Abstract
Benign breast diseases constitute a heterogeneous group of lesions arising in the mammary epithelium or in other mammary tissues, and they may also be linked to vascular, inflammatory or traumatic pathologies. Most lesions found in women consulting a physician are benign. Ultrasound (US) diagnostic criteria indicating a benign lesion are described as well as US findings in the most frequent benign breast lesions.
Keywords: Breast, Ultrasound, Benign breast lesions
Sommario
Le lesioni mammarie benigne costituiscono un gruppo eterogeneo di manifestazioni, sia proprie dell’epitelio mammario, sia con origine dagli altri tessuti che costituiscono l’organo, sia con altra patogenesi (vascolare, flogistica e traumatica).
Esse costituiscono il reperto più frequente che si osserva nella maggior parte dei casi nei quali una donna pensa di avere un problema al seno e si rivolge al medico o al radiologo.
Vengono riproposti da un punto di vista ecografico i criteri diagnostici che orientano per la benignità ed i quadri iconografici che si riscontrano nelle principali lesioni mammarie benigne ecograficamente identificabili.
Introduction
Benign breast diseases constitute a heterogeneous group of lesions arising in the mammary epithelium or in other mammary tissues and they may also be linked to vascular, inflammatory or traumatic pathologies. (Table 1) [1]. Some lesions are palpable masses, which may be nodular, sometimes with specific or unspecific characteristics, but often (particularly in lesions of greater prognostic significance such as atypical hyperplasia) there are no specific clinical signs, and detection is difficult also at diagnostic imaging examinations.
Table 1.
Classification of benign breast lesions according to histological origin.
| Terminal and lobular ducts |
| a. Acinar distension |
| i. Cyst |
| b. Intralobular connective tissue proliferation |
| i. Sclerosing adenosis |
| ii. Fibroadenoma |
| iii. Phyllodes tumor |
| iv. Hamartoma |
| c. Epitelial changes in terminal duct lobular units (TDLUs) |
| i. Apocrine metaplasia |
| ii. Ductal and lobular hyperplasia, usual and atypical |
| iii. Papillomatosis |
| iv. Intracystic papilloma |
| Ductal system |
| d. Ductal ectasia |
| e. Intraductal papilloma |
| Lesions of different origin |
| f. Fatty tissue lesions |
| i. Lipoma |
| ii. Liponecrosis |
| g. Fibrous tissue lesions |
| i. Focal fibrosis |
| ii. Diabetic mastopathy |
| iii. Pseudoangiomatous stromal hyperplasia (PASH) |
| iv. Myofibroblastoma |
| h. Vascular origin |
| i. Hemangioma |
| i. Inflammatory origin |
| i. Mastitis/abscess |
| ii. Tuberculosis and sarcoidosis |
| iii. Foreign body granuloma and siliconoma |
| j. Lymph node origin |
| i. Intramammary lymph nodes |
This paper will not deal with mammary dysplasia or fibrocystic mastopathy which is the most common breast lesion in women of childbearing age. This denomination is inappropriate, as it does not refer to a specific histological condition. It is a variable combination of some basic proliferative and regressive lesions induced by hormonal factors affecting both the epithelium and the connective tissue, and in the 1980s Hughes therefore introduced the concept of ANDI (Aberrations of Normal Development and Involution) [2].
It is important to recognize benign breast diseases from the clinical signs as well as mammographic and ultrasound (US) findings, since most lesions found in women consulting a physician are benign. In order to reduce the number of unnecessary biopsies and to avoid inappropriate diagnostic delays, the breast radiologist should also be aware that benign breast diseases may present atypical findings and that possible overlap of findings between certain types of benign and malignant diseases may occur.
Diagnostic US criteria indicating benign lesions are described in this paper as well as US findings in the most frequent benign breast lesions.
US parameters in the diagnosis of a probably benign lesion
US scanning of the breast has long been considered as just an additional examination for identifying the nature of any abnormalities detected at physical examination or at mammography in order to classify them in the following categories:
-
•
Normal tissue
-
•
Simple cyst
-
•
Complicated or complex cyst
-
•
Indeterminate solid or cystic lesion
-
•
Solid lesion [3,4]
However, thanks to the technological progress, the diagnostic potential of US examination is far more complete now, and this procedure is very useful in the workup of a lesion and essential in guiding interventional and bioptical procedures [5].
In the field of US examinations, a systematic analysis of the findings in solid breast nodules in order to differentiate benign from malignant lesions was proposed by Stavros in 1995 [6,7], and in 2003 the Breast Imaging Reporting And Data System (BI-RADS) was developed by the American College of Radiology [8].
Application of a standard terminology related to US, as indicated by the BI-RADS lexicon, should ensure uniformity of interpretation and consequently an appropriate management of diagnostic US findings (follow-up or ascertainment) [9].
Simple cysts and some specific solid lesions have pathognomonic characteristics, so the diagnosis of benign disease is easy and also BI-RADS classification in Category 2. However, in most solid lesions, the diagnosis of probably benign lesion is based on the exclusion of suspicious signs but also on the confirmation of certain parameters showing benign lesions such as:
-
•
elliptical shape and horizontal orientation;
-
•
well-defined curvilinear or only slightly lobated margins; the presence of a complete, thin echogenic capsule;
-
•
echotexture almost completely hyperechoic.
Classification in BI-RADS category 3 (<2% of malignancy) should lead to a follow-up of an appropriate duration, although a needle biopsy or surgical excision should be considered according to the patient and/or her physician’s preference. If there are no clearly suspicious signs, but the signs of a benign lesion as mentioned above are not certain, the lesion should be classified as BI-RADS category 4a and biopsy should be performed [3].
A standard examination carried out by experienced operators using equipment of the latest technology has shown an elevated degree of sensitivity and specificity due to the possibility of compound and harmonic imaging which improves the visibility of the margins and the echostructure of the lesion [10]. Technological progress has also over the years provided software that allows second-level evaluation, which can further improve a non-invasive diagnostic approach in order to avoid an excessive use of biopsy as a definitive diagnostic tool.
In the 1990s, color/power Doppler examination was introduced, and only more recently equipment has been developed for three-dimensional (3D) studies and sonoelastography. US contrast agents do not seem to be widely used, also due to the high cost involved; their use seems to be more appropriate particularly in the study of breast lesions undergoing neoadjuvant chemotherapy in order to document the response to treatment [11–13] rather than in the differentiation between benign and malignant lesions and in the detection of small impalpable lesions.
Doppler study was initially considered very promising in the differential diagnosis between benign and malignant masses on the basis of morphological criteria (number of penetrating vessels as well as central or peripheral distribution) and semiquantitative criteria (resistance and pulsatility indices, peak systolic velocity) which may identify the characteristic neoangiogenesis of malignant lesions [14–16]. Power Doppler is better than color Doppler for detecting small vessels with slow blood flow and is therefore able to distinguish between solid and complicated cystic lesions, but it is more sensitive to artifacts [17]. However, the literature has revealed a substantial overlap of aspects in the vascularity of benign and malignant lesions. The hypothesis that more vascularization means a higher probability that the lesion is malignant is absolutely not valid (for example, also benign papillary lesions are highly vascularized). However, vascularization within a solid lesion detected at color/power Doppler should increase the US operator’s attention and suspicion.
Software for 3D reconstruction of the superficial tissues is also applicable to the study of breast lesions and can be useful in the differential diagnosis between benign and malignant nodules. Full thickness visualization of the region containing the focal lesion and subsequent processing of the images allows assessment of the shape and margins of the lesion in three orthogonal planes evidencing expansive or infiltrative growth [18,19]. After morphological evaluation, the 3D vascular image of the mass can be evaluated in a panoramic view that can facilitate quantification of the vessels and their relationship with the lesion.
Sonoelastography evaluates the different elastic properties of the tissues and is best applied in the evaluation of breast lesions, as there is a substantial difference between fibroglandular tissue and nodules of different types; malignant lesions are generally less elastic than benign masses [20,21]. A slight rhythmic compression is applied using the transducer positioned perpendicular to the breast, so that the relative deformation of the underlying tissues can be reconstructed and displayed on the monitor sometimes in black and white images; otherwise in color images which yield a better view of the degree of tissue elasticity. In the first case, benign lesions are white and malignant lesions are dark, while cysts show a bull’s eye appearance with a white center and a surrounding dark halo. In color images, the lesions present characteristics which have been assigned a score from 1 to 5 (similar to the BI-RADS classification), where benign or probably benign lesions (more elastic) are green with possible blue points, whereas malignant lesions (less elastic) are almost entirely blue with a possible halo which can be correlated with a desmoplastic reaction in the surrounding tissues [22,23]. Typically, simple and complicated cystic lesions present a triphasic pattern with a blue, more rigid layer at the surface, an intermediate green layer and a deep layer which is red.
Sonoelastography is very useful in non-palpable lesions, where it assumes the role of clinical examination in assessing the consistency of the palpable mass, thereby improving the perception of any US changes in shape and thickness of the lesion which become evident when the degree of probe compression is changed. Sensitivity of sonoelastography seems to improve in solid lesions of small size, and sensitivity is independent of the depth of the lesion, thickness and echogenicity of the breast. Sensitivity values reported in the literature are 70%–96% and specificity is 24%–90% [13].
Sonographic features of the most common benign breast lesions
Table 1 shows the classification of benign breast lesions on the basis of histological origin [1].
Cysts
Cysts are caused by over-distension of the terminal duct lobular units (TDLU) due to progressive filling with liquid, fibrosclerosis of the loose connective intralobular tissue and coalescence of single dilated ductules in a polylobated mass up to a single tensive cyst [3].
Cysts can be divided into three groups: simple, complicated and complex cysts [24,25].
Simple cysts present five basic characteristics: a well-circumscribed appearance, anechoic contents, a thin echogenic external capsule, enhanced through-transmission and subtle acoustic shadows at the edges (Fig. 1). Cysts with these features are very common in women of 30–50 years of age, and unless they are symptomatic they do not require evacuation or monitoring. If the mass is mammographically visible or palpable, it is important to make sure that this finding corresponds to the cyst and that there are no adjacent, more important, solid lesions [3].
Fig. 1.
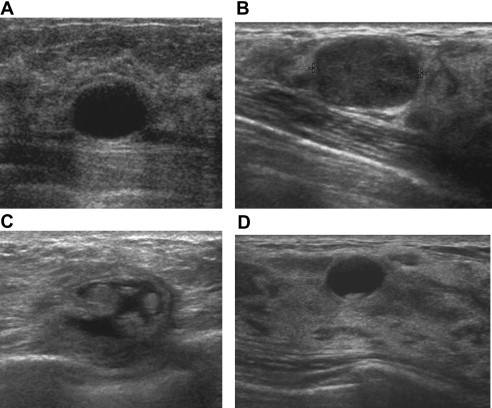
Simple cyst (A): US shows a mass which appears well-circumscribed, with anechoic contents, thin echogenic exterior capsule, enhanced through-transmission, subtle acoustic shadowing at the edges. Complicated cyst (B): US shows that the content is not purely anechoic (like a simple cyst), but there are diffused echoes of low amplitude. Complex cyst (C): US shows a well-circumscribed anechoic mass containing solid components. Cysts with sediment (D): US shows an anechoic mass with sharp margins and posterior hyperechoic sediment.
There may be possible echoes within the cyst due to technical artifacts or improper adjustment of the gain or due to excessive probe compression, which can generally be eliminated in harmonic imaging. There are also complicated cysts (so called because the content is not purely anechoic), which show diffused echoes of low amplitude, mainly due to the presence of amorphous material with cellular debris, blood cells and macrophages with foamy cytoplasm (Fig. 1B) or liquid–liquid levels (e.g. galactocele) [24]. They should be evacuated if they are symptomatic or if there are diagnostic doubts.
High-grade invasive ductal carcinomas and medullary carcinomas, as well as extra mammary metastatic lesions can show a marked hypoechoic image and have a rounded shape with enhanced through-transmission, thereby simulating a complicated cyst. However, a careful analysis of the shape and contour of the lesion as well as Doppler evaluation will raise diagnostic suspicion [3].
Complex cysts (Fig. 1C), which are generally less worrisome when located in the breast than those arising in other organs, may indicate the presence of malignancy or infection.
Morphological features which are suspicious for malignancy are thick isoechoic intracystic septations, mural nodules, fibrovascular stalk in the solid components and a microcystic appearance or microlobulated contour [3,24,25]. In 85%–90% of cases the lesion is a benign intracystic papilloma, and in the remaining 10%–15% of cases it is a papillary lesion with atypia or intracystic papillary carcinoma.
Definitive diagnosis requires histological analysis after core biopsy or preferably using vacuum-assisted device, leaving a marker to identify the sampling location. Morphological features indicating inflammation or infection are: relatively uniform isoechoic circumferential cyst wall thickening, hyperemia of the cyst wall and presence of blood in the cyst sediment (Fig. 1D) with the image showing fluid-debris level. If these three features coexist and the patient is symptomatic, the sediment is likely to be purulent. In this case the patient should undergo needle aspiration with subsequent bacterial culture and antibiogram [3].
Abscess
Abscesses may originate from infection of the subareolar ducts and/or preexisting galactocele (puerperal mastitis), or from ruptured ectatic ducts or cysts with initial chemical inflammation and subsequent bacterial superinfection. US features are similar (Fig. 2) and distinction is based on whether or not the patient is breastfeeding [3,26,27].
Fig. 2.
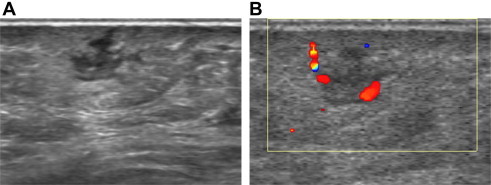
Abscess: US shows a hypo-anechoic mass with irregular margins (A) and peripheral hypervascularity (B).
Puerperal mastitis (Fig. 3) has an acute onset and although it is of lobar or sublobar origin, the classic inflammatory signs may involve the whole organ. Penetration of germs - more commonly Staphylococcus – occurs through a crack or fissure in the area of the nipple and it finds an excellent culture medium in the milk contained in the subareolar ducts or galactocele. If mastitis is not treated properly, an abscess will develop with necrotic tissue and denatured milk floating within the pus contained in the abscess cavity. When originating from a preexisting galactocele, the abscess may develop earlier and tend to be more demarcated with a roughly oval or multilobulated shape. When the subareolar ducts are involved, the abscess is often multiloculated due to the confluence of several small abscesses.
Fig. 3.
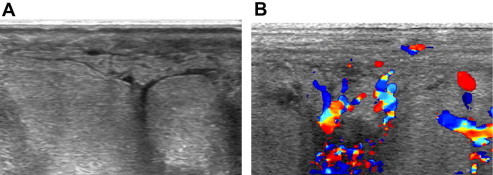
Mastitis: US shows subcutaneous and parenchymal edema associated with small fluid collections (A) and diffuse hypervascularity (B).
When the original event is a focal chemical mastitis caused by rupture of ducts or cysts and consequent release of lipid-rich secretions, it is an inflammatory condition involving the large ducts, known as ductal ectasia, which affects perimenopausal women. This often causes multilobar bilateral involvement with episodes of focal periductal mastitis and abscesses which are typically located around the areola. Bacterial infection is a result of aspiration attempts or communication with ducts which are already colonized by germs or hematogenous seeding.
US image of these abscesses is elongated and follows the axis of the duct of origin, with markedly thickened walls, early involvement of the nipple and marked inflammation of the surrounding tissues.
Nonpuerperal abscesses tend to recur easily and become chronic with cutaneous fistula formation which can be difficult to eradicate. A marked fibrotic reaction causes permanent retraction of the nipple. In this case neoplastic disorder should be included in a differential diagnosis.
However, several other benign breast conditions may during their evolution go through phases mimicking complex cysts, such as galactocele, seroma, hematoma, liponecrosis and hemangioma.
Galactocele
A painless lump developing during or a few weeks after ended breastfeeding is generally thought to be a galactocele. US monitoring may show spontaneous resolution, or a targeted aspiration may be carried out in cases of diagnostic doubt [3,28].
A galactocele is a cystic dilatation of the terminal ducts and ductules containing milk, so the appearance of a galactocele may vary during the monitoring. At first it appears as a anechoic cyst with possible septation, as the milk is fresh with homogeneously emulsified fat globules in a liquid component. Later the content becomes moderately echogenic, when the fat tends to form increasingly large and less emulsified globules, which are distributed unevenly or are suspended above the liquid component (Fig. 4), sometimes forming the classic fat-fluid level which is also mammographically visible in the mediolateral view. When the milk is curdled, the galactocele may mimic a solid nodule; however, it is easily compressed, there is no vascular signal at color Doppler, and the contents may move according to the pressure. A chronic galactocele, whose liquid component has been completely reabsorbed, may appear as a simple or complex lipid cyst.
Fig. 4.
Galactocele in the intermediate stage: US shows moderately echogenic contents with fat tending to form voluminous unemulsified globules, which are distributed heterogeneously in the liquid component.
Seroma and hematoma
Seromas are collections of serous fluid arising unpredictably after interventional procedures [29], and they commonly occur after surgical resection or breast augmentation [3]. After breast resection they occur at the level of the wound proportionally to the extension of the operation. After breast augmentation they tend to occur between the prosthetic shell and the fibrous capsule, more or less completely surrounding the prosthesis. Axillary seromas occurring due to lymph node dissection are actually lymphoceles. In both cases hemorrhagic extravasation may occur with consequent hematoma, particularly if the patient is receiving anticoagulant treatment [30].
These collections may be round or oval shaped if they are distended, whereas they may look angular and flat if the surgical cavity is slightly anfractuous.
Seromas may initially be anechoic or markedly hypoechoic, but diffuse low-level echoes or thin fibrin septations subsequently appear within them. The presence of blood which tends to clot leads to sediment, pseudonodule, wall thickening and coarse septa formation showing no vascularity at power Doppler and a hypoechoic heterogeneous pattern at US becomes hyperechoic after coagulation.
Hematoma may resolve spontaneously or become chronic with varying aspects mimicking also a solid mass, and it may develop liponecrosis with possible wall calcification. In blunt trauma cases there may not be a real pseudocystic hematoma, but an edema in the adipose tissue may be observed in the presence of superficial skin bruising.
Liponecrosis
Liponecrosis is a nonsuppurative inflammatory process resulting from saponification of the adipose tissue after biopsy and/or surgical resection with seroma or hematoma formation and possibly exacerbated by subsequent radiotherapy [31,32]. Extravasation of blood causes edema and stromal thickening with ischemia and necrosis due to increased local pressure and consequent adipocyte rupture. Accumulation of macrophages and plurinucleate giant cells containing necrotic lipid vacuoles results in an “oil cyst”, mammographically visible as a “soap bubble” image [33]. If the initial US image shows a pseudonodular hyperechoic edematous area, fat liquefaction gives raise to a complex cyst (Fig. 5). It is sometimes multilocular and has the appearance already described for seroma and hematoma (presence of echogenic bands and mural pseudonodules, often mobile when the patient changes position, yielding no vascular signals at color-power Doppler). During healing, cyst wall fibrosis develops, and parietal calcification occurs showing an egg-shell shape. Fibrous tissue may replace the fat content inside the cavity, and if the extent of necrosis and inflammation is important, the final stage may show an area of well-circumscribed hyperechoic fibrosis of irregular shape and angular edges, sometimes with acoustic absorption and shrinking or deformation of the surrounding tissues. In this case scirrhous carcinoma should be included in a differential diagnosis.
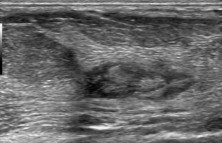
Fig. 5.
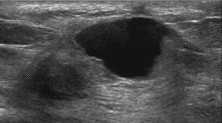
Liponecrosis: US shows a nodule mimicking a complex cyst.
Hemangioma
This benign vascular tumor is often clinically invisible, although it is situated subcutaneously and can measure more than 1 cm in diameter. It is an oval or polylobed pseudonodular mass, and US appearance depends on the caliber of the blood vessels. Capillary hemangiomas are largely homogeneously hyperechoic and cavernous hemangiomas present a mixed echotexture [34,35]. Both have a soft texture and can easily be compressed by probe pressure. In case of thrombosis, hypoechoic areas can be observed, often with phlebolithic calcifications, which are granular or amorphous.
Fibroadenoma
Fibroadenomas are benign solid tumors developing from a terminal duct lobular unit due to uncoordinated proliferation of the epithelial and stromal component (presumably due to estrogen stimulation) which involves part of the surrounding tissues. These tissues are partially compressed by the expansive growth, thereby creating a sort of a pseudocapsule.
Fibroadenomas have an internal structure composed of stromal and epithelial elements. The stromal element may undergo a myxoid degeneration, such as sclerosis, hyalinization and calcification, whereas the epithelial element may present all possible proliferative and non-proliferative aspects of the breast parenchyma, such as apocrine metaplasia, ductal hyperplasia, sclerosing and florid adenosis [36–38]. Fibroadenomas characterized by apocrine metaplasia, ductal hyperplasia, sclerosing adenosis or cysts are defined as “complex” [39,40].
Fibroadenomas have two peaks of incidence: in the third and in the fifth decade of life, but they may also occur after menopause as a result of hormone replacement therapy. They can grow rapidly but usually up to max. 2–3 cm. Giant and juvenile fibroadenomas are exceptions which may reach 6–10 cm. They have a highly cellular stroma and should be distinguished from benign phyllodes tumor [41,42]. They can be multiple and bilateral in approximately 20%–25% of patients. During pregnancy and breastfeeding these lesions may become more irregular due to episodes of infarctions and therefore more difficult to distinguish from carcinomas [43]. However, carcinoma rarely develops within a fibroadenoma; this occurs in 1 out of 1000 cases with an increased risk related to “complex” fibroadenomas, and in that case they are mainly in situ while infiltrating carcinomas occur more rarely [44]. At US examination, classic fibroadenomas, which are mobile and smooth, present the following characteristics [36–38]: elliptical or slightly lobulated shape, horizontal orientation (transverse diameter greater than the anterior-posterior diameter), isoechoic or mildly hypoechoic echotexture, well-defined curvilinear margin with a complete thin, echogenic capsule, unaltered US beam transmission beyond the lesion and subtle acoustic shadows on both sides of the nodule (Fig. 6A).
Fig. 6.
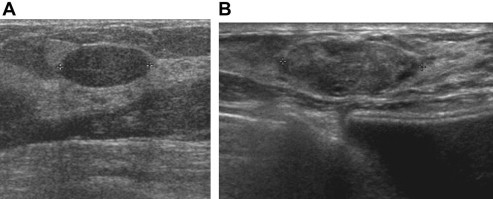
Fibroadenoma (A): US shows an elliptic mass with horizontal orientation, slightly isoechoic echotexture, sharp rounded margins with a complete thin echogenic capsule, unaltered US beam transmission beyond the lesion, subtle edge shadowing on both sides of the nodule. Complex fibroadenoma (B): US shows a lesion of heterogeneous echotexture with the presence of microcysts (apocrine metaplasia) or small hyperechoic areas (sclerosing adenosis).
If the nodule presents a microlobulated appearance, it differs from a classic or presumed fibroadenoma (BI-RADS 2 or 3) and becomes suspicious for malignancy (BI-RADS 4a), and needle biopsy with micro-histological analysis is therefore required [3].
“Complex” fibroadenomas also require histological analysis due to the presence of microcalcifications (associated with ductal hyperplasia), or heterogeneous echotexture due to the presence of microcysts (apocrine metaplasia) or small hyperechoic areas (sclerosing adenosis) (Fig. 6B) [3,39].
Fibroadenoma variants with evident epithelial hyperplasia and a very little stromal component include tubular adenomas and lactating adenomas. The latter occurs particularly during breastfeeding and in the third trimester of pregnancy; it is sometimes quite big, elastic in consistency and therefore compressible almost like a lipoma [3]. These lesions are scattered in the parenchyma, which presents widespread changes due to lactation; only rarely an evident pseudocapsule is seen, the shape is oval and generally microlobulated due to the presence of multiple microlobules or microcysts which contain milk. The echotexture is slightly hypoechoic, often mixed, but sometimes also hyperechoic, when the lipid component is not sufficiently emulsified in the liquid. Lactating adenomas present an elevated vascularization at color/power Doppler.
Phyllodes tumor
The term “phyllodes tumor” was introduced by Lemonaco in 1960. This lesion has so far been called by 62 different names, thereby indicating the difficulty encountered by the pathologists in characterizing the lesion since it was described by Muller for the first time in 1838 as a “cystosarcoma”. Later, Lee and Pack described the first case of malignant phyllodes tumor in 1931, and in 1946 Foote and Stewart concluded that malignant transformation arose from the stromal elements and not from the epithelial cells. In 1951, Treves and Sunderland divided phyllodes tumors into three subclasses: benign, borderline and malignant (classification still being used) based on the number of mitosis, the type of cellularity and nuclear atypia [45].
Phyllodes tumors account for 2%–3% of all fibroepithelial lesions and have a peak incidence in the perimenopausal age and another peak before the age of 20. Histologically there is a marked intraductal growth of intralobular stroma with leaflike projections (the term “phyllodes” originates from the Greek word “phyllos” = leaf) that are pathognomonic of this lesion. Among these hyperplastic stromal cells, distorted slitlike pseudocystic spaces are visible [45–47]. Phyllodes tumors are very similar to intracanalicular fibroadenomas, and histological underestimation is possible when a limited amount of sampling material is available (e.g. cytological sampling but also core biopsy). In these cases, diagnosis is assumed when the nodules are larger than 3 cm in diameter or fast-growing (benign lesions have an estimated doubling time of about 4 months, malignant lesions a little over one month).
Phyllodes tumors presumably arise ex-novo and have an expansive growth that leads to the formation of a pseudocapsule consisting of compressed adjacent parenchyma. This aspect may also be present in the malignant variant, which more frequently presents infiltrating margins. It is therefore difficult to distinguish them taking into account that the histological features may vary from area to area within the mass. In the past, phyllodes tumors could exceed 10 cm in diameter, but nowadays diagnosis is made much earlier. Malignant transformation may lead to hematogenous distant metastases, such as sarcomas, without involving regional lymph nodes [45,48].
Excision must necessarily be large to prevent local recurrence.
Mammography shows a circumscribed mass of high density with lobulated margins, rarely with a few coarse calcifications. At US, a phyllodes tumor appears as a mildly hypoechoic mass, often pseudocapsulated, without posterior acoustic shadowing. Inside the mass, pseudocystic spaces are sometimes so compressed that they look like characteristic hyperechoic striations (Fig. 7). Cystic spaces tend to be thinner and more horizontally oriented compared to the oval or round ones found in complex fibroadenomas. In the malignant variants, these pseudocystic spaces are coarser, and the echotexture may be rather heterogeneous due to areas of colliquative necrosis and reparative fibrosis [3].
Fig. 7.
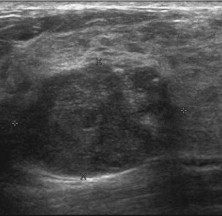
Phyllodes tumor. US shows moderately hypoechoic nodules with pseudocapsule, unaltered US beam transmission beyond the lesion; characteristic hyperechoic striations within the lesion, expression of pseudocystic spaces.
Other stromal proliferations
Focal fibrosis corresponds to a focal area of homogeneous fibrous tissue with no glandular structures, and the US image therefore shows an intensely and homogeneously hyperechoic mass, well-circumscribed but not encapsulated, which is drop-shaped or spindle-shaped with a horizontal axis [3].
Diabetic mastopathy, which typically occurs about 20 years after diagnosis of type I diabetes, is a result of an altered collagen metabolism and it is a hard, palpable, painless nodule. Mammographic image is non-specific, but US appearance is very suspicious, similar to spiculated malignant lesions (a taller than wide hypoechoic mass with irregular margins and acoustic absorbtion) [49]. Like lobular carcinoma, diabetic mastopathy can be multifocal, multicentric and bilateral. Color Doppler US and particularly MRI may show a lack of vascularity and enhancement, but the US appearance still requires biopsy.
Pseudoangiomatous stromal hyperplasia (PASH), which is probably caused by excessive progestinic stimulation, is frequently a microscopic incidental finding or it may be a real mass. Histological analysis shows dense breast stromal tissue containing a complex pattern of linear spaces caused by the separation of collagen fibrils, which resemble vascular spaces (hence the name “pseudoangiomatous”) and they may suggest a low grade malignant angiosarcoma (distinction is achieved by immunohistochemical markers for vascular tumors) [50,51].
At US a number of PASH nodules are similar to complex fibroadenomas with heterogeneous echotexture and sometimes a few microcysts, or similar to phyllodes tumors, so they can be classified as BI-RADS 3, but in most cases the masses have irregular or microlobulated margins and they require biopsy.
Granular cell tumors (mioblastomas) are stromal tumors probably originating from Schwann cells, as they yield a positive reaction to S-100 protein. They may arise anywhere in the body, particularly in the tongue, but also in the breast, predominantly in the upper internal quadrant, i.e. the area which is innervated by the supraclavicular nerve [35]. US image shows a hypoechoic or slightly hyperechoic nodule depending on whether the scan is parallel or perpendicular to the interior fibrils (anisotropy). The mass has an oval shape and a horizontal axis but seemingly the margins are infiltrative also at histological examination, often resulting in overlying skin dimpling, and biopsy is therefore always indicated.
Hamartoma
Breast hamartomas are roughly oval masses with a thin pseudocapsule. They can be of varying size and contain variable amounts of fat, glandular tissue and fibrous connective tissue, all of normal histology. US image (Fig. 8) is usually heterogeneous with a variable mixture of isoechoic elements (fat and glandular tissue) and hyperechoic elements (fibrous connective tissue); it sometimes shows a target or multilayered appearance which is pathognomonic like the mammographic features [52,53]. Consistency and compressibility depend on the fat component, which is extremely variable. Diagnosis may be difficult when the masses are small with a low component of fat and an incomplete pseudocapsule. Hamartomas are most common in women over 40 years of age, and they are generally asymptomatic. They are not at risk of malignancy, so in cases of classic hamartomas further investigation or a specific follow-up is not required.
Fig. 8.
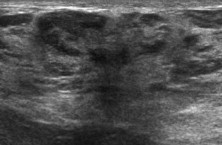
Hamartoma: US shows heterogeneous appearance of the lesion with a mixture of isoechoic (adipose tissue and glandular lobules) and hyperechoic (fibrous tissue) elements.
Papilloma
Papillomas are intraductal epithelial proliferations of papillary appearance; they have a fibrovascular stalk and are therefore well vascularized and highly cellular, being extremely soft and fragile. A distinction is usually made between papillomas which arise as single lesions in the large retro-periareolar ducts, most frequently in the perimenopausal period, and papillomas which arise in the peripheral ducts, most frequently seen in younger patients and most often as multiple lesions. The latter are associated with various proliferative aspects of the surrounding terminal ductal-lobular units, also with atypical characteristics; they are therefore considered at high risk of malignant transformation [3,54–56]. Papillomas occurring in the large ducts may vary in size from a few millimeters up to extending over a variable length of the duct lumen involving the ramifications. They tend to release secretion resulting in the expansion of the duct itself and frequent spontaneous secretion from the nipple. The secretion is most commonly serous but due to partial infarction and necrosis of the papilloma, secretion may contain blood. As a result of hypersecretion and expansive growth of the mass, duct obstruction may also occur resulting in cystic dilatation of the excretory duct and intracystic papilloma. Given the variable appearance and extent of intraductal papillomas, US diagnosis requires the presence of circumscribed ectasia of a milk duct whose lumen contains echoic material. In the early stages it may look like an isoechoic or slightly hypoechoic nodule with a microlobulated or lobulated surface (Fig. 9) and Color Doppler examination will show a marked vascular signal at the fibrovascular stalk. Later there will be signs linked to the expansion of the papilloma along the duct with involvement of the ramifications, and subsequently transformation to intracystic papilloma. Intraductal papilloma should be studied with scans performed along radial and antiradial planes and with alternated compression and decompression of the duct using the US probe to differentiate it from any mobile intraductal echoes associated with thickened secretions due to ductal ectasia. Color/power Doppler is essential to detect the absence of intraluminal vascular signals typical of all stages of ductal ectasia, which involves multiple milk ducts bilaterally, symmetrically or asymmetrically. Probe compression during Doppler examination should always be mild. The extension of the papilloma should be studied carefully at US examination using maneuvers aimed at following the course of the ducts, which are often tortuous, and at minimizing the possible interference of the nipple on the visibility of the most central portions of the ducts [3]. Sometimes the papilloma may be masked by dense secretions or blood clots, which may be mobile and more compressible than the papilloma. Papilloma may therefore be assumed if there is still a residual circumscribed dilatation of the duct under compression. When papilloma becomes intracystic (Fig. 10), the extension should be carefully investigated toward the proximal duct, where the mass arises. The lack of ductal extension suggests a cyst containing papillary apocrine metaplasia.
Fig. 9.
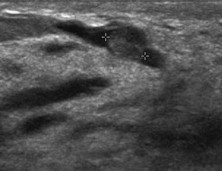
Intraductal papilloma: US shows a well-circumscribed subareolar duct ectasia with an isoechoic nodule with microlobulated surface in the lumen.
Fig. 10.
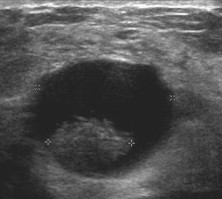
Intracystic papilloma: US shows a cyst with a prominent mass inside.
In the presence of significant discharge from the nipple, the finding of an intraductal vegetating mass will in most cases suggest a papilloma, whereas malignant forms are found in only 10% of cases. Like galactography, also US cannot clearly differentiate between papilloma, ductal carcinoma in situ (DCIS) and papillary carcinoma (usually intracystic), although the latter is more likely to occur with a palpable or mammographically visible mass, rather than with nipple discharge.
Papillomas of peripheral ducts very rarely give rise to duct ectasia and nipple discharge. They may be invisible at US, otherwise they should be included in the differential diagnosis between other benign and malignant solid nodules.
US is often preferred to galactography in the study of nipple discharge, as it may provide a diagnosis of papilloma and localize the lesion with a view to surgical biopsy. US is also used as a guide for needle biopsy with vacuum-assisted devices, which is preferred to core biopsies because a greater amount of tissue is obtained (sometimes the entire lesion) with the possibility to make a more accurate histological diagnosis and often definitively stop the secretion [3,57].
Inflammatory, infectious and reactive disorders
Inflammatory, infectious and reactive diseases rarely affect the breast, and these lesions generally occur as secondary locations in patients with known systemic or target-organ specific diseases.
The radiological and clinical features of breast lesions caused by immunologic, reactive, and infectious diseases (sarcoidosis, tuberculosis, Wegener’s granulomatosis) often mimic those of malignancy, and they therefore frequently require biopsy considering the possible coexistence of both diseases [58]. Some infectious diseases (echinococcosis, actinomycosis, blastomycosis) may result in localized lesions with US appearance of complex cysts [58]. However, foreign body granulomatous lesions are more frequent, sometimes containing cholesterol crystals, and siliconomas are quite characteristic. Silicone gel leaking from a broken breast implant capsule or due to continuous transcapsular leakage gives rise to pericapsular granulomatous lesions, which often present the typical “snow-storm” US pattern [3]. However, initially US image may be similar to that of a complex cyst; in the intermediate stage a granulomatous lesion may mimic an isoechoic nodule, and later, an ill-defined hypoechoic pseudonodular image may develop due to foreign body reaction and fibrosis with sound absorption, similar to carcinoma.
Intramammary lymph node
These lesions most frequently occur in the upper outer peripheral quadrant toward the axillary tail, but they can occur everywhere, even in the inner marginal edge. The classic appearance of an oval or lobulated, isoechoic or hypoechoic nodule with well-circumscribed margins and a hyperechoic central fatty hilum provides the diagnosis without the need for further investigation [3].
Conclusions
The above descriptions make it clear that benign breast lesions form an extremely heterogeneous group. Some lesions are easily diagnosed due to their charactistic appearance, other lesions present varying images during different stages of their evolution, and others again are difficult to differentiate from malignant lesions.
A last comment should be dedicated to high risk lesions and particularly atypical hyperplasia, which involve a more important prognostic significance albeit with unspecific US characteristics. Macroscopic findings show those proliferative conditions in whose context atypical hyperplasia has its origin, i.e. papillary hyperplasia, intraductal papilloma and radial scar. In atypical hyperplasia, diagnosis is exclusively histological and this pathology is more easily detected during mammographically guided investigation of clustered microcalcifications. However, the findings are difficult to interpret and also histological analysis may be controversial.
Technologically advanced US equipment provides a better evaluation of lesions and therefore a potentially reduced number of diagnostic biopsies. However, when further investigation is appropriate, US guided biopsy is performed and micro-histological diagnosis is obtained. This procedure is still based mainly on core biopsy but recently also sampling using vacuum-assisted biopsy devices with a larger caliber has been introduced to ensure a better sample for histological evaluation, and in some cases of a circumscribed benign lesion this procedure has performed definitive resection of the mass.
Conflict of interest
The authors have no conflict of interest.
Appendix. Supplementary data
The following are the Supplementary data related to this article:
References
- 1.Lanyi M. Mammography. Springer-Verlag; Berlin, Heidelberg, New York: 2003. Diagnosis and pathological analysis. [Google Scholar]
- 2.Hughes L.E., Mansel R.E., Webster D.J. Aberrations of normal development and involution (ANDI); a new perspective on pathogenesis and nomenclature of benign breast disorders. Lancet. 1987;2(8571):1316–1319. doi: 10.1016/s0140-6736(87)91204-9. [DOI] [PubMed] [Google Scholar]
- 3.Stavros A.T. Lippincott Williams & Wilkins; Philadelphia: 2004. Breast ultrasound. [Google Scholar]
- 4.Dennis M.A., Parker S.H., Klaus A.J., Stavros A.T., Kaske T.J., Clark S.B. Breast biopsy avoidance: the value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219:186–191. doi: 10.1148/radiology.219.1.r01ap35186. [DOI] [PubMed] [Google Scholar]
- 5.Jackson V.P. Management of solid nodules: what is the role of sonography? Radiology. 1995;196:14–15. doi: 10.1148/radiology.196.1.7784557. [DOI] [PubMed] [Google Scholar]
- 6.Stavros A.T., Thickman D., Rapp C.L., Dennis M.A., Parker S.H., Sisney G.A. Solid breast nodules: use of sonography to distinguish between benign and malignant nodules. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 7.Rahbar G., Sie A.C., Hansen G.C., Prince J.S., Melany M.L., Reynolds H.E. Benign versus malignant solid breast masses: US differentiation. Radiology. 1999;213:889–894. doi: 10.1148/radiology.213.3.r99dc20889. [DOI] [PubMed] [Google Scholar]
- 8.American College of Radiology (ACR) 4th ed. American College of Radiology; Reston, VA: 2003. Illustrated breast imaging reporting and data system (BI-RADS) [Google Scholar]
- 9.Levy L., Suissa M., Chiche J.F., Teman G., Martin B. BIRADS ultrasonography. Eur J Radiol. 2007;61:202–211. doi: 10.1016/j.ejrad.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Szopinski K.T., Pajk A.M., Wysocki M., Amy D., Szopinska M., Jakubowski W. Tissue harmonic imaging utility in breast sonography. J Ultrasound Med. 2003;22:479–487. doi: 10.7863/jum.2003.22.5.479. [DOI] [PubMed] [Google Scholar]
- 11.Sehgal C.M., Weinstein S.P., Arger P.H., Conant E.F. A review of breast ultrasound. J Mammary Gland Biol Neoplasia. 2006;11:113–123. doi: 10.1007/s10911-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 12.Athanasiou A., Tardivon A., Ollivier L., Thibault F., El Khoury C., Neuenschwander S. How to optimize breast ultrasound. Eur J Radiol. 2009;69:6–13. doi: 10.1016/j.ejrad.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Piccoli C.W., Forsberg F. Advanced ultrasound techniques for breast imaging. Semin Roentgenol. 2011;46:60–67. doi: 10.1053/j.ro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Cosgrove D.O., Kedar R.P., Bamber J.C., al-Murrani B., Davey J.B., Fisher C. Breast diseases: color Doppler US in differential diagnosis. Radiology. 1993;189:99–104. doi: 10.1148/radiology.189.1.8372225. [DOI] [PubMed] [Google Scholar]
- 15.Calliada F., Raieli G., Sala G., Conti M.P., Bottinelli O., La Fianza A. Doppler color-echo in the echographic evaluation of solid neoplasms of the breast: 5 years of experience. Radiol Med. 1994;87:28–35. [PubMed] [Google Scholar]
- 16.Draghi F., Coopmans de Yoldi G. Poletto; Milano: 1995. Atlante eco color-Doppler della mammella. [PubMed] [Google Scholar]
- 17.Hayashi N., Miyamoto Y., Nakata N., Irie T., Ikegami M., Asao K. Breast masses: color Doppler, power Doppler, and spectral analysis findings. J Clin Ultrasound. 1998;26:231–238. doi: 10.1002/(sici)1097-0096(199806)26:5<231::aid-jcu1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Cho K.R., Seo B.K., Lee J.Y., Pisano E.D., Je B.K., Lee J.Y. A comparative study of 2D and 3D ultrasonography for evaluation of solid breast masses. Eur J Radiol. 2005;54:365–370. doi: 10.1016/j.ejrad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Abbattista T., Serri L., Busilacchi P. Studio ecografico 3D delle lesioni mammarie. Three dimensional sonographic study of breast nodules. J Ultrasound. 2007;10:93–98. doi: 10.1016/j.jus.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samani A., Zubovits J., Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 2007;52:1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- 21.Ginat D.T., Destounis S.V., Barr R.G., Castaneda B., Strang J.G., Rubens D.J. US Elastography of breast and prostate lesions. RadioGraphics. 2009;29:2007–2016. doi: 10.1148/rg.297095058. [DOI] [PubMed] [Google Scholar]
- 22.Itoh A., Ueno E., Tohno E., Kamma H., Takahashi H., Shiina T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 23.Scaperrotta G., Ferranti C., Costa C., Mariani L., Marchesini M., Suman L. Role of sonoelastography in non-palpable breast lesions. Eur Radiol. 2008;18:2381–2389. doi: 10.1007/s00330-008-1032-8. [DOI] [PubMed] [Google Scholar]
- 24.Berg W.A., Campassi C.I., Ioffe O.B. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003;227:183–191. doi: 10.1148/radiol.2272020660. [DOI] [PubMed] [Google Scholar]
- 25.Houssami N., Irwig L., Ung O. Review of complex breast cysts: implications for cancer detection and clinical practice. ANZ J Surg. 2005;75:1080–1085. doi: 10.1111/j.1445-2197.2005.03608.x. [DOI] [PubMed] [Google Scholar]
- 26.Hayes R., Michell M., Nunnerley H.B. Acute inflammation of the breast: the role of breast ultrasound in diagnosis and management. Clin Radiol. 1991;44:253–256. doi: 10.1016/s0009-9260(05)80190-4. [DOI] [PubMed] [Google Scholar]
- 27.Boisserie-Lacroix M., Lafitte J.J., Sirben C., Latrabe V., Grelet P., Zeinoun R. Inflammatory and infectious lesions of the breast: contribution of ultrasonography. J Chir (Paris) 1993;130:408–415. [PubMed] [Google Scholar]
- 28.Salvador R., Salvador M., Jimenez J.A., Martinez M., Casas L. Galactocele of the breast: radiologic and ultrasonographic findings. Br J Radiol. 1990;63:140–142. doi: 10.1259/0007-1285-63-746-140. [DOI] [PubMed] [Google Scholar]
- 29.Harlow C.L., Schackmuth E.M., Bregman P.S., Zeligman B.E., Coffin C.T. Sonographic detection of hematomas and fluid after imaging-guided core breast biopsy. J Ultrasound Med. 1994;13:877–882. doi: 10.7863/jum.1994.13.11.877. [DOI] [PubMed] [Google Scholar]
- 30.Pignatelli V., Basolo F., Bagnolesi A., Cartei F., Grassi L., Savino A. Hematoma and fat necrosis of the breast: mammographic and echographic features. Radiol Med. 1995;89:36–41. [PubMed] [Google Scholar]
- 31.Soo M.S., Kornguth P.J., Hertzberg B.S. Fat necrosis in the breast: sonographic features. Radiology. 1998;206:261–269. doi: 10.1148/radiology.206.1.9423681. [DOI] [PubMed] [Google Scholar]
- 32.Tan P.H., Lai L.M., Carrington E.V., Opaluwa A.S., Ravikumar K.H., Chetty N. Fat necrosis of the breast - A review. The Breast. 2006;15:313–318. doi: 10.1016/j.breast.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Harvey J.A., Moran R.E., Maurer E.J., De Angelis G.A. Sonographic features of mammary oil cysts. J Ultrasound Med. 1997;16:719–724. doi: 10.7863/jum.1997.16.11.719. [DOI] [PubMed] [Google Scholar]
- 34.Webb L.A., Young J.R. Case report: haemangioma of the breast- appearances on mammography and ultrasound. Clin Radiol. 1996;51:523–524. doi: 10.1016/s0009-9260(96)80198-x. [DOI] [PubMed] [Google Scholar]
- 35.Porter G.J., Evans A.J., Lee A.H., Hamilton L.J., James J.J. Unusual benign breast lesions. Clin Radiol. 2006;61:562–569. doi: 10.1016/j.crad.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Cole-Beuglet C., Soriano R.Z., Kurtz A.B., Goldberg B.B. Fibroadenoma of the breast: sonomammography correlated with pathology in 122 patients. AJR. 1983;140:369–375. doi: 10.2214/ajr.140.2.369. [DOI] [PubMed] [Google Scholar]
- 37.Jackson V.P., Rothschild P.A., Kreipke D.L., Mail J.T., Holden R.W. The spectrum of sonographic findings of fibroadenoma of the breast. Invest Radiol. 1986;21:34–40. doi: 10.1097/00004424-198601000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Fornage B.D., Lorigan J.G., Andry E. Fibroadenoma of the breast: sonographic appearance. Radiology. 1989;172:671–675. doi: 10.1148/radiology.172.3.2549564. [DOI] [PubMed] [Google Scholar]
- 39.Sklair-Levy M., Sella T., Alweiss T., Craciun I., Libson E., Mally B. Incidence and management of complex fibroadenomas. AJR. 2008;190:214–218. doi: 10.2214/AJR.07.2330. [DOI] [PubMed] [Google Scholar]
- 40.Kuijper A., Mommers E.C., van der Wall E., van Dienst P.J. Histopathology of fibroadenoma of the breast. Am J Clin Pathol. 2001;115:736–742. doi: 10.1309/F523-FMJV-W886-3J38. [DOI] [PubMed] [Google Scholar]
- 41.Steinbock R.T., Stomper P.C., Meyer J.E., Kopans D.B. The ultrasound appearance of giant fibroadenoma. J Clin Ultrasound. 1983;11:451–454. doi: 10.1002/jcu.1870110810. [DOI] [PubMed] [Google Scholar]
- 42.Kronemer K.A., Rhee K., Siegel M.J., Sievert L., Hildebolt C.F. Gray scale sonography of breast masses in adolescent girls. J Ultrasound Med. 2001;20:491–496. doi: 10.7863/jum.2001.20.5.491. [DOI] [PubMed] [Google Scholar]
- 43.Skaane P., Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR. 1998;170:109–114. doi: 10.2214/ajr.170.1.9423610. [DOI] [PubMed] [Google Scholar]
- 44.DuPont W.D., Page D.L., Parl F.F., Vnencak-Jones C.L., Plummer W.D., Jr., Rados M.S. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10–15. doi: 10.1056/NEJM199407073310103. [DOI] [PubMed] [Google Scholar]
- 45.Giri D. Recurrent challenges in the evaluation of fibroepithelial lesions. Arch Pathol Lab Med. 2009;133:713–721. doi: 10.5858/133.5.713. [DOI] [PubMed] [Google Scholar]
- 46.Cole-Beuglet C., Soriano R., Kurtz A.B., Meyer J.E., Kopans D.B., Goldberg B.B. Ultrasound, X-ray mammography and histopathology of cystosarcoma phylloides. Radiology. 1983;146:481–486. doi: 10.1148/radiology.146.2.6294737. [DOI] [PubMed] [Google Scholar]
- 47.Buchberger W., Strasser K., Heim K., Muller E., Schrocksnadel H. Phylloides tumor: findings on mammography, sonography and aspiration cytology in 10 cases. AJR. 1991;157:715–719. doi: 10.2214/ajr.157.4.1654022. [DOI] [PubMed] [Google Scholar]
- 48.Liberman L., Bonaccio E., Hamele-Bena D., Abramson A.F., Cohen M.A., Dershaw D.D. Benign and malignant phyllodes tumors mammographic and sonographic findings. Radiology. 1996;198:121–124. doi: 10.1148/radiology.198.1.8539362. [DOI] [PubMed] [Google Scholar]
- 49.Thorncroft K., Forsyth L., Desmond S., Audisio R.A. The diagnosis and management of diabetic mastopathy. Breast J. 2007;13:607–613. doi: 10.1111/j.1524-4741.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 50.Mercado C.-L., Naidrich S.A., Hamele-Bena D., Fineberg S.A., Buchbinder S.S. Pseudoangiomatous stromal hyperplasia of the breast: sonographic features with histopathologic correlation. Breast J. 2004;10:427–432. doi: 10.1111/j.1075-122X.2004.21373.x. [DOI] [PubMed] [Google Scholar]
- 51.Jones K.N., Glazebrook K.N., Reynolds C. Pseudoangiomatous stromal hyperplasia: imaging findings with pathologic and clinical correlation. AJR. 2010;195:1036–1042. doi: 10.2214/AJR.09.3284. [DOI] [PubMed] [Google Scholar]
- 52.Adler D.D., Jeffries D.O., Helvie M.A. Sonographic features of breast hamartomas. J Ultrasound Med. 1990;9:85–90. doi: 10.7863/jum.1990.9.2.85. [DOI] [PubMed] [Google Scholar]
- 53.Georgian-Smith D., Kricun B., McKee G., Yeh E., Rafferty E.A., D’Alessandro H.A. The mammary hamartoma: appreciation of additional imaging characteristics. J Ultrasound Med. 2004;23:1267–1273. doi: 10.7863/jum.2004.23.10.1267. [DOI] [PubMed] [Google Scholar]
- 54.Han B.K., Choe Y.H., Ko Y.H., Yang J.H., Nam S.J. Benign papillary lesions of the breast: sonographic-pathologic correlation. J Ultrasound Med. 1999;18:217–223. doi: 10.7863/jum.1999.18.3.217. [DOI] [PubMed] [Google Scholar]
- 55.Ganesan S., Karthik G., Joshi M., Damodaran V. Ultrasound spectrum in intraductal papillary neoplasms of breast. Br J Radiol. 2006;79:843–849. doi: 10.1259/bjr/69395941. [DOI] [PubMed] [Google Scholar]
- 56.Brookes M.J., Bourke A.G. Radiological appearances of papillary breast lesions. Clin Radiol. 2008;63:1265–1273. doi: 10.1016/j.crad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Puglisi F., Zuiani C., Bazzocchi M., Valent F., Aprile G., Pertoldi B. Role of mammography, ultrasound and large core biopsy in the diagnostic evaluation of papillary breast lesions. Oncology. 2003;65:311–315. doi: 10.1159/000074643. [DOI] [PubMed] [Google Scholar]
- 58.Sabatè J.M., Closet M., Gomez A., De Las Heras P., Torrubia S., Salinas T. Radiologic evaluation of uncommon inflammatory and reactive breast disorders. RadioGraphics. 2005;25:411–424. doi: 10.1148/rg.252045077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


