Abstract
Introduction
Real-time elastography (RTE) is a novel technique for measuring tissue elasticity. The aims of this study were to prospectively measure liver stiffness with RTE in patients with chronic viral hepatitis and to evaluate the possible correlation between RTE data and the extent of fibrosis based on liver biopsy findings (Ishak score).
Material and methods
Between February and October 2011, 26 patients (18M, 8F, mean age 41 ± 13 [standard deviation], range 22–62) with chronic viral hepatitis were prospectively evaluated with ultrasonography (US) that included RTE. All patients then underwent US-guided percutaneous liver biopsy (right lobe) for evaluation of fibrosis. Examinations were performed with a iU22 scanner (Philips, Bothell, WA, USA); a convex transducer (C5-1) was used for the US examination, and a linear transducer (L12-5) for RTE. In the RTE images, relative tissue stiffness is expressed according to a color scale with soft areas represented in green/red and hard areas in blue. Patients were examined in the supine position in suspended normal respiration; three loops of 20 RTE frames were recorded for each case. For each patient, we calculated the mean strain ratio (MSR) for the 3 loops. The Spearman correlation coefficient was used to assess correlation between the ASR and fibrosis stage (F) reflected by the Ishak score.
Results
The Spearman coefficient showed significant correlation between the MSR and F (Rho = 0.470, p = 0.015).
Conclusions
RTE appears to be a useful tool for noninvasive evaluation of fibrosis in patients with chronic viral hepatitis although these findings need to be confirmed in larger case series.
Keywords: Real-time elastography, Liver fibrosis, Liver disease
Sommario
Introduzione
L'elastosonografia real-time (ERT) è una nuova tecnologia integrata all’ultrasonografia convenzionale in grado di rappresentare la rigidità dei tessuti. Scopo dello studio è quantificare la rigidità epatica tramite ERT in pazienti con epatopatia cronica virale e ricercare una correlazione con lo staging istologico di malattia.
Materiali e metodi
Da Febbraio a Ottobre 2011, 26 pazienti (18M, 8F, età media 41 ± 13 [deviazione standard], range 22–62) sono stati valutati prospetticamente con ecografia addominale (US) ed ERT e sono stati sottoposti a biopsia epatica (lobo destro) secondo indicazione clinica. Gli esami sono stati eseguiti con l'ecografo iU22 (Philips, Bothell, WA, USA) usando per l'US una sonda convex (C5-1) e per l'ERT una lineare (L12-5). L'apparecchiatura rappresenta la rigidità relativa dei tessuti esplorati secondo una scala cromatica, mostrando le porzioni più rigide in blu e quelle più morbide in verde/rosso. Per tutti i pazienti, sono stati registrati 3 loop di 20 frame di ERT; è stata ottenuta la media delle medie degli strain ratio dei 3 loop (MSR) ed è stato calcolato l’indice di correlazione di Spearman (IS) tra la MSR e lo stadio di fibrosi (F) secondo Ishak.
Risultati
L’IS è risultato significativo (rho = 0.470, p = 0.015) mostrando una buona correlazione tra F e MSR.
Conclusioni
l'ERT potrebbe essere utile per la quantificazione non invasiva della fibrosi nei pazienti con epatopatia cronica su base virale, ma per confermare questa ipotesi è necessaria una casistica più ampia.
Introduction
Assessment of hepatic fibrosis is fundamental for staging chronic viral liver disease. The information it provides is indispensable for planning drug therapy and follow-up in these cases, especially when antiviral drugs are prescribed [1,2]. The extent of fibrosis is one of the main predictors of response to drug therapy in patients with HCV-related liver disease [1]. The “gold standard” method for quantifying fibrosis is still percutaneous liver biopsy, although owing to its invasiveness, this procedure is not without risks [3]. Up to 25% of patients experience postprocedural abdominal pain, but the incidence of serious complications is low (1 in 4000-10,000) [4–6]. Other limitations involve sampling errors, which can occur in several types of liver disease, including those caused by HCV [7–10]; operator-dependent variability in the interpretation of the morphological features of the specimen [11], which can lead to under- and overestimates of the actual extent of fibrosis; costs; the limited repeatability of biopsy (which makes it unsuitable for monitoring the evolution of the disease); and contraindications to the procedure (which include coagulation disorders, lack of patient cooperation, refusal to submit to the procedure).
For these reasons, numerous attempts have been made in the past few years to develop noninvasive methods for assessing hepatic fibrosis, some based on laboratory tests, others on instrumental techniques. One of the first ultrasound-based methods investigated for the measurement of liver stiffness (as an indirect index of fibrosis) was transient elastography (FibroScan), which is based on the use of mechanical waves generated by vibration [12]. Its diagnostic reliability has been validated in patients with HCV-related liver disease (in three important multicenter studies) [13–15] and in patients with nonalcoholic fatty liver disease [16]. It is important to recall, however, that in some cases (10–20%), reliable data cannot be obtained due to excess body weight, narrow intercostal spaces, or excessive measurement variability [17,18].
Acoustic radiation force impulse (ARFI) imaging is an elastosonographic technique that furnishes quantitative measurements of hepatic elasticity during conventional abdominal B-mode sonography. It involves the transmission of a high-intensity ultrasound pulse, which causes tissue displacement and thereby produces a new mechanical wave that propagates transversely to the transmitted pulse. The propagation velocity of this wave (referred to as shear wave velocity) can be measured, and it correlates with the stiffness of the hepatic parenchyma. ARFI elastography has proved to be a reliable tool for assessing hepatic fibrosis in patients with chronic liver disease [19,20].
Real-time elastography (RTE) is another new technique that is used during conventional B-mode sonography to evaluate tissue stiffness. It provides qualitative (colorimetric) and quantitative readouts. This method has been used to differentiate benign and malignant lesions of the thyroid, breast, and prostate [21] and to study the liver [22,23]. The aims of this study were to measure hepatic stiffness using RTE in patients with chronic viral liver disease and to assess the possible correlation between these data and the extent of fibrosis assessed with liver biopsy (the reference method).
Materials and methods
We prospectively enrolled 26 patients with chronic viral liver disease (18 men, 8 women, mean age 41 ± 13 years [standard deviation], range 22–62) with clinical indications for ultrasound-guided percutaneous liver biopsy. The inclusion criteria were as follows: 1) chronic liver disease related to viral infection; 2) indication for liver biopsy; 3) patient consent. Candidates were excluded if they had any of the following: 1) contraindications for liver biopsy; 2) history of liver transplant; 3) presence of ascites or liver tumor; 4) nonconsent to participation in the study.
During the outpatient visit, each patient underwent abdominal ultrasonography, RTE, and liver biopsy. Abdominal sonography was performed with a commercial scanner (Philips iU22, Bothell, WA, USA) and a convex transducer (C5-1). RTE was carried out with the same system (which was equipped with elastographic software) and a linear transducer (L12-5). RTE is a noninvasive technique that provides information on the elastic properties of tissues. It provides analysis of the radio frequency signal of each line of view of the transducer.
The signal is then represented on the screen in a color-coded manner: blue for harder tissues, red/green for those that are softer. The elastographic module installed on the system is based on real-time analysis of the tissue displacement (strain) induced by dynamic excitation of the zone of interest (by heart beats or respiratory movements). The result is visualized within a box, where it can be represented in different chromatic scales (Fig. 1). Software provided by the manufacturer (QLAB®, Philips) furnishes a numerical value that represents the stiffness of the tissue being examined (strain ratio, SR) (Fig. 2).
Figure 1.
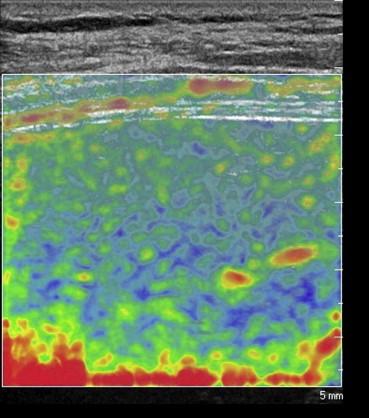
Elastographic image obtained during an intercostal scan performed with a linear transducer. It provides real-time colorimetric representation of liver stiffness: blue indicating the stiffer areas of the parenchyma and red/green those that are softer and more compliant.
Figure 2.
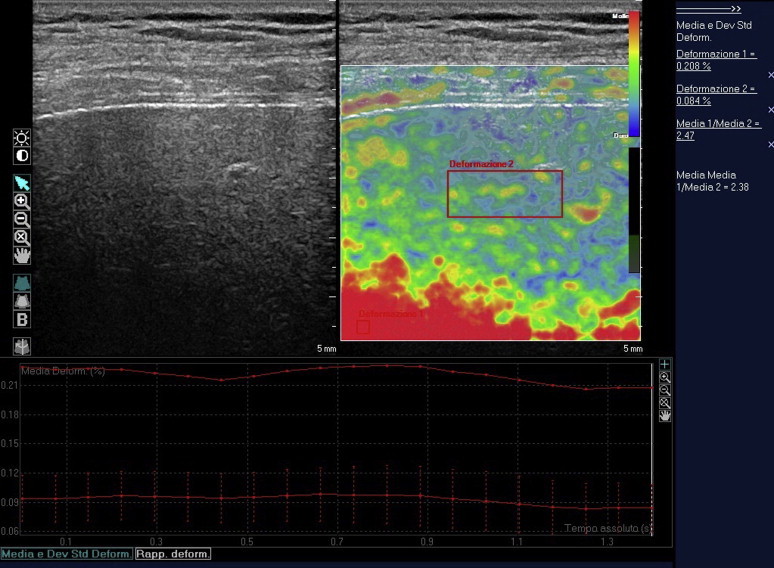
Elaboration of the recorded images using QLAB software. Tissue stiffness can be quantified by placing a ROI over the area that appears softest (i.e., red, used as a reference) and a second ROI over the parenchyma. The ratio of the deformation values for the two ROIs is the strain ratio.
In our study, the transducer was positioned over the intercostal space that offered the best view of the parenchyma; the same space was then used to perform the liver biopsy. Patients were examined in the supine position during suspended normal respiration. When the elastographic image had stabilized (as confirmed by an indicator on the side of the screen), we recorded three 20-frame loops of RTE. The size and position of the elastographic box were selected to include only parenchyma (blood vessels and the gallbladder were excluded whenever possible) with the deepest portion of the image represented in red; this was achieved with the preinstalled optimization algorithm “Elasto 2”.
Using the software provided by the manufacturer, we calculated the SR (expressed as an absolute number) for each frame, obtained from the ratio of a first region of interest (ROI-1) deformation value to a second ROI (ROI-2) deformation value; ROI-1 was placed over the tissue that was most uniformly red (that represented as the bottom of the scale and considered the reference value), ROI-2 included hepatic parenchyma located 1 cm from the capsule. For each loop of 20 images, we calculated the mean SR (mSR), and the mean of the mSRs of the three loops was calculated for each patient (MSR).
Immediately after the elastographic images were acquired, liver biopsy was done at the same site used for the sonographic examination. All biopsies were carried out percutaneously with ultrasound guidance by the same operator (CS), using an 18G needle. All of the histologic specimens were adequate for evaluation. They were examined by a pathologist who was unaware of the elastographic findings. Histological features were classified using the Ishak scoring system [24].
The Spearman correlation coefficient was used to assess the possible correlation between the MSR and the Ishak score for fibrosis (F).
The study was conducted in accordance with the ethical standards of the Helsinki Declaration adopted in June 1964, and the protocol was approved by the competent commissions. All patients provided written informed consent to take part in the study.
Results
Of the 26 patients we examined, 14 were HCV-positive, 5 were HBV-positive, 3 were positive for HBV and HDV, 3 for HCV and HIV, and 1 was positive for HBV, HCV, and HIV. Histologic examination revealed F1 fibrosis in 9 patients, F2 in 7, F3 in 6, F4 in 1, F5 in 1, and F6 in 2. The mean MSR for the entire study population was 2.77 ± 0.44 (range 2.24–4.08, median 2.63).
The mean areas of ROI1 and ROI2 were 3.7 ± 0.1 mm2 and ROI2 146.9 ± 0.7 mm2, respectively. The Spearman correlation coefficient revealed significant correlation between the stage of fibrosis and the MSR (Rho = 0.470, p = 0.015) (Fig. 3).
Figure 3.
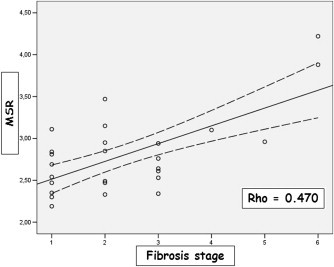
Correlation between the MSR (mean of the mean strain ratios) and the stage of fibrosis.
Discussion
Several instrumental methods have been used to quantify hepatic fibrosis in a noninvasive manner. The most widely used is FibroScan, which is mentioned in recent practice guidelines among the techniques available for this purpose [1,2]. Nonetheless, percutaneous liver biopsy remains the reference standard for this type of evaluation [25].
There is also an increasing body of evidence on the use of ARFI, which seems to offer reproducible results that correlate well with hepatic fibrosis [26,27].
The more recently developed technique known as RTE also seems to be producing promising results [22,23,28].
In our study we used this technique to quantify hepatic fibrosis in patients with chronic viral liver disease. Although our study population was small, the data we obtained indicate that the MSR of each patient correlates well with the degree of fibrosis, as indicated by histologic findings (Rho = 0.470, p = 0.015), and the MSR range was similar to that reported by Koizumi et al. [22]. Unlike Koizumi and colleagues, we used three 20-frame loops to calculate the MSR in our patients. The recordings were made at a point in time when the elastographic image on the screen and the indicator at the edge of the screen were stable. In this manner, with a brief acquisition period, we obtained a total of 60 measurements that were used to calculate the patient's MSR with the least possible variability.
The operator should apply only enough pressure to the transducer to ensure good contact with the skin surface: additional pressure is not necessary to obtain quality images.
In the system we used, the RTE module was designed to be used with a linear transducer. For this reason, we were able to analyze only a limited portion of the liver parenchyma, which lay at a depth of no more than about 6 cm. To maximize our chances of collecting elastographic and histologic data from the same area of the liver, the images were acquired using a single point of measurement, i.e., the intercostal space that (in the operator's opinion) offered the best view of the parenchyma, the one also used as the access point for the liver biopsy. However, Koizumi and colleagues [22] did not find any significant variation in the data they collected at different points in the parenchyma.
To calculate the SR, we positioned the ROI1 on an area of the image represented by the color at the bottom of the scale and located in the deepest part of the elastographic box, as specified above. This was done to render the elastographic image of the parenchyma as large as possible so that the ROI2 could be positioned with ease in an area free of artifacts.
Although the patients we studied had disease caused by different viruses, none had potential confounding factors like significant sonographic evidence of steatosis or significantly elevated transaminase levels (over twice the upper limit of normal). In any case, the influence of such factors on elastographic measurements remains to be confirmed. Owing to the small size of our sample, we were not able to identify reference values for distinguishing the various degrees of fibrosis.
In conclusion, although its diagnostic performance needs to be confirmed in larger patient populations, RTE appears to be useful for the noninvasive quantification of fibrosis in patients with chronic viral liver disease.
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
Best Poster Award at the 2012 National Congress of the SIUMB.
Appendix A. Supplementary data
References
- 1.EASL Clinical Practice Guidelines management of hepatitis C virus infection. European association for the study of the liver. J Hepatol. 2011 Aug;55(2):245–264. doi: 10.1016/j.jhep.2011.02.023. Epub 2011 Mar 1. [DOI] [PubMed] [Google Scholar]
- 2.EASL Clinical Practice Guidelines Management of chronic hepatitis B. European association for the study of the liver. J Hepatol. 2009 Feb;50(2):227–242. doi: 10.1016/j.jhep.2008.10.001. Epub 2008 Oct 29. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen D., Talwalkar J.A. Noninvasive assessment of liver fibrosis. Hepatology. 2011 Jun;53(6):2107–2110. doi: 10.1002/hep.24401. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G., Boyer J.L. Outpatient liver biopsy: how safe is it? Ann Intern Med. 1993;118(2):150–153. doi: 10.7326/0003-4819-118-2-199301150-00013. [DOI] [PubMed] [Google Scholar]
- 5.Janes C.H., Lindor K.D. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med. 1993;118(2):96–98. doi: 10.7326/0003-4819-118-2-199301150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Castéra L., Nègre I., Samii K., Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30(6):1529–1530. doi: 10.1002/hep.510300624. [DOI] [PubMed] [Google Scholar]
- 7.Regev A., Berho M., Jeffers L.J., Milikowski C., Molina E.G., Pyrsopoulos N.T. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 8.Maharaj B., Maharaj R.J., Leary W.P., Cooppan R.M., Naran A.D., Pirie D. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 9.Poniachik J., Bernstein D.E., Reddy K.R., Jeffers L.J., Coelho-Little M.E., Civantos F. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 10.Guido M., Rugge M. Liver biopsy sampling in chronic viral hepatitis. Semin Liver Dis. 2004;24:89–97. doi: 10.1055/s-2004-823103. [DOI] [PubMed] [Google Scholar]
- 11.Bedossa P., Poynard T. Metavir cooperative group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 12.Sandrin L., Fourquet B., Hasquenoph J.M., Yon S., Fournier C., Mal F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Ziol M., Handra-Luca A., Kettaneh A., Christidis C., Mal F., Kazemi F. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 14.Castera L., Vergniol J., Foucher J., Le Bail B., Chanteloup E., Haaser M. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Arena U., Vizzutti F., Abraldes J.G., Corti G., Stasi C., Moscarella S. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57(9):1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 16.Wong V.W., Vergniol J., Wong G.L., Foucher J., Chan H.L., Le Bail B. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 17.Castera L., Forns X., Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Castera L., Foucher J., Bernard P.H., Carvalho F., Allaix D., Merrouche W. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H., Ono N., Eguchi Y., Eguchi T., Kitajima Y., Kawaguchi Y. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30(4):538–545. doi: 10.1111/j.1478-3231.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 20.Piscaglia F., Salvatore V., Di Donato R., D'Onofrio M., Gualandi S., Gallotti A. Accuracy of virtual touch acoustic radiation force impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32(2):167–175. doi: 10.1055/s-0029-1245948. Epub 2011 Feb 14. [DOI] [PubMed] [Google Scholar]
- 21.Rago T., Vitti P. Potential value of elastosonography in the diagnosis of malignancy in thyroid nodules. Q J Nucl Med Mol Imaging. 2009;53:455–464. [PubMed] [Google Scholar]
- 22.Koizumi Y., Hirooka M., Kisaka Y., Konishi I., Abe M., Murakami H. Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography-establishment of the method for measurement. Radiology. 2011;258(2):610–617. doi: 10.1148/radiol.10100319. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich-Rust M., Ong M.F., Herrmann E., Dries V., Samaras P., Zeuzem S. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758–764. doi: 10.2214/AJR.06.0322. [DOI] [PubMed] [Google Scholar]
- 24.Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 25.Ghany M.G., Strader D.B., Thomas D.L., Seeff L.B. American association for the study of liver diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fierbinteanu-Braticevici C., Andronescu D., Usvat R., Cretoiu D., Baicus C., Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525–5532. doi: 10.3748/wjg.15.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boursier J., Isselin G., Fouchard-Hubert I., Oberti F., Dib N., Lebigot J. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074–1084. doi: 10.1097/MEG.0b013e328339e0a1. [DOI] [PubMed] [Google Scholar]
- 28.Colombo S., Buonocore M., Del Poggio A., Jamoletti C., Elia S., Mattiello M. Head-to-head comparison of transient elastography (TE), real-time tissue elastography(RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J Gastroenterol. 2012 Jan 6 doi: 10.1007/s00535-011-0509-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


