Abstract
Introduction
Breast cancer (BC) is the most common malignancy in women. Various studies [5,6] have shown that surgical resection of single liver or lung metastases in patients with metastases from BC increases survival. Radiofrequency ablation (RFA) can be an alternative to resection in some patients when resection is not feasible.
Materials and methods
From January 2002 to December 2008, 491 patients with liver metastases underwent US-guided percutaneous RFA. Of these patients 5 (5/491; 1%) had BC. In the same period, 32 patients with pulmonary metastases underwent CT-guided RFA. Of these patients 3 (3/32; 9%) had BC. Mean age was 61.3 years. All patients were postmenopausal and receiving polychemotherapy according to international guidelines. Inclusion criteria for RFA treatment of metastases from BC applied are identical or in some cases more restrictive than those reported in the literature.
Results
There were no deaths or severe complications and no treatment failures. Disease free and overall median survival were respectively 7.65 and 25.7 months after US-guided RFA and 13.4 and 34.8 months after CT-guided RFA. During follow-up (mean follow-up 26 months, range 4–63 months) 5/8 (62.5%) patients exhibited recurrence: 3/5 (60%) had local recurrence and 2/5 (40%) had non-local recurrence; 4/5 patients with recurrence were re-treated.
Discussion
The authors' experience confirms that RFA is an effective, safe and repeatable technique in the treatment of metastases from BC. Metastatic recurrence rate confirms that metastatic BC is a disease which requires a multidisciplinary approach and that the role of chemotherapy is indisputable. Effects on survival are promising but further confirmation is needed through prospective randomized studies.
Keywords: Breast carcinoma, Liver metastases, Pulmonary metastases, Radiofrequency ablation
Sommario
Introduzione
Il carcinoma della mammella (BC) è la più frequente neoplasia nel sesso femminile. Vari studi hanno dimostrato che la resezione chirurgica delle MTS epatiche o polmonari singole determina un allungamento della sopravvivenza. La termoablazione a radiofrequenze (RFA) potrebbe rappresentare una valida alternativa alla resezione quando la prima non è fattibile.
Materiali e metodi
Presso il nostro centro nel periodo compreso tra gennaio 2002 e dicembre 2008 sono stati trattati con RFA percutanea US-guidata 491 pazienti affetti da MTS epatiche di cui 5 (5/491; 1%) da BC. Nello stesso periodo sono stati trattati con RFA CT-guidata 32 pazienti con MTS polmonari: 3 (3/32; 9%) da BC. L’età media delle pazienti era di 61,3 anni. Tutte le pazienti erano in fase post-menopausale e in trattamento polichemioterapico in accordo con le linee guida internazionali. Le indicazioni per il trattamento delle metastasi da BC utilizzate nel nostro centro sono sovrapponibili o in alcuni casi maggiormente restrittive rispetto a quelle presenti in letteratura.
Risultati
Non si sono verificati decessi o complicanze gravi. Non ci sono stati fallimenti terapeutici. La median survival, libera da malattia e complessiva, è rispettivamente di 7,65 e 25,7 mesi dopo RFA US-guidata e 13.4 e 34.8 mesi dopo RFA CT-guidata. Durante il follow-up (in media 26 mesi; range 4–63 mesi) 5/8 (62,5%) pazienti hanno avuto recidive: 3 su 5 (60%) locali e 2/5 (40%) non-locali. Quattro delle 5 recidive sono state nuovamente trattate.
Discussione
La nostra esperienza conferma che la termoablazione a radiofrequenze è una tecnica efficace, sicura e ripetibile per il trattamento delle metastasi da BC. Il numero di recidive conferma che il BC metastatico è una patologia che va affrontata in un contesto multidisciplinare in cui il ruolo della chemioterapia è indiscutibile. I risultati sulla sopravvivenza sembrano promettenti ma necessitano comunque di ulteriori conferme attraverso studi prospettici anche randomizzati.
Introduction
Breast cancer (BC) is the most common malignancy in women and it is the leading cause of death in women between 20 and 59 years [1]. Approximately 50% of patients with BC develop metastases [2].
The earliest and most frequent site of BC metastasis is the bones and lungs, and no more than 5%–20% of cases have only liver metastases [3]. Metastatic BC is worldwide considered a systemic disease and therefore treated with chemotherapy [4].
Over the years various studies [5,6] have shown that surgical resection of single liver or lung metastases in patients with BC increases survival resulting in a 5-year survival of 24% and 54.5%, respectively.
Radiofrequency ablation (RFA) is a good alternative to resection in some patients when resection is not feasible for clinical or technical reasons [7]. RFA has in the recent years been performed under ultrasound (US) guidance [8,9] and computed tomography (CT) guidance [10] in the treatment of lung and liver metastases from BC. The aim of this paper was to compare the results of RFA performed by the authors in the treatment of metastases from BC with the most recent literature on the subject.
Materials and methods
Ethical approval for this study was granted by the Medical Research Ethics Committee of the authors' institution, and informed consent was obtained from all patients.
Patients
From January 2002 to December 2008, 491 patients with metastases in the liver underwent US-guided percutaneous RFA: 469 (469/491; 96%) had colon cancer, 17 (17/491; 3%) had kidney cancer and 5 (5/491; 1%) had BC.
In the same period, 32 patients with pulmonary metastases underwent CT-guided RFA: 15 had colon cancer (15/32; 47%), 14 had urogenital malignancies (14/32; 44%) and 3 (3/32; 9%) had BC.
In the authors' department inclusion criteria for US-guided treatment of liver metastases are the following:
-
1.
Number of lesions ≤ 5
-
2.
Diameter ≤ 5 cm
-
3.
Absence of extrahepatic metastases
-
4.
Exclusion of surgical treatment (surgical resection not indicated, general contraindications to surgery or patient's refusal of surgery)
-
5.
Prothrombin time ≥ 50% (or International Normalized Ratio (INR) < 1.7) and platelet count ≥ 50 × 109/L
-
6.
Good liver function (Child-Pugh classification ≤ B7) [11]
-
7.
Patient ability to collaborate during the procedure
-
8.
Good US visualization of the lesion
These inclusion criteria are identical or in some cases more restrictive than those reported in the literature [8,9].
Inclusion criteria for CT-guided treatment of lung metastases are the following [12]:
-
1.
Number of lesions ≤ 2
-
2.
Diameter ≤ 3.5 cm
-
3.
Absence of extrapulmonary metastases
-
4.
Exclusion of surgical treatment (surgical resection not indicated, general contraindications to surgery or patient's refusal of surgery)
-
5.
Prothrombin time ≥ 50% (or International Normalized Ratio (INR) < 1.7) and platelet count ≥ 50 × 109/L
-
6.
The lesion must be surrounded by aerated parenchyma; no infiltration of the main bronchus, trachea and/or mediastinum
The patients with hepatic metastases from BC submitted to RFA presented a mean number of liver metastases of 1.8 (range 1–3), mean diameter 20 mm (range 10–45 mm). The lesions were located as follows: 3 in the right lobe of the liver and 2 in the left lobe. Mean number of lung metastases was 1.7 (range 1–2), mean diameter 23 mm (range 10–35 mm). The lesions were located as follows: 1 in the lower lobe of the right lung, 2 in the lower lobe of the left lung.
Mean age of these 8 patients was 61.3 years (range 55–67; mode 65 years, median 65 years). All patients were postmenopausal and receiving polychemotherapy according to international guidelines [13,14].
US-guided RFA of liver lesions
RFA was performed using a radiofrequency generator and 14 to 19 gauge expandable needle electrodes (Fig. 1) able to produce thermal lesions of a diameter between 2.5 and 3.5 cm [15–17]. The type of needle electrode was chosen on the basis of the size and location of the metastasis (Fig. 2a–b). Duration of hospitalization was 3 nights (unless complications occurred) in accordance with the regional Italian regulations. Patients undergoing RFA were required to be fasting from the previous evening.
Figure 1.
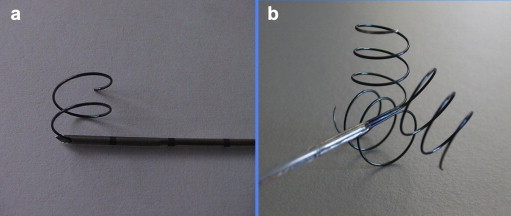
Expandable needle electrodes for US-guided RFA: the exposed tip may contain: a) a single spiral array; b) three spiral array.
Figure 2.
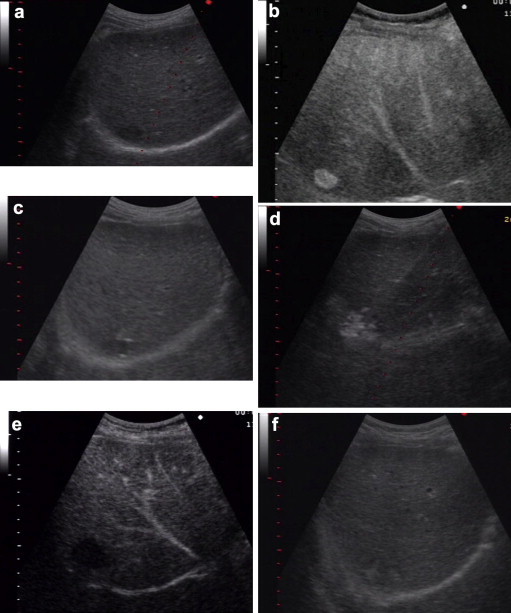
US-guided RFA of a liver metastasis: a) US image of the metastasis before treatment; b) pretreatment CEUS; c) placement of the needle under US guidance; d) creation of a thermal lesion; e) Follow-up CEUS; f) US follow-up of an RFA treated liver metastasis.
Percutaneous procedure was performed under local anesthesia or using a mild sedation if necessary [15,16]. The needle electrode was positioned under US guidance after which the hooks were extracted (Fig. 2C). During each session, the needle electrode was inserted into the lesion 1–3 times and at each insertion 1 to 3 thermal lesions were created using the pull-back technique [14,15]. The session was terminated when the hyperechoic area appearing around the active tip of the needle electrode positioned in the lesion was the size of the tumor or larger (Fig. 2d) [15,16].
CT-guided RFA of pulmonary lesions
The equipment used for RFA of lung lesions consisted of a radiofrequency generator [12], a needle electrode (Fig. 3) and grounding pads. The active electrodes were able to create thermal lesions measuring 30–35 mm in diameter [18].
Figure 3.
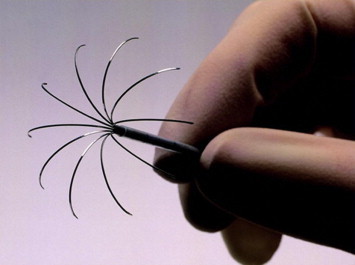
Expandable needle electrode for CT-guided RFA.
The RFA procedure was performed without general anesthesia and patients had been fasting for at least one night. Prophylactic antibiotictherapy was administered 3 h before the procedure and continued for 72 h (1 g of cefotaxime 3 times a day). Up to four grounding pads were placed on the patient's back or lower limbs and connected to the radiofrequency generator. The patient was placed in prone position, on one side or in supine position according to needle electrode insertion path which was determined before the procedure. Generally, the chosen path is the shortest and most direct access route to the metastasis that does not cut larger blood vessels or respiratory ways. A 22 gauge Chiba needle was inserted under CT-guidance (Fig. 4a).
Figure 4.
The needle electrode path is anesthetized: a) insertion of the needle to anesthetize the path; b) creation a subpleural swelling.
During the advancement of the needle, the path was anesthetized and when the tip of the needle reached the subpleural space of the parietal pleura, an additional bolus of 10 ml anesthetic drug was injected. This final injection anesthetized the parietal pleura and compressed the lung parenchyma, thus reducing the distance to be covered to reach the lesion (Fig. 4b). The needle electrode was then introduced still under CT-guidance. The needle tip was positioned in the center of the nodule and the final position was evaluated in all the planes (Fig. 5). The radiofrequency generator was then activated and the power was gradually increased until impedance values prevented the flow of further energy [18]. According to the shape and size of the tumor one or more thermal lesions were created by using the pull-back technique [14,15] before the electrode was retracted sterilizing the insertion route. During the entire procedure blood pressure, heart rate, electrocardiogram and blood oxygen level were continuously monitored.
Figure 5.
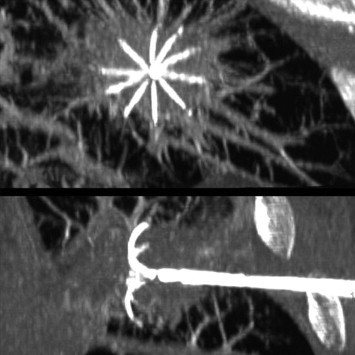
The correct positioning of the needle electrode is checked in all the spatial planes.
Immediate results and complications
In US-guided RFA, the insertion path was examined by color Doppler US after removal of the needle electrode in order to detect possible bleeding. About 3 h after the RFA procedure, US examination and blood count were carried out. After 24 h, contrast enhanced US (CEUS) was performed (Fig. 2e), and hemoglobin, lactate dehydrogenase and aminotransferase values were assessed. At the end of the CT-guided RFA procedure, CT was performed to evaluate the result of the procedure and to detect possible complications (Fig. 6).
Figure 6.
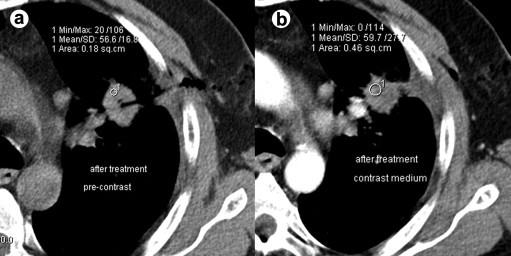
Immediate CT evaluation showing complete radiological necrosis of an RFA treated lung metastasis. The lack of contrast enhancement is indicative of complete radiological necrosis: a) Evaluation of pre-contrast density; b) Evaluation of contrast enhancement.
Follow-up
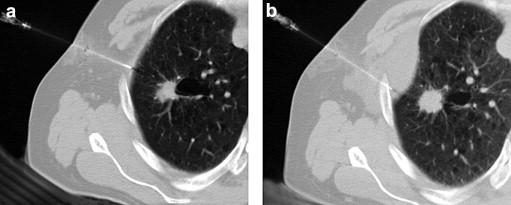
Treatment effectiveness was evaluated by CT (in case of pulmonary metastases) (Fig. 7) or US (in case of liver metastases) (Fig. 2f) with subsequent administration of contrast medium (CEUS) performed 30 ± 5 days after RFA treatment. The presence of enhancement on the site of the treated lesion was considered indicative of incomplete treatment, whereas an unenhanced area larger than the treated metastasis was regarded as complete radiological necrosis and therefore complete response to therapy.
Figure 7.
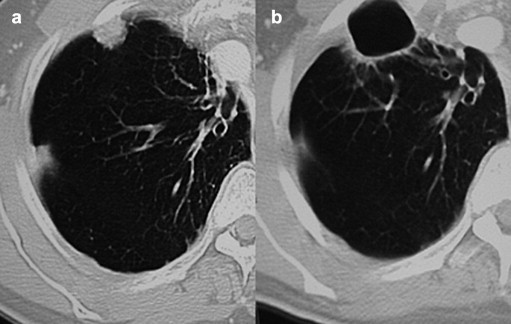
CT follow-up of an RFA treated lung metastasis: a) the lesion before RFA; b) CT shows complete ablation of the lesion 1 year after treatment with cystic degeneration of the perilesional parenchyma.
In case of incomplete treatment, RFA was repeated if the inclusion criteria were still met. If follow-up 30 days after the second RFA session showed residual tumor tissue the case was classified as treatment failure. Patients with complete response underwent clinical and radiological follow-up which included CT (in case of pulmonary metastases), magnetic resonance imaging (MRI) and/or CEUS (in case of liver metastases) after 3, 6, 12 months during the first year and every 6 months thereafter.
Statistical analysis
Median survival was calculated with recurrence (median disease free survival) and death (median overall survival) as end points. Statistical analysis was carried out using MedCalc 12 (MedCalc Software, Mariakerke, Belgium).
Results
There were no deaths or severe complications (which required treatment). In one case after RFA of a liver metastasis, a small fluid collection appeared in Morrison's pouch which cleared up without treatment. There were no treatment failures. During follow-up (mean 26 months, range 4–63 months) 5/8 (62.5%) patients had recurrence: 3/5 (60%) had local recurrence and 2/5 (40%) had non-local recurrence; 4/5 patients with recurrence underwent repeated treatment, whereas repeated treatment was not performed in one case as the patient refused. Of the 4 recurrent lesions, 2 were local liver lesions and 2 were non-local liver lesions. In all cases complete radiological necrosis was obtained. Median disease free and median overall survival were respectively 7.65 and 25.7 months after US-guided RFA and 13.4 and 34.8 months after CT-guided RFA. Median disease free and median overall survival are shown in Fig. 8a–b, respectively, using the Kaplan–Meier method.
Figure 8.
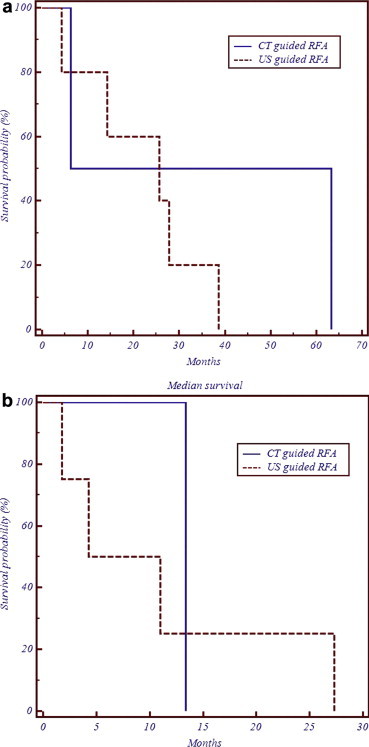
A Kaplan–Meier graphic illustration of median survival: a) Overall; b) Disease free.
Discussion
The authors' experience confirms that RFA is an effective, safe and repeatable treatment of metastases from BC. The percentage of complete radiological necrosis is indicative of the effectiveness of RFA in the ablation of metastases from BC and coincides with the results reported in the literature [8–10]. Moreover, the lack of complications confirms that RFA is an extremely safe technique [12,19]. This should be kept in mind particularly in view of the complication rate linked to surgery which is much higher [20–22]. In addition, RFA was repeated in nearly 80% of cases [9] as opposed to resective surgery [21]. These data confirm that RFA is an extremely versatile technique which can be repeated in the same patient to treat new metastases and in cases of incomplete treatment.
The present study has limitations, mostly related to sample size and standardization of the systemic therapies administered. Furthermore, the very restrictive inclusion criteria probably induced selection bias. However, comparison of the results of this study and those reported in the literature (which are still not related to particularly large patient populations) [8,9] show that the present experience is in agreement with the results reported by other authors [8–10] confirming that RFA may complement surgical resection in the treatment of metastases from BC.
The number of non-local recurrent lesions (40% of total recurrences) confirms that metastatic BC is a disease which should be addressed in a multidisciplinary context in which the role of chemotherapy is indisputable [4]. There is an increase in survival of selected patients receiving combined systemic and local therapy [9] compared with survival of patients treated with chemotherapy alone [2]. These results, as well as those related to surgical series [5,6], show that local treatment of metastases from BC may increase survival in selected patients with liver or lung metastases from BC.
RFA compared with surgical resection has several important advantages: it is less expensive, it does not require long periods of hospitalization, it is not associated with significant mortality and morbidity and it has a high repeatability rate. Seen from a “test of time approach” [23] point of view RFA may anticipate surgery when possible. These results still require further validation through prospective randomized studies of larger patient populations.
Conflict of interests
The authors have no conflict of interests to disclose.
Appendix A. Supplementary material
References
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T. Cancer statistics. CA Cancer J Clin 2008. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Pentheroudakis G., Fountzilas G., Bafaloukos D., Koutsoukou V., Pectasides D., Skarlos D. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat. 2006;97(3):237–244. doi: 10.1007/s10549-005-9117-4. [DOI] [PubMed] [Google Scholar]
- 3.Jakobs T.F., Hoffmann R.T., Schrader A., Stemmler H.J., Trumm C., Lubienski A. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32(1):38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 4.Beslija S., Bonneterre J., Burstein H.J., Cocquyt V., Gnant M., Heinemann V. Central European Cooperative Oncology Group (CECOG). Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009;20(11):1771–1785. doi: 10.1093/annonc/mdp261. [DOI] [PubMed] [Google Scholar]
- 5.Elias D., Lasser P.H., Montrucolli D., Bonvallot S., Spielmann M. Hepatectomy for liver metastases from breast cancer. Eur J Surg Oncol. 1995;21:510–513. doi: 10.1016/s0748-7983(95)96972-1. [DOI] [PubMed] [Google Scholar]
- 6.Kycler W., Laski P. Surgical approach to pulmonary metastases from breast cancer. Breast J. 2012;18(1):52–57. doi: 10.1111/j.1524-4741.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 7.Rossi S., Ravetta V., Rosa L., Ghittoni G., Viera F.T., Garbagnati F. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53(1):136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T., Goldberg S.N., Solbiati L., Meloni F., Ierace T., Gazelle G.S. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220(1):145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- 9.Meloni M.F., Andreano A., Laeseke P.F., Livraghi T., Sironi S., Lee F.T., Jr. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation–intermediate and long-term survival rates. Radiology. 2009;253(3):861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennathur A., Abbas G., Qureshi I., Schuchert M.J., Wang Y., Gilbert S. Radiofrequency ablation for the treatment of pulmonary metastases. Ann Thorac Surg. 2009;87(4):1030–1036. doi: 10.1016/j.athoracsur.2008.12.061. discussion 1036–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.Rossi S., Dore R., Cascina A., Vespro V., Garbagnati F., Rosa L. Percutaneous computed tomography-guided radiofrequency thermal ablation of small unresectable lung tumours. Eur Respir J. 2006;27(3):556–563. doi: 10.1183/09031936.06.00052905. [DOI] [PubMed] [Google Scholar]
- 13.Beslija S., Bonneterre J., Burstein H.J., Gnant M., Goodwin P., Heinemann V. Consensus on medical treatment of metastatic breast cancer (statement for Central European Cooperative Oncology Group) Breast Cancer Res Treat. 2003;81(Suppl. 1):S1–S7. [Google Scholar]
- 14.Beslija S., Bonneterre J., Burstein H., Cocquyt V., Gnant M., Goodwin P. Second consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2007;18(2):215–225. doi: 10.1093/annonc/mdl155. Epub 2006 Jul 10. [DOI] [PubMed] [Google Scholar]
- 15.Rossi S., Garbagnati F., Lencioni R., Allgaier H.P., Marchianò A., Fornari F. Percutaneous radiofrequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood flow. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 16.Rossi S., Buscarini E., Garbagnati F., Di Stasi M., Quaretti P., Rago M. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 17.Head H.W., Dood G.D., 3rd Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127:S167–S178. doi: 10.1053/j.gastro.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Curley S.A., Izzo F., Delrio P., Ellis L.M., Granchi J., Vallone P. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawes D., Chopada A., Gillams A., Lees W., Taylor I. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88(7):639–642. doi: 10.1308/003588406X149129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schepers A., Mieog S., van de Burg B.B., van Schaik J., Liefers G.J., Marang-van de Mheen P.J. Impact of complications after surgery for colorectal liver metastasis on patient survival. J Surg Res. 2010;164(1):e91–e97. doi: 10.1016/j.jss.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Shah S.A., Haddad R., Al-Sukhni W., Kim R.D., Greig P.D., Grant D.R. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202(3):468–475. doi: 10.1016/j.jamcollsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Baron O., Amini M., Duveau D., Despins P., Sagan C.A., Michaud J.L. Surgical resection of pulmonary metastases from colorectal carcinoma. Five-year survival and main prognostic factors. Eur J Cardiothorac Surg. 1996;10(5):347–351. doi: 10.1016/s1010-7940(96)80093-5. [DOI] [PubMed] [Google Scholar]
- 23.Livraghi T., Solbiati L., Meloni F., Ierace T., Goldberg S.N., Gazelle G.S. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003;97:3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


