Abstract
Interest has been increasing in the use of transthoracic ultrasound for the study of the pleuropulmonary disease. US imaging depends mainly on the physical interactions between ultrasound waves and the tissues being examined. In the thoracic region, the prescence of the chest wall and the air-containing pulmonary tissues cause various artifacts that strongly influence the resulting images. At the interface between tissues and air, the ultrasound beam is totally reflected and produces simple reverberation, comet-tail artifacts, and ring-down artifacts.
We report the findings of transthoracic ultrasound in normal healthy subjects and in those who had undergone pneumonectomy.This experience shows that, in terms of the ultrasound artifacts mentioned above, the postpneumonectomy cavity is not significantly different from the healthy lung.
Keywords: Transthoracic, Normal pleuropulmonary, Pneumonectomy space, Ultrasound artifacts
Sommario
SommarioL’interesse nell’utilità dell’ecografia del torace si è di recente accresciuto specialmente nello studio delle malattie pleuro-polmonari. L’immagine ecografica dipende prevalentemente dalle interazioni fisiche tra gli ultrasuoni e i tessuti esaminati; pertanto nello studio del torace la presenza della gabbia toracica e del tessuto polmonare contenente aria influenza fortemente le immagini generando molteplici artefatti. Il fascio ultrasonoro passando attraverso l’interfaccia tessuti/aria è quasi totalmente riflesso, producendo semplici riverberazioni (artefatti orizzontali), “comet-tail” e “ring-down” (artefatti verticali). Riportiamo gli effetti fisici implicati nella formazione di artefatti durante l’ecografia del torace in soggetti normali confrontati con lo spazio residuo post-pneumonectomia. In tutti i soggetti sani esaminati così come nella cavità residua dopo pneumonectomia abbiamo rilevato tutti gli artefatti descritti in accordo con i principi fisici degli ultrasuoni.
Introduction
In the last few years, transthoracic ultrasound (TU) has aroused increasing interest among emergency-room physicians, pneumologists, and even veterinarians as a useful diagnostic tool for studying pleuropulmonary disease [1]. The physics of ultrasound has been studied since the introduction of diagnostic sonography [2]. The appearance of ultrasound images is determined by physical interactions between sound waves and body tissues.
In spite of its limitations, which are related to the basic physical principles of ultrasound [3], TU has proved to be useful as a complementary imaging modality for the study of pleural lesions, including effusions, pleural and subpleural lesions and invasive tumors [4].
Pleuropulmonary ultrasonography is limited not only by the chest wall but by the constant presence of artifacts related to the air in the lungs. These artifacts are caused the substantial difference in acoustic impedance that occurs at an air/soft–tissue interface, and they include simple reverberation as well as the “comet-tail” and “ring-down” phenomena. Artifacts, by definition, are images that lack anatomic correspondents. They are related to the physical effects of ultrasound and, especially in the lung, to the cardiac and respiratory movements, and they can be a source of confusion for the examiner.
The aim of this study was to evaluate the US artifacts observed during examination of the lungs of healthy subjects and the residual cavity of patients who had undergone pneumonectomy.
Materials and methods
Between November 2003 and December 2009, we enrolled 360 healthy subjects (M/F 138/122, aged 18–57 years) and 96 patients who had undergone total pneumonectomy (M/F 49/7, aged 49–68 years). The healthy subjects were nonsmoking hospital employees who had recently (within 1 week of enrolment) undergone yearly physical examinations that included a detailed medical history, a complete physical examination, a chest x-ray, and an electrocardiogram. Candidates were excluded if they presented any type of cardiac disease, hypertension, pulmonary or pleural diseases, allergic diseases, renal and hepatic chronic diseases. The pneumonectomy patients had been operated on for stage T1-2, N0 M0 lung tumors, and they were recruited from the Thoracic Surgery Outpatient Clinic of our hospital. All 96 underwent TU (as described below) 3, 6, and 12 months after surgery; in 37 cases, TU was also performed 18, 24, and 30 months postoperatively. The study was approved by the ethics board of our hospital. All patients provided written informed consent.
Transthoracic ultrasound was performed by an internal medicine specialist (operator 1) with specific training in lung sonography (MS). An Esaote Technos MPX scanner (Esaote, Genoa, Italy) was used with multifrequency linear (8–12 MHz) and convex (3.5–5 MHz) transducers. The ultrasound examination was performed with patients in the seated position or lying down (supine, prone, lateral decubitus as needed), and scans were made through all ventral and dorsal intercostal spaces. The scanner was preset for use with a convex probe, scanning depth of 7–15 cm, a gain of 55%, and a frequency of 3.5–5 MHz.
The following scans were performed on each participant: 2 anterior parasternal scans, 2 latero-basal scans on the posterior axillary line, and 2 posterior paravertebral scans done on the midscapular line. The entire examination lasted approximately 25 min (range 20–35 min). Each examination was recorded and the video reviewed independently and under blinded conditions by a second examiner (operator 2), a radiologist with over 20 years of experience in the study of pleuropulmonary disease.
TU findings in the pneumonectomy group were compared with follow-up radiographs, which were performed 1 month and 3 months after surgery and with chest CT scans obtained 6, 12, and 24 months after surgery, as part of the standard postoperative follow up protocol.
Statistical methods
Poisson regression models for count data were used to compare the number of artifacts detected in the healthy subjects and those who had undergone total pneumonectomy. Generalized estimating equations [5] were used to account for repeated measures within each patient.
Results were reported as estimated means with 95% confidence intervals (CI). P-values are reported for differences between the two groups of subjects in terms of estimated mean numbers of reverberation, comet-tail, and ring-down artifacts and of total artifacts (calculated as the sum of the three previously cited artifacts). Results obtained by the two observers were analyzed separately.
P-values <0.05 were considered significant. All analyses were carried out with SAS® software, version 9.1.
Results
All three types of sonographic artifacts were observed at TU in all of the healthy subjects and pneumonectomy patients. In all of the healthy subjects, scans through each intercostal space revealed reverberation, comet-tail, and ring-down artifacts (Fig. 1a,b). The mean thickness of the pleural line in this group (measured with the convex transducer) was 1.6 mm (range 1.2–2.4 mm), and there was no statistically significant difference according to sex or age (>30 years vs. ≤ 30 years). In the pneumonectomy patients, ultrasound examination of the pneumonectomy space revealed all the artifacts (Fig. 2). The pneumonectomy space appeared as a non-mobile hyperechoic line. In these patients, chest X-rays obtained during the 1-month and 3-month follow-up visits showed opacity of the hemithorax that had been operated on, which masked the border of the diaphragm outline; the presence of an air-fluid level; mediastinal and tracheal deviation; and initial herniation of the intact lung into the pneumonectomy cavity. Thoracic CT scans performed 6 months after pneumonectomy showed effusions in remaining cavity, deviation of mediastinum, and a shift inthe contralateral lung toward the remaining cavity (Fig. 3). The 12-month postoperative CT scan showed small effusions in the cavity. In 57 of the 96 pneumonectomy patients, the presence of ring-down artifacts was increased on the 6 and 12-month scans.
Fig. 1.
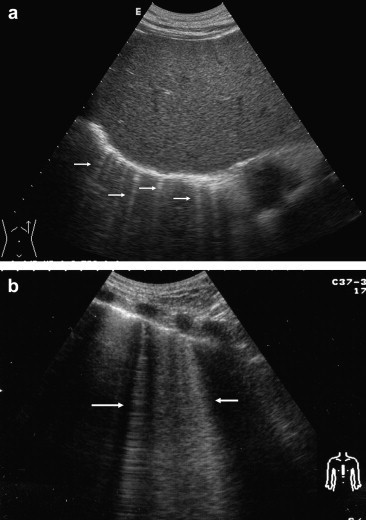
Transthoracic ultrasound scan in healthy subjects (supine position). a) Subcostal scan shows “comet-tail” artifacts (arrows) beyond the diaphragmatic line. b) Parasternal scan in a different subject shows “ring-down” artifacts (arrows).
Fig. 2.
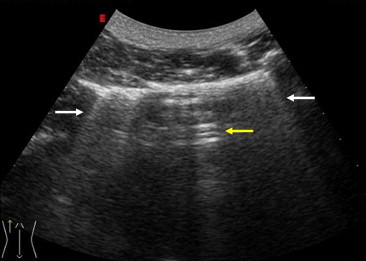
Laterobasal US scan in a pneumonectomy patient (examined in the seated position) reveal “ring-down” (white arrow) and reverberation artifacts (yellow arrows).
Fig. 3.
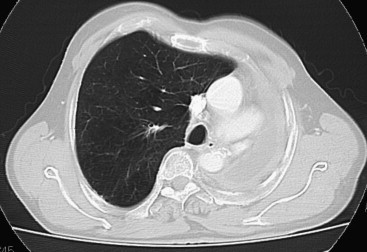
Computed tomography of the chest in a pneumonectomy patient reveals pleural effusion in the residual space with mediastinal sliding. The intact lung is shifted toward the residual cavity.
Table 1 shows the ultrasound findings for both groups. The estimated mean number of artifacts (comet-tail, ring-down, simple reverberation, and total artifacts) observed through each intercostal space are reported separately for operators 1 and 2. The results calculated by Operator 1 revealed no significant differences between the two groups (means for healthy subjects: 2.25 comet-tail, 1.95 ring-down, 3.40 reverberations,total artifacts 7.60 versus means for pneumonectomy group: 2.28 comet-tail, 1.91 ring-down, 3.38 reverberations, 7.57 total artifacts), and similar results were obtained by Operator 2,suggesting that transthoracic ultrasonography cannot distinguish between normal subjects and pneumonectomy patients.
Table 1.
Estimated number of artifacts detected with convex transducer (3.5–5 MHz) during intercostal US scans in the two groups.
| Artifact | Operatora | Healthy subjects (Estimated Mean)b | Pneumonectomy patients (Estimated Mean)b | p-valuea |
|---|---|---|---|---|
| Comet-tail | Operator 1 | 2.25 | 2.28 | 0.28 |
| Operator 2 | 2.05 | 2.10 | 0.19 | |
| Ring-down | Operator 1 | 1.95 | 1.91 | 0.10 |
| Operator 2 | 1.85 | 1.89 | 0.20 | |
| Simple reverberation | Operator 1 | 3.40 | 3.38 | 0.56 |
| Operator 2 | 3.37 | 3.37 | 0.79 | |
| Total Artifacts | Operator 1 | 7.60 | 7.57 | 0.59 |
| Operator 2 | 7.26 | 7.37 | 0.10 |
Operator 2 was blinded to the group origin of the scan.
Estimated means for the healthy subjects versus pneumonectomy patients (Poisson regression models).
Discussion
The basic principles of ultrasonography are related to the physics of ultrasound and to differences between different tissues in their ability to impede the passage of the ultrasound waves (acoustic impedance, i.e., the product of the density of the material and propagation speed of the sound waves). Between the kidney and the liver, for example, this difference is very small), but if the difference in acoustic impedance is very large, the entire ultrasound beam will be reflected. At a intercostal tissue-pulmonary air interface, over 90% of the beam is reflected, leaving almost none for further imaging.
Some artifacts are well known, such as posterior acoustic shadowing of the chest (ribs, sternum, scapula) [6] and the “mirror effect” whereby mirror images of subdiaphragmatic hepatic lesions are projected onto the pulmonary side of the diaphragm [7]. Ring-down artifacts have been misinterpreted by some authors as comet-tail artifacts [8,9]. These artifacts are always generated by substantial differences in the acoustic impedance of two adjacent tissues [10]. This interface is encountered by the ultrasound beam in the pleural space, and the result is hyperechoic “pleural” line that moves during respiration (gliding or sliding sign). The same principle is responsible for reverberation artifacts, echoes of decreasing intensity that lie parallel to the pleural line. The echoes are spaced at a fixed interval, which depends on the time elapsing between emission and registration of the signal (Fig. 4).
Fig. 4.
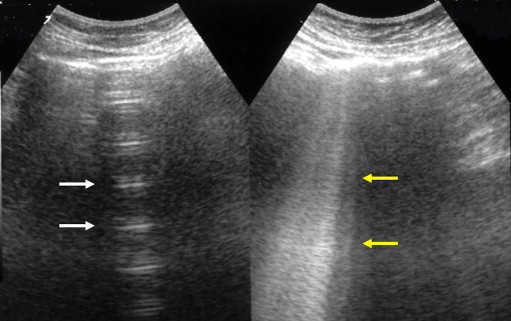
Posterior US scan in a healthy subject shows simple reverberation (white arrow) and ring-down artifacts (yellow arrow).
Comet-tail artifacts were first described by Wendell and Athey [11] and later studied in depth by Thickmann and Ziskin [12]. They are high-amplitude echoes that fan out from their source along the US beam like the tail of a comet. These artifacts arise from hyperechoic interfaces (e.g., cholesterol crystals in the anterior wall of the gallbladder, foreign bodies like metal clips in organ parenchyma, small gas bubbles within the biliary tree) [13]. In pleuropulmonary ultrasonography, comet-tail artifacts are caused by the difference in acoustic impedance at the soft tissue/ pulmonary air interface. In fact, they are observed in the normally ventilated lung and in all the cases where these interfaces exist, including distal subpleural lesions of any nature and lesions within the pleural space (inflammatory foci, tumors, diffuse or focal pleural thickening) [14].
Ring-down artifacts have been described by Avruch and Cooperberg [15] as a series of continuous hyperechoic lines or parallel bands radiating along the ultrasound beam. They are readily distinguished from comet-tail reverberations. They can be seen behind mixed air-fluid effusions, for example, in intestinal loops.
Wilson [16] hypothesized that ring-down artifacts were caused by the prescence of small amounts of fluid containing microbubbles. In fact when the incident US beam encounters a mixture of gas and fluid, it is converted into resonance and a new US beam is produced, with a characteristic frequency that is different from the incident one, which is reflected back towards the transducer. This theory might explain why ring-down artifacts are more numerous (>6) in different lung disorders characterized by altered fluid-to-gas ratios, such as interstitial lung diseases (pulmonary edema, pulmonary fibrosis), acute inflammatory bronchopneumonia, and neoplastic lymphangitis, exacerbations of chronic broncopneumopathy, hydrothorax, acute bronchial asthma [9,17–20].
All of these artifacts-simple reverberations and ring down artifacts—were also observed in the healthy subjects we examined. This is consistent with the normal physical behavior of ultrasound waves in this area of the body. In patients who had undergone pneumonectomy, the pneumonectomy space displayed all the different types of ultrasound artifacts in variable percentages. In fact, the residual cavity contains both gas (air) and fluid (effusion liquid), which are the physical basis for the creation of ultrasound artifacts. The difference in acoustic impedance at the soft tissue–residual cavity interface causes simple reverberations and comet-tail artifacts, whereas the mixture of effusion liquid and residual air is the source of the ring-down pattern. In our pneumonectomy patients, CT scans confirmed the presence of liquid or exudate, supporting the view that ring-down artifacts occur when the ultrasound beam passes through a mixture of liquid and air, such as that which sometimes forms in in the residual cavity after pneumonectomy.
All of our pneumonectomy patients also presented a fixed hyperechoic line at the soft tissue/residual cavity interface, which resembled the pleural line except that it remained motionless during respiration (Fig. 5).The physical explanation for this hyperechoic line is, once again, an interface—in this case, between soft tissues and an air-containing cavity-characterized by a substantial difference in acoustic impedance. In the healthy lung, this hyperechoic line moves with respiratory movements (“gliding” or sliding sign”), and it is conventionally referred to as the pleural line although it is not the anatomical equivalent of the visceral and parietal pleurae (thickness 200-400 microns) [21]. In the residual cavity of pneumonectomy patients, the line is fixed and does not move with respiratory movements.
Fig. 5.
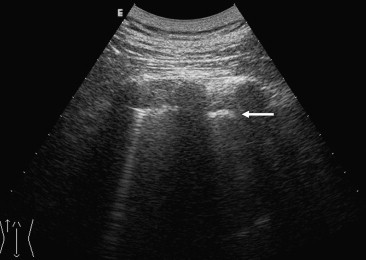
Posterior US scan in a pneumonectomy patient reveals a hyperechoic pleural line (arrow) and one “ring-down”.
Conclusion
In our study, the well-known artifacts were observed in variable percentages of the healthy, aerated lungs we examined with transthoracic ultrasound. These artifacts arise when an ultrasound beam encounters an interface characterized by a marked difference in acoustic impedance.
The usefulness of pleuropulmonary ultrasonography is an open issue that continues to be debated. In the absence of data from large case series and in-depth studies of healthy subjects, caution should be used in validating artifacts as diagnostic patterns. Thanks to the ongoing improvement of ultrasound technology, sonographic images can now be automatically optimized with elimination of artifacts like the second harmonic and the digital compound. This trend could further modify normal visualization of anatomic tissues, eliminating the interference and misleading effects of the numerous artifacts that are normally present in the thoracic cavity [22]. Artifacts are “imaging errors” caused by physical processes that affect the ultrasound beam and in some way alter the basic assumptions the operator makes about the beam [23].
For the time being, pleuropulmonary ultrasonography can be considered a complementary method for diagnosing several chest diseases; it is also an optimal guide during percutaneous procedures involving the aspiration of pleural effusions or the biopsy of peripheral pleural and lung consolidations [24,25]. The enthusiasm raised by the well-known advantages of ultrasound -widespread availability, noninvasiveness, low costs have to be reconciled with the limitations of this imaging modality in the chest. Due to the presence of these artifacts, ultrasonography can never be the sole basis of imaging diagnosis of pleural and lung diseases.
Conflict of interest statement
The authors have no conflict of interest.
AppendixSupplementary material
References
- 1.Flock M. Diagnostic ultrasonography in cattle with thoracic disease. Vet J. 2004;167:272–280. doi: 10.1016/S1090-0233(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 2.Mikhak Z., Pedersen P.C. Acoustic attenuation properties of the lung: an open question. Ultrasound Med Biol. 2002;28:1209–1216. doi: 10.1016/s0301-5629(02)00561-6. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen P.C., Ozcan H.S. Ultrasound properties of lung tissue and their measurements. Ultrasound Med Biol. 1986;12:483–499. doi: 10.1016/0301-5629(86)90220-6. [DOI] [PubMed] [Google Scholar]
- 4.Meuwly J.Y., Gudinchet F. Sonography of the thoracic and abdominal walls. J Clin Ultrasound. 2004;32:500–510. doi: 10.1002/jcu.20070. [DOI] [PubMed] [Google Scholar]
- 5.Diggle P.J., Liang K.Y., Zeger S.L. Oxford University Press; Oxford: 1994. Analysis of Longitudinal data. [Google Scholar]
- 6.Aldrich J.E. Basic physics of ultrasound imaging. Crit Care Med. 2007;35:S131–S137. doi: 10.1097/01.CCM.0000260624.99430.22. [DOI] [PubMed] [Google Scholar]
- 7.Hedrick W.R. Image artifacts in Real-time ultrasound. J Diagn Med Son. 1995;11:300–308. [Google Scholar]
- 8.Skolnick M.L., Meire H.B., Lecky J.W. Common artifacts in ultrasound scanning. J ClinUtrasound. 1975;3:273–280. doi: 10.1002/jcu.1870030408. [DOI] [PubMed] [Google Scholar]
- 9.Chan S.S. Comet tail artifacts in emergency chest ultrasound. Am J Emerg Med. 2007;25:724–725. doi: 10.1016/j.ajem.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Hangiandreou N.J. AAPM/RSNA physic tutorial for resident. Topics in US: B-modeUS: basic concepts and new technology. Radiographics. 2003;23:1019–1033. doi: 10.1148/rg.234035034. [DOI] [PubMed] [Google Scholar]
- 11.Wendell B.A., Athey P.A. Ultrasonic appearance of metallic foreign bodies in parenchimal organs. J Clin Ultrasound. 1981;9:133–135. doi: 10.1002/jcu.1870090307. [DOI] [PubMed] [Google Scholar]
- 12.Thickman D.I., Ziskin M.C., Goldemberg N.J., Linder B.E. Clinical manifestation of the comet tail artifact. J Ultrasound Med. 1983;2:225–230. doi: 10.7863/jum.1983.2.5.225. [DOI] [PubMed] [Google Scholar]
- 13.Kremkau F., Taylor K.J. Artifacts in ultrasound imaging. J Ultrasound Med. 1986;5:227–237. doi: 10.7863/jum.1986.5.4.227. [DOI] [PubMed] [Google Scholar]
- 14.Doelken P., Strange C. Chest ultrasound for “Dummies”. Chest. 2003;123:332–333. doi: 10.1378/chest.123.2.332. [DOI] [PubMed] [Google Scholar]
- 15.Avruch L., Cooperberg P.L. The ring-down artefact. J Ultrasound Med. 1985;4:21–28. doi: 10.7863/jum.1985.4.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Wilson S.R., Burns P.N., Wilkinson L.M., Simpson D.H., Muradali D. Gas at abdominal US: appearance, relevance, and analysis artifacts. Radiology. 1999;210:113–123. doi: 10.1148/radiology.210.1.r99ja12113. [DOI] [PubMed] [Google Scholar]
- 17.Rajan G.R. Ultrasound lung comets: a clinically useful sign in acute respiratory distress syndrome/acute lung injury. Crit Care Med. 2007;35:2869–2870. doi: 10.1097/01.CCM.0000288094.70325.E4. [DOI] [PubMed] [Google Scholar]
- 18.Bedetti G., Gargani L., Corbisiero A., Frassi F., Poggianti E., Mottola G. Evaluation of ultrasound lung comet by hand-held echocardiography. Cardiovasc Ultrasound. 2006;4:34. doi: 10.1186/1476-7120-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouhemad B., Zhang M., Lu Q., Rouby J.J. Clinical review: bedside lung ultrasound in critical care practice. Crit Care. 2007;11:205. doi: 10.1186/cc5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louvet A., Bourgeois J.M. Lung ring-down artifact as a sign of pulmonary alveolar-interstitial disease. Vet Radiol Ultrasound. 2008;49:374–377. doi: 10.1111/j.1740-8261.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 21.De Luca C., Valentino M., Rimondi M.R., Branchini M., CasadioBaleni M., Barozzi L. Use of chest sonography in acute-care radiology. JUS. 2008;11(4):1–12. doi: 10.1016/j.jus.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperandeo M., Varriale A., Sperandeo G., Filabozzi P., Piattelli M.L., Carnevale V. Transthoracic ultrasound in the evaluation of pulmonary fibrosis: our experience. Ultrasound Med Biol. 2009;35:723–729. doi: 10.1016/j.ultrasmedbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Treece G.M., Gee A.H., Prager R.W. Ultrasound compounding with automatic attenuation compensation using paired angle scans. Ultrasound Med Biol. 2007;33:630–642. doi: 10.1016/j.ultrasmedbio.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Sperandeo M., Carnevale V., Varriale A. Response to pleura-pulmonary US examination artifacts: “errors in images”. Ultrasound Med Biol. 2010;36:357. [Google Scholar]
- 25.Sperandeo M., Filabozzi P., Varriale A., Carnevale V., Piattelli M.L., Sperandeo G. Role of thoracic ultrasound in the assessment of pleural and pulmonary diseases. JUS. 2008;11(2):39–46. doi: 10.1016/j.jus.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


