a new 2.5-cm HCC nodule (white arrowheads). (b) 3D CEUS image of the new nodule displaying its arterial vascularization. The nodule was treated with radiofrequency ablation, and the immediate post-procedural CEUS (c) and 24-h CT (d) images showed no residual tumor tissue, but (e) nodular recurrence (white arrowheads) was evident on the 3-month follow-up CT.
Table 2.
Diagnostic accuracy of the immediate post-procedural CEUS.
| Immediate post-procedural CEUS | Residual tumor | No residual tumor |
|---|---|---|
| Positive | 4 | 3 |
| Negative | 8 | 39 |
| Value | 95% confidence interval | |
| Sensitivity | 33.3% | 13.8%–60.9% |
| Specificity | 92.9% | 81.0%–97.5% |
| Positive predictive value | 57.1% | 25.1%–84.2% |
| Negative predictive value | 83.0% | 69.9%–91.1% |
| Positive likelihood ratio | 4.7 | 1.4–15.4 |
| Negative likelihood ratio | 0.7 | 0.5–1.1 |
| Error rate | 20.4% | 11.8%–32.9% |
| Accuracy | 79.6% | 67.1%–88.2% |
| Area under ROC curve | 0.63 |
The 24-h CEUS examination correctly predicted the 3-month follow-up imaging results for 45 (83%) of the 54 tumors (sensitivity 33%, specificity 98%) (Table 3). The diagnostic odds-ratio was 17.3 (95% CI, 2.08–516.02). The 24-h MDCT findings predicted the 3-month follow-up imaging results for 46 (85%) of the 54 tumors (sensitivity 42%, specificity 98%) (Table 3). The diagnostic odds-ratio was 24.26 (95% CI, 3.15–707.96).
Table 3.
Diagnostic accuracy of the 24-h CEUS and MDCT examinations.
| 24 h CEUS |
24 h MDCT |
|||
|---|---|---|---|---|
| Residual tumor | No residual tumor | Residual tumor | No residual tumor | |
| Positive | 4 | 1 | 5 | 1 |
| Negative | 8 | 41 | 7 | 41 |
| Value | 95% confidence interval | Value | 95% confidence interval | |
| Sensitivity | 33.3% | 13.81–60.94% | 41.7% | 19.3–68.0% |
| Specificity | 97.6% | 87.68–99.58% | 97.6% | 87.7–99.6% |
| Positive predictive value | 80.0% | 37.55–96.38% | 83.3% | 43.6–97.0% |
| Negative predictive value | 83.7% | 70.96–91.49% | 85.4% | 72.8–92.7% |
| Positive likelihood ratio | 14.0 | 1.91–102.64 | 17.5 | 2.4–127.4 |
| Negative likelihood ratio | 0.7 | 0.46–1.02 | 0.6 | 0.4–1.0 |
| Error rate | 16.7% | 9.02–28.74% | 14.8% | 7.7–26.6% |
| Accuracy | 83.3% | 71.26–90.98% | 85.2% | 73.4–92.3% |
| Area under ROC curve | 0.65 | 0.69 | ||
The diagnostic accuracy parameters of the immediate post-procedure CEUS, the 24-h CEUS, and the 24-h MDCT examinations were not significantly different.
Discussion
Attempts to verify the completeness of thermal tumor ablation procedures are often thwarted by the reactive hyperemia that develops in tissue surrounding the ablated lesion. This represents an inflammatory reaction to thermal injury and occurs immediately after ablation. The rim of enhancement resulting from this reactive hyperemia is usually uniform in thickness and surrounds the entire ablated lesion. In contrast, peripheral enhancement reflecting residual tumor tissue is focal and irregular. In addition, reactive hyperemia is iso-enhancing and does not undergo washout during the portal and late phases [9]. However, within the reactive rim there may be a small area of enhancement resulting from residual tumor tissue, and failure to distinguish this area results in a false negative diagnosis. False positives can also occur when the border of the ablation zone is irregular since the reactive hyperemic rim will also appear irregular.
In our study, the immediate post-procedural CEUS underestimated the size of the obtained necrosis compared with that observed at 24-h on both the CEUS and MDCT examinations. This finding is probably due to the hyperemic reaction discussed above, which creates a thick hypervascular peri-lesional halo, and to the fact that a certain amount of sub-lethally injured tissue at the periphery of the ablation is known to die in the hours following the ablation.
Underestimation of the ablation area size did not seem to impair the diagnostic ability of immediate post-procedural CEUS (compared with those of the 24-h CEUS and CT studies).
We also observed a high percentage of low-quality examinations (43%). We hypothesize that hemodynamic changes induced by the general anesthesia may have altered the dynamics of contrast transit to the liver, negatively impacting the image quality. However, performing CEUS when the patient is awake is useless since the intent of the examination is to allow retreatment when needed during the same session.
Data on the role of CEUS in the immediate evaluation of tumor ablation are scarce. In a pilot study, postprocedural CEUS performed with perflutren lipid microspheres (Definity, Bristol-Myers Squibb) an hour or less after ablation correctly predicted the results of follow-up MDCT or magnetic resonance in 21 of patients (sensitivity 40%, specificity of 94%) [16]. Another recent study focused on small HCCs treated with HIFU ablation. The immediate evaluation of ablation completeness was made with 3D CEUS using a lipid-stabilized perfluorobutane agent (Sonazoid, Amersham Health). Compared with findings obtained at the 1-month follow-up with MDCT or MR in 21 tumors, the post-procedural CEUS examination displayed sensitivity, specificity, and accuracy of 100%, 75%, and 95%, respectively [17]. The sensitivity reported in this study is higher than that observed in our patients. This might be related to the reaction of normal tissues to HIFU ablation, the 3D nature of the CEUS in the previous study, and/or the pharmacokinetics of the contrast agent used. Kupffer cells phagocytize a high percentage of Sonazoid, and the result is a delayed parenchymal phase that starts about 10 min after injection. In this phase, lesions that do not contain Kupffer cells, such as HCC or metastases, are depicted as contrast defects surrounded by enhanced normal hepatic parenchyma [18].
Imaging studies are performed 24 h after ablation to rule-out complications and assess the local results [19–22]. The hyperemic halo persists for several days, and it may be the cause of the low sensitivity of our 24-h CEUS and MDCT examination in predicting local recurrence at the 3-month follow-up. Even if the 24-h study indicates that complete ablation has been achieved, a few viable neoplastic cells may still be present, and in 3 months, these cells can proliferate sufficiently to generate a small recurrent lesion that can be detected with diagnostic imaging.
This study has some limitations. First, it is a retrospective study (although assessment of imaging was done prospectively and in blinded fashion) and second, the 3-month follow-up imaging studies used as a reference standard may still have missed some of the recurrences.
Conclusions
In patients undergoing thermal ablation of liver tumors, immediate post-procedural CEUS findings were comparable to those of 24-h CEUS and MDCT studies for predicting local recurrence at three months. The low sensitivity of CEUS is offset to some extent by low cost, ready availability, and absence of radiation exposure. Therefore, post-procedural CEUS can be used to detect at least some incomplete ablations and allow re-treatment in the same session. Immediate post-procedural CEUS may even substitute the 24-h CEUS examination. As for the 24-h MDCT examination, its diagnostic accuracy in detecting persistent disease is similar to that of CEUS but it is important to detect complications.
Conflict of interest
The authors have no conflicts of interest to declare with reference to this article.
Footnotes
SIUMB Award for the Best Poster at the National SIUMB Congress 2010.
Appendix A. Supplementary material
References
- 1.Llovet J.M., Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl. 1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Mulier S., Ni Y., Jamart J., Ruers T., Marchal G., Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meloni M.F., Andreano A., Laeseke P.F., Livraghi T., Sironi S., Lee F.T. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation – intermediate and long-term survival rates. Radiology. 2009;253:861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillams A.R., Lees W.R. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol. 2008;19:712–717. doi: 10.1016/j.jvir.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg S.N. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001 Jun;13(2):129–147. doi: 10.1016/s0929-8266(01)00126-4. Review. [DOI] [PubMed] [Google Scholar]
- 6.Shiina S. Image-guided percutaneous ablation therapies for hepatocellular carcinoma. J Gastroenterol. 2009;44(Suppl. 19):122–131. doi: 10.1007/s00535-008-2263-9. [DOI] [PubMed] [Google Scholar]
- 7.Nikfarjam M., Christophi C. Interstitial laser thermotherapy for liver tumours. Br J Surg. 2003;90:1033–1047. doi: 10.1002/bjs.4326. [DOI] [PubMed] [Google Scholar]
- 8.Ong S.L., Gravante G., Metcalfe M.S., Strickland A.D., Dennison A.R., Lloyd D.M. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605. doi: 10.1097/MEG.0b013e328318ed04. [DOI] [PubMed] [Google Scholar]
- 9.Kim S.K., Lim H.K., Kim Y.H., Lee W.J., Lee S.J., Kim S.H. Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics. 2003;23:107–121. doi: 10.1148/rg.231025055. [DOI] [PubMed] [Google Scholar]
- 10.Park M., Rhim H., Kim Y., Choi D., Lim H.K., Lee W.J. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics. 2008;28:379–390. doi: 10.1148/rg.282075038. [DOI] [PubMed] [Google Scholar]
- 11.Schraml C., Clasen S., Schwenzer N.F., Koenigsrainer I., Herberts T., Claussen C.D. Diagnostic performance of contrast-enhanced computed tomography in the immediate assessment of radiofrequency ablation success in colorectal liver metastases. Abdom Imaging. 2008;33:643–651. doi: 10.1007/s00261-007-9351-9. [DOI] [PubMed] [Google Scholar]
- 12.Breen M.S., Lazebnik R.S., Fitzmaurice M., Nour S.G., Lewin J.S., Wilson D.L. Radiofrequency thermal ablation: correlation of hyperacute MR lesion images with tissue response. J Magn Reson Imaging. 2004;20:475–486. doi: 10.1002/jmri.20143. [DOI] [PubMed] [Google Scholar]
- 13.Cha C.H., Lee F.T., Gurney J.M., Markhardt B.K., Warner T.F., Kelcz F. CT versus sonography for monitoring radiofrequency ablation in a porcine liver. AJR Am J Roentgenol. 2000;175:705–711. doi: 10.2214/ajr.175.3.1750705. [DOI] [PubMed] [Google Scholar]
- 14.Raman S.S., Lu D.S., Vodopich D.J., Sayre J., Lassman C. Creation of radiofrequency lesions in a porcine model: correlation with sonography, MDCT, and histopathology. AJR Am J Roentgenol. 2000;175:1253–1258. doi: 10.2214/ajr.175.5.1751253. [DOI] [PubMed] [Google Scholar]
- 15.Leyendecker J.R., Dodd G.D., Halff G.A., McCoy V.A., Napier D.H., Hubbard L.G. Sonographically observed echogenic response during intraoperative radiofrequency ablation of cirrhotic livers: pathologic correlation. AJR Am J Roentgenol. 2002;178:1147–1151. doi: 10.2214/ajr.178.5.1781147. [DOI] [PubMed] [Google Scholar]
- 16.Dill-Macky M.J., Asch M., Burns P., Wilson S. Radiofrequency ablation of hepatocellular carcinoma: predicting success using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:S287–S295. doi: 10.2214/AJR.04.1916. [DOI] [PubMed] [Google Scholar]
- 17.Numata K., Fukuda H., Ohto M., Itou R., Nozaki A., Kondou M. Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur J Radiol. 2010;75:e67–e75. doi: 10.1016/j.ejrad.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Yanagisawa K., Moriyasu F., Miyahara T., Yuki M., Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318–325. doi: 10.1016/j.ultrasmedbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Kim C.K., Choi D., Lim H.K., Kim S.H., Lee W.J., Kim M.J. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol. 2005;56:66–73. doi: 10.1016/j.ejrad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Choi D., Lim H.K., Lee W.J., Kim S.H., Kim Y.H., Kim S.H. Early assessment of the therapeutic response to radio frequency ablation for hepatocellular carcinoma: utility of gray scale harmonic ultrasonography with a microbubble contrast agent. J Ultrasound Med. 2003;22:1163–1172. doi: 10.7863/jum.2003.22.11.1163. [DOI] [PubMed] [Google Scholar]
- 21.Meloni M.F., Goldberg S.N., Livraghi T., Calliada F., Ricci P., Rossi M. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol. 2001;177:375–380. doi: 10.2214/ajr.177.2.1770375. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto M., Nozawa A., Numata K., Shirato K., Sugimori K., Kokawa A. Evaluation using contrast-enhanced harmonic gray scale sonography after radio frequency ablation of small hepatocellular carcinoma: sonographic-histopathologic correlation. J Ultrasound Med. 2005;24:273–283. doi: 10.7863/jum.2005.24.3.273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


