Abstract
Kaposi's sarcoma (KS) is an aggressive, multifocal oncologic disease, which frequently involves skin and internal organs, predominantly affecting homosexual men with AIDS. Hepatic KS is rarely reported in living patients, while autopsies show liver involvement in 35% of patients with KS. Ultrasound (US) of the liver in AIDS patients shows hyperechoic nodules with periportal bands; CT shows a hypodense lesion before and after contrast administration, but in the late phase after iodinated contrast agent injection the nodules are enhanced. Those findings are considered indicative of hepatic KS [1–3].
Keywords: AIDS, Liver, Kaposi's sarcoma, Ultrasound imaging
Sommario
Il sarcoma di Kaposi (KS) è una patologia oncologica multicentrica e aggressiva che coinvolge frequentemente cute e organi interni, prevalentemente in soggetti di sesso maschile e omosessuali affetti da AIDS. Il KS epatico è raramente identificato in pazienti viventi, mentre le autopsie mostrano un coinvolgimento epatico nel 35% dei casi. L’ecografia epatica (US) in pazienti con AIDS mostra noduli iperecogeni con bande periportali, e la scansione TC mostra una lesione ipodensa prima e dopo contrasto, mentre nelle fasi tardive i noduli presentano enhancement. Questi dati sono considerati come indicatori di KS epatico [1–3].
Introduction
The form of Kaposi's sarcoma (KS) that appears in patients with acquired immunodeficiency syndrome (AIDS) is called “epidemic” and presents epidemiological and clinical characteristics different from the “classic”, “African or endemic” and “iatrogenic” forms. The “classic” form typically affects elderly people (>60 years), mainly in the Eastern Europe and Mediterranean area, and it is usually confined to the lower limbs with skin or visceral involvement occurring late. The “African endemic” form affects young adults of equatorial Africa and is characterized by localized nodular lesions; in one third of cases the disease spreads to other organs involving also the bones. The “iatrogenic” form which is caused by immunosuppressive drugs administered after organ transplant is aggressive and tends to spread.
“Epidemic” KS was for many years the most common form of cancer diagnosed in human immunodeficiency virus (HIV)-seropositive patients. However, after the introduction of highly active antiretroviral therapy (HAART), the incidence of this disease has been reduced to less than 1% of patients with AIDS. The spread of this disease among HIV-seropositive patients highlights the role of a risky sexual behavior linked to homosexuality and bisexuality. This has lead to the hypothesis that epidemic KS has a complex pathogenesis that requires sexually transmitted infectious agents as cofactors.
The genome of a member of the Herpesviridae family, Gammaherpesviridae subfamily, Herpes virus 8 (HHV-8) has been identified in KS cells. This virus has been found in more than 90% of KS lesions in AIDS patients and in some cases of lymphomas. The pathogenetic model suggests that in HIV-infected patients, T lymphocyte activation causes the release of sarcomatous cells known as spindle cells. HIV replication would then cause proliferation of these cells with consequent release of cytokines capable of inducing neoangiogenesis and proliferation of mesenchymal cells. The natural history of AIDS-related KS is highly variable.
In the early stage the lesions are small, flat, and macular and may be reddish, pink, purplish, or brown involving the skin and mucous membranes. In a few weeks or months they enlarge and develop into papules or plaques. Cutaneous lesions may be uncharacteristic and associated with asymptomatic visceral localizations of which the most common are the lymph nodes, spleen, oral mucosa, gastrointestinal tract, lung and liver. These sites can be affected even in the absence of cutaneous manifestations. Liver involvement is often associated with hepatomegaly and abdominal pain.
Abdominal CT or abdominal US with contrast agent can reveal the presence of lesions in the hepatic capsular, hilum and portal area with invasion of the liver parenchyma. However, a definitive diagnosis of KS requires biopsy and histological examination. In most cases, antiretroviral therapy alone is effective in controlling this neoplastic disease. Specific therapy is indicated in lesions causing aesthetic problems or functional impotence (radiotherapy, cryotherapy or intralesional vinblastine) while systemic therapy is used in patients with a large number of lesions or visceral involvement. The authors describe the diagnostic and therapeutic management of a case of KS in a HIV-seropositive patient admitted to their department for abdominal pain and hepatomegaly.
Materials, methods and results
A young man from Senegal, recently arrived in Italy, was admitted to the authors' hospital because of fever, weight loss, anorexia, abdominal pain, nausea and vomiting. Physical examination revealed nodular lesions on the back, spots on the tongue, hepatomegaly and abdominal bloating (Fig. 1). Laboratory tests showed gamma-glutamyl transpeptidase (GGT) 109, alkaline phosphatase (AP) 230, alanine transaminase (ALT) 54 UI/L, aspartate transaminase (AST) 65 UI/L, white blood cell count 3100/mmc, total leukocyte count 800/mmc, hemoglobin (Hb) 10.5 g/dl, HIV-ribonucleic acid (RNA) 1.6 × 106 cp/ml, cluster of differentiation 4 (CD4) 23/mmc. The following tests yielded normal results: hepatitis C antibody test (HCV-Ab), hepatitis B surface antigen (HBsAg), venereal disease research laboratory test (VDRL), carcinoembryonic antigen (CEA), cancer antigen 19/9, coproculture for bacteria, fungi, mycobacteria, protozoa and malaria blood test.
Figure 1.

Slightly protruding nodular KS lesions on the back.
Gray-scale US showed inhomogeneous hepatomegaly caused by several hyperechoic nodules as well as periportal hyperechogenicity (Fig. 2) and colon distension. Gall bladder, kidneys, pancreas and spleen were unremarkable.
Figure 2.
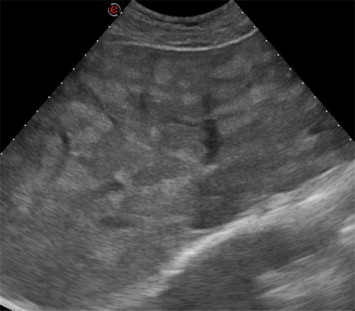
Right subcostal oblique scan: gray-scale US shows diffuse inhomogeneity of the liver because of hyperechoic nodules.
CT scan showed an enlarged inhomogeneous liver with multiple hypodense lesions (Fig. 3) and distension of the colon. No nodule enhancement after contrast administration and the vascular structure appeared undamaged (Fig. 4a, b). Colonoscopy with retrograde ileoscopy showed no mucous alterations in colon and terminal ileum.
Figure 3.
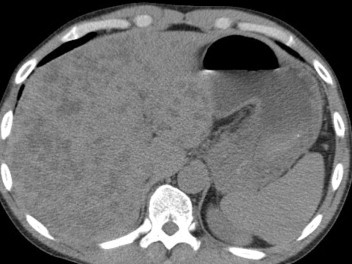
CT scan shows multiple hypodense nodules, normal spleen.
Figure 4.
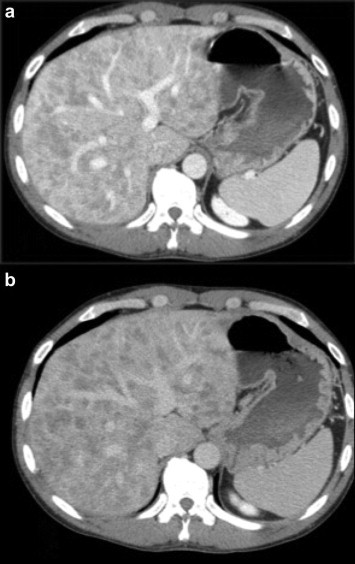
CT portal (a) and late (b) phase: no nodule enhancement after administration of contrast agent; the vascular structure appears undamaged.
Magnetic resonance imaging (MRI) T1-weighted in-phase images showed hyperintense liver nodules (but no mass effect on the vascular structures) and T1-weighted out-of-phase images showed a decrease in the signal of the nodules, which appeared hypointense due to fat accumulation in the hepatocytes. In the T2-weighted images there were no changes in the signal, and in the hepatobiliary phase (20 minutes) there was no contrast agent uptake (Gd-EOB-DTPA) in the fatty nodules (Fig. 5a–d).
Figure 5.
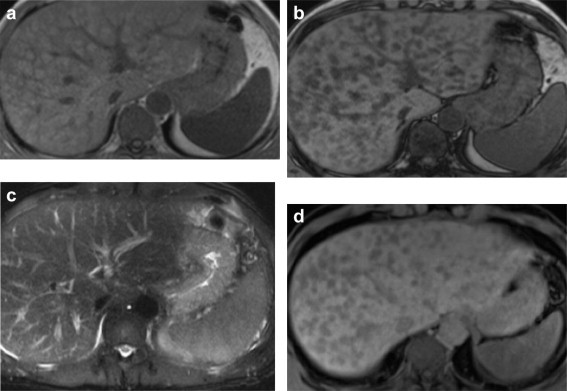
a) T1/in-phase (axial in-phase gradient-echo (GRE) T1-weighted): multiple hyperintense nodules with no mass effect on the vascular structures; b) T1/out-of-phase (axial out-of-phase gradient-echo T1-weighted): no enhancement of the nodules which appear hypointense because of intra-hepatocyte fat; c) T2-weighted sequence (HASTE Fast Spin-Echo): no change in signal; d) VIBE hepatobiliary phase (20 minutes): no uptake of liver-specific contrast agent (Gd-EOB-DTPA) in the fatty nodules.
Contrast enhanced US (CEUS) using SonoVue (Bracco, Milan, Italy) showed homogeneous arterial enhancement without hyperenhancement. The nodules remained isoechoic in the late phase, while the perilesional tissue was enhanced showing washout in the late portal phase (Fig. 6a, b).
Figure 6.
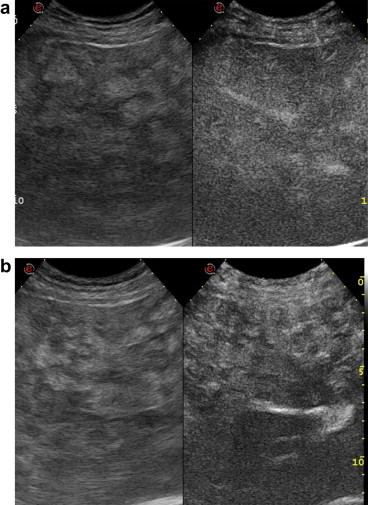
a) Right subcostal oblique scan: CEUS of the liver shows contrast uptake in the arterial phase. b) Right subcostal oblique scan in the late phase: US of the liver shows nodules with contrast uptake and washout of the surrounding tissue.
The patient underwent US-guided biopsy of the hyperechoic nodules and perinodular tissue; 4 biopsy samples were taken. Microbiological analysis revealed no parasitic inclusions in 2 samples. Histological analysis of the nodules showed diffuse macrovacuolar steatosis with large fibrotic portal spaces, bile duct ectasia and neoductogenesis. Histological analysis of the perinodular tissue showed small vascular structures with expression of specific monoclonal markers: cluster of differentiation31 (CD31), CD34+ and FVIII (Fig. 7a, b).
Figure 7.
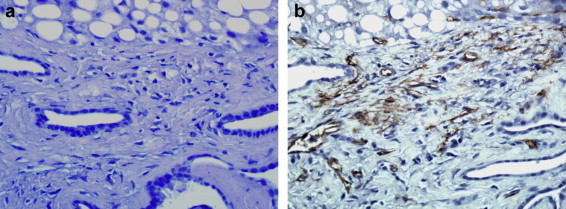
a, b) US-guided liver biopsy: diffuse macrovacuolar steatosis with periportal fibrosis, bile duct ectasia with neoductogenesis and reticulum of small vessels showing CD31, CD34+ and FVIII.
These findings suggested diagnosis of hepatic KS with multifocal steatosis confirmed by skin and mucous membrane lesions positive for HHV-8. The patient started antiretroviral treatment: emtricitabine/tenofovir disoproxil combined with atazanavir/ritonavir. Symptoms as well as skin and tongue lesions completely disappeared in 2 months, and the patient regained weight. Biochemical parameters returned to normal values, HIV-RNA levels decreased (7300 copies/ml) and CD4 count was 237 cells/mmc. Gray-scale US examination of the liver showed no hyperechoic lesions (Fig. 8). Liver biopsy showed significantly reduced steatosis with mild dilatation of the bile ducts and no lesions in the portal area.
Figure 8.
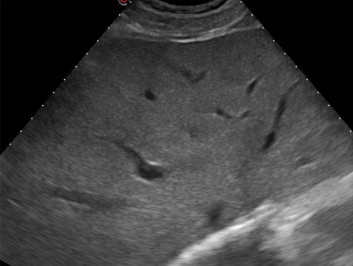
Right subcostal oblique US scan: no nodules are visible.
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Discussion
KS affects the blood and lymphatic vessels, and more often the skin and mucosa, but it is frequently observed also in other organs. There are 4 versions of the disease, which present different characteristics: classic or isolated or Mediterranean KS; African or endemic KS; iatrogenic or transplantation-related KS; and AIDS-related KS.
In the pre-AIDS era, a growing incidence of KS was caused by the increase in transplants and the consequent use of immunosuppressive drugs, although the mechanism is not very clear. However, also HHV-8 infection and other factors are involved in the development of this neoplastic disease. Epidemiological studies have postulated that transmission of HHV-8 occurs mainly during sexual activity among homosexual men. KS generally affects individuals with low CD4 counts (<150–200 cells/mm3), and the most common sites are lymph nodes (72%), lung (51%), gastrointestinal tract (48%), liver (34%) and spleen (24%) [2].
After the introduction of HAART the incidence of some HIV-related forms of cancer has significantly declined compared to the past. However, the incidence of KS has increased in patients at risk, e.g. patients with concurrent HHV-8 infection, high viral loads and angiogenic factors. The skin is nearly always involved, but this finding is of little use in the clinical approach to hepatic KS, and a differential diagnosis including other infectious and neoplastic diseases is necessary [3–6].
In hepatic KS, the US image of the liver is inhomogeneous with multiple hyperechoic periportal bands and nodules. CT reveals numerous small liver nodules, often in the periportal area with portal and hilar contrast enhancement. MRI shows nodules that are hyperintense on T1-weighted in-phase images and hypointense on T1-weighted out-of-phase images owing to the presence of fat. T2-weighted images do not show signal changes, and late hepatobiliary volumetric interpolated breath hold examination (VIBE) shows no specific uptake of contrast by the fatty nodules. This was confirmed by biopsy, which showed diffuse steatosis surrounded by typical angiosarcoma-like lesions which were easily identified by specific monoclonal markers (CD31, CD34 and FVIII) [7,8].
In this case, the patient had digestive tract palsy with abdominal lymphoadenomegaly. These are signs of widespread lymph node and vascular involvement of the disease, which usually resolves through antiretroviral treatment. US and CT identified hepatic involvement which permitted prompt implementation of therapy.
In patients with KS, HAART therapy allows a rapid viral load reduction and decrease in angiosarcomatose lesions in all organs, as ritonavir-boosted protease inhibitors (PIs) have a specific antiangiogenetic effect permitting rapid immune reconstitution with recovery of CD4 cell count. This leads to a decrease in proinflammatory cytokines which are essential for the growth of spindle-like KS cells.
Conflict of interest
The authors have no conflicts of interest to disclose.
Appendix A. Supplementary data
References
- 1.Tappero J.W., Conant M.A., Wolfe S.F., Berger T.G. Kaposi's sarcoma. Epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371–395. doi: 10.1016/0190-9622(93)70057-z. [DOI] [PubMed] [Google Scholar]
- 2.Saltz R.K., Kurtz R.C., Lightdale C.J., Myskowski P., Cunnigham-Rundles S., Urmacher C. Kaposi's sarcoma. Gastrointestinal involvement correlation with skin findings and immunological function. Dig Dis Sci. 1984;29:817–823. doi: 10.1007/BF01318424. [DOI] [PubMed] [Google Scholar]
- 3.Valls C., Cañas C., Turell L.G., Pruna X. Hepatosplenic AIDS-related Kaposi's sarcoma. Gastrointest Radiol. 1991;16:342–344. doi: 10.1007/BF01887385. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T., Masuda G., Takechi A., Kobayashi H., Tanaka S., Koike M. A case of AIDS-related Kaposi's sarcoma. J Gastroenterol. 1995;30:268–272. doi: 10.1007/BF02348677. [DOI] [PubMed] [Google Scholar]
- 5.Luburich P., Bru C., Ayuso M.C., Azón A., Condom E. Hepatic Kaposi sarcoma in AIDS: US and CT findings. Radiology. 1990;175:172–174. doi: 10.1148/radiology.175.1.2179988. [DOI] [PubMed] [Google Scholar]
- 6.Defalque D., Menu Y., Matheron S., Girare P.M., Nahum H. Hepatic Kaposi's sarcoma and AIDS. Ultrasonographic and x-ray computed tomographic aspects. J Radiol. 1988;69:617–620. [PubMed] [Google Scholar]
- 7.Restrepo C.S., Martínez S., Lemos J.A., Carrillo J.A., Lemos D.F., Ojeda P. Imaging manifestations of Kaposi sarcoma. Radiographics. 2006;26:1169–1185. doi: 10.1148/rg.264055129. [DOI] [PubMed] [Google Scholar]
- 8.Nobrega da Costa D., Viana P.C.C., Maciel R.P., Santiago Gebrim E.M.M., de Souza Roche M. Kaposi sarcoma related to acquired immunodeficiency syndrome: hepatic findings on computer tomography and magnetic resonance imaging. Radiol Bras. 2008;41:139–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


