Abstract
Purpose
Septic arthritis (SA), frequently involving hand and wrist, is common in rheumatoid arthritis (RA) patients due to immunomediated etiology of RA and immunosuppressive drug use. Clinical and laboratory features might not be useful to differentiate between RA relapse and superimposed SA. The role of magnetic resonance imaging (MRI) has been described in several studies. Our aim is to evaluate the role of ultrasonography (US).
Material and methods
In the last 4 years 31 MRI of hand and wrist has been performed in the suspect of SA complicating RA. A 1.5 T unit (Siemens Symphony, Erlangen, Germany) with standardized protocol, involving the administration of contrast medium, was used. Also US with power Doppler evaluation was performed. A Philips IU22 US scanner was used.
Results
Eleven points (according to Graif's study) were analyzed for every MRI and US. At MRI joint effusion (37.5% of RA relapse vs 100% superimposed SA) and soft tissue edema (25% vs 100%) were indicative of SA. At US joint effusion (31.3% of RA relapse vs 73.3% superimposed SA) and soft tissue edema (12.5% vs 60%) were indicative of SA.
Conclusion
Our results suggest that joint effusion and soft tissue edema are markers suggestive for superimposed SA and that MRI is more sensitive in their evaluation. Although US is less sensitive than MRI, the former is important in guiding invasive procedure and evaluating patients that cannot undergo MRI.
Keywords: Rheumatoid arthritis, Septic arthritis, Ultrasonography, Magnetic resonance imaging
Sommario
Scopo del lavoro
L'artrite settica (SA), che spesso coinvolge mano e polso, è comune nei pazienti con artrite reumatoide (AR) a causa dell'eziologia immunomediata della AR e dell'uso di farmaci immunosoppressivi. Le caratteristiche cliniche e di laboratorio possono non essere utili per distinguere tra recidiva di AR e sovrapposizione di SA. Il ruolo della risonanza magnetica (RM) è stata descritto in diversi studi. Il nostro obiettivo è quello di valutare il ruolo della ecografia (US). Materiali e Metodi. Negli ultimi 4 anni sono stati eseguiti 31 esami di risonanza magnetica della mano e del polso nel sospetto di SA come complicanza di AR. È stata utilizzata una risonanza magnetica da 1.5 T (Siemens Symphony, Erlangen, Germania) con protocollo standardizzato, che comprendeva la somministrazione di mezzo di contrasto; è stata eseguita anche un'ecografia con power Doppler utilizzando un ecografo Philips IU22.
Risultati
Sono stati analizzati undici punti (secondo lo studio di Graif) per ogni esame di RM e di US. Alla RM il versamento articolare (37,5% di recidiva AR vs 100% sovrapposizioni di SA) e l'edema dei tessuti molli (25% vs 100%) erano indicativi di SA. Anche ecograficamente il versamento articolare (31.3% di AR recidiva 73,3% vs sovrapposizione di SA) e l'edema dei tessuti molli (12,5% vs 60%) erano indicativi di SA.
Conclusione
I nostri risultati confermano che il versamento articolare e l'edema dei tessuti molli sono suggestivi per sovrapposizione di SA e che la RM è più sensibile nella loro valutazione. Anche se l'US è meno sensibile della RM, essa è importante nel guidare procedure invasive e nella valutazione dei pazienti che non possono essere sottoposti a RM.
Introduction
Rheumatoid Arthritis (RA) is a progressive systemic disease more common in women (3:1) and affecting 0.5–1.0% of the population [1]. The incidence varies on racial and geographical basis [2] and the onset is usually in the fourth and fifth decades [3].
Etiology is largely unknown but several hypotheses have been taken into account due to the probably multifactorial nature of the disease [4]: autoimmune, infective and genetic factors were considered, but their contribution is yet to be defined [5].
Most patients have shown a fluctuating course of the disease, characterized by a symmetric bilateral arthritis of more than three small hand joints. This may be complicated by vasculitis, atherosclerosis [6], pulmonary fibrosis and infection [2].
RA is characterized by a high [7] frequency of joint infections, commonly involving hand and wrist. This can be related to different factors: immune-mediated etiology of the disease, corticosteroids [8] or invasive procedure (either diagnostic or therapeutic) without asepsis.
Literature has reported that 46% of patients with septic arthritis (SA) had a pre-existing joint disease and, of these patients, 14% had RA and 10% other inflammatory arthritis [9].
Assessing septic complications in RA patients can be difficult because clinical and laboratory signs are confounded by underlying disease or by therapy (e.g. corticosteroids increase white blood cell count as well as infection). Imaging is the best way to distinguish between patients with reactivated RA and those with septic arthritis, without invasive procedure which, nevertheless, are necessary for final diagnosis. As a matter of fact, since 1987 American College of Rheumatology's classification [10], until 2010 European League Against Rheumatism's criteria, imaging has always had a key role in evaluating RA [11]. Every classification stresses the importance of an accurate and early evaluation of articular involvement and this goal, nowadays, can be achieved only through imaging: ultrasound (US) or magnetic resonance imaging (MRI).
First level imaging techniques, such as US, are important in order to evaluate soft tissue involvement and tendons, muscle or ligaments disorders [12], but fails in evaluating synovial and articular early modifications. Only MRI imaging can provide visualization of intra-articular structure and proved to be very sensitive in showing alteration at this site [13].
This techiniques are important not only in diagnosis, staging or therapy evaluation but also, and even more, in detecting articular complication. Misdiagnosis of local complication can lead to relevant therapeutic delay so it's necessary to evaluate the real contribute of MRI and US imaging in detecting septic complication in patients with pre-existing RA. The role of magnetic resonance imaging (MRI) has been described in several studies. Our aim is to evaluate the role of ultrasonography (US). To address this issue we decided to review the experience of our center taking into account the latest scientific literature.
Material and methods
During the last 4 years (October 2006–May 2011) our Department performed 182 MRI of hand and wrist. Among these 182 MRI, 31 (31/182, 17.0%) were referred to our structure because there was a suspect of SA complicating RA.
All 31 patients had a previous diagnosis of inflammatory arthritis: 24 (24/31, 77.4%) of RA, 2 (2/31, 6.5%) of atypical LES and 4 (5/31, 16.1%) of an undetermined inflammatory arthritis (on MRI very suggestive to be RA).
The 31 patients under examination had very polarized demographic characteristic. The mean age was 56.2 year (median 57.5 years, mode 45 years). If we consider that the mean age of onset of RA is during the fourth and fifth decades, we can therefore note that, after a 10 year history of disease, our patients start to develop complications such as infective arthritis. With regards to sex, 23 patients were female and 8 patients were male (74.2% vs. 25.8%; ∼3:1): very close to the ratio reported in the literature.
Imaging technique
All 31 patients were examined on a 1.5 T unit (Siemens Symphony MAGNETOM, Siemens Medical Solutions, Erlangen, Germany) with superconductive magnet and with a Philips IU22 US scanner.
MRI protocol used has obviously been subject to several changes during the period 2006–2011, but for all of them an unchangeable core of sequence was acquired. Paramagnetic contrast medium (Dotarem, Guerbet, Roissy CdG, France) was administrated. Every exam consisted in, at least, one T1w sequence (in transverse or coronal plane: generally for the wrist transverse plane was used, while for hand coronal plane was applied) and 2 high intrinsic contrast sequence (T2w with fat suppression or TIRM) on two perpendicular plane. When administration of contrast medium was possible [28 of 31 cases; 90.3%], T1w sequence with fat suppression were repeated on the most meaningful plane. When possible, this protocol was integrated with other plane or other kind of sequences (T2w and PD), which were considered important to evaluate specific aspects of the single case. Nevertheless, some patients' claustrophobia or uncontrollable movements permitted to acquire only partial examinations or low quality images. In these cases if the exam was judged not diagnostic by the radiologist these patients were excluded from our revision.
Methods
All the exams were evaluated by a small pool of radiologists (5 members) with at least 10 years of experience in musculoskeletal US and 5 years of experience in MRI imaging. Challenging cases were revised collegially.
During the analysis, eleven findings were taken from Graif et al. [14] article, in which the prevalence of these (and other) findings in septic and non-septic joint were reported. Graif's findings covered all the components of joints and adjacent tissues: synovia, articular space, cortical bone, bone marrow and soft tissue.
The 11 findings evaluated in our study were effusion presence and heterogeneity, synovial thickening and contrast enhancement (c.e.), cartilage loss, bone erosions and their c.e., bone marrow edema and his c.e., soft tissue edema and c.e. Graif's eleven findings were modified to fit US imaging: instead of contrast enhancement was considered power-Doppler (PD) behavior. Bone marrow edema and contrast enhancement were not assessed on US.
With regards to our research, investigators used a semi-quantitative scale to report their findings: they divided pathologic manifestation in mild (+), moderate (++) and severe (+++). Only severe manifestations were used in the subsequent analysis.
Final diagnosis (15 SA and 16 RA reactivation) was determinate by ex adiuvantibus criteria and, if necessary, through biopsy.
Results
Our MRI findings were taken into account separately for reactivated RA and infective complications (respectively) [Table 1]. The same was done for US findings [Table 2]. Relevant fluid heterogeneity was never observed and will not be taken into account in the subsequent discussion.
Table 1.
MRI findings.
| Characteristic | RA reactivation % | No. patients | Infective complication % | No. patients |
|---|---|---|---|---|
| Effusion | 37.5 | 6/16 | 100.0 | 15/15 |
| Fluid heterogeneity | 0.0 | 0/16 | 0.0 | 0/15 |
| Synovial thickening | 75.0 | 12/16 | 73.3 | 11/15 |
| Synovial c.e. | 93.3 | 14/15 | 84.6 | 11/13 |
| Cartilage loss | 37.5 | 6/16 | 40.0 | 6/15 |
| Bone erosion | 50.0 | 8/16 | 60.0 | 9/15 |
| Bone erosion c.e. | 53.3 | 8/15 | 69.2 | 9/13 |
| Bone marrow edema | 25.0 | 4/16 | 33.3 | 5/15 |
| Bone marrow c.e. | 20.0 | 3/15 | 23.1 | 3/13 |
| Soft tissue edema | 25.0 | 4/16 | 100.0 | 15/15 |
| Soft tissue c.e. | 20.0 | 3/15 | 100.0 | 13/13 |
Table 2.
US findings.
| Characteristic | RA reactivation % | No. patients | Infective complication % | No. patients |
|---|---|---|---|---|
| Effusion | 31.3 | 5/16 | 73.3 | 11/15 |
| Fluid heterogeneity | 12.5 | 2/16 | 6.7 | 1/15 |
| Synovial thickening | 81.3 | 13/16 | 66.7 | 10/15 |
| Synovial PD | 93.8 | 15/16 | 100.0 | 15/15 |
| Cartilage loss | 37.5 | 6/16 | 26.7 | 4/15 |
| Bone erosion | 43.8 | 7/16 | 66.7 | 10/15 |
| Bone erosion PD | 37.5 | 6/16 | 33.3 | 5/15 |
| Bone marrow edema | ||||
| Bone marrow c.e. | ||||
| Soft tissue edema | 12.5 | 2/16 | 60.0 | 9/15 |
| Soft tissue PD | 37.5 | 6/16 | 86.7 | 13/15 |
The analysis of Tables 1 and 2 indicates that presence or absence of effusion and soft tissue edema seems to be more significant.
High grade articular or tendon effusion shows significant difference between the two groups both on MRI [Figs. 1 and 2] and US [Figs. 3 and 4]. Also soft tissue edema showed the same behavior [Figs. 5 and 6 and Fig. 7 for MRI and US respectively]. Administration of contrast medium increased all the differences, making them more evident (e.g. soft tissue contrast enhancement was almost only reported during infective complication (20% of RA Reactivation vs. 100% of SA cases)).
Figure 1.
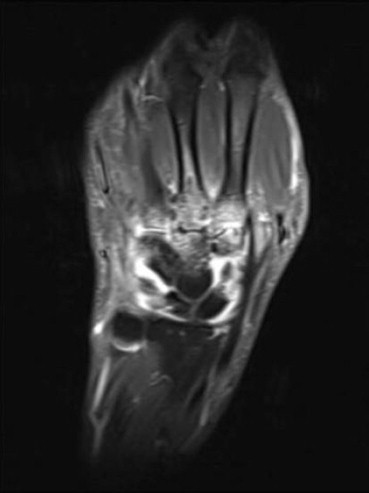
A 47 years old male patient with septic arthritis complicating RA after corticosteroid joint infiltration occurred 3–4-weeks earlier. Coronal T1-weighted (598/18) with fat suppression sequence after gadolinium (Dotarem) administration showing bone morphologic and structural alteration and significant effusion.
Figure 2.
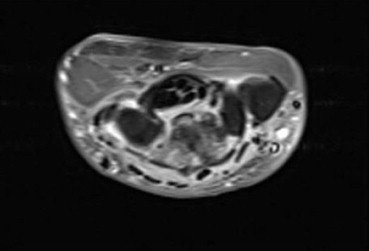
The same patient after 1 month (during this time diagnosis was clarified and antibiotics therapy started). Axial T1-weighted (436/21) with fat suppression sequence after gadolinium (Dotarem) administration shows significant reduction of effusion.
Figure 3.
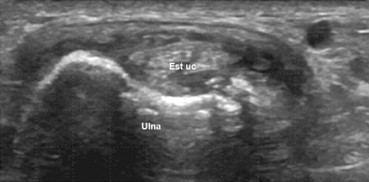
Extensor carpi ulnaris (Est uc) tenosynovitis. US shows effusion and synovial hypertrophy.
Figure 4.
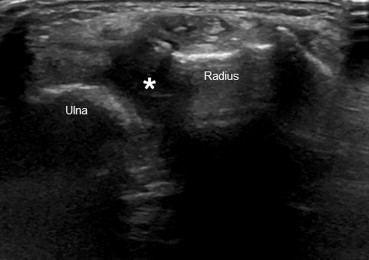
Joint effusion. US shows an anechoic collection within radio-ulnar distal joint (*).
Figure 5.
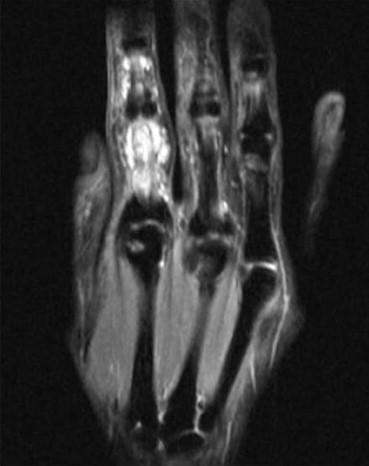
A 41 years old female patient with pain and functional impairment risen up during summer holiday without any relevant trauma. Coronal (Short Tau Inversion Recovery) (3970/13/160) shows low grade signs of RA, such as little subcortical cysts on the head of II metacarpal bone and high grade signs of SA such as soft tissue edema.
Figure 6.
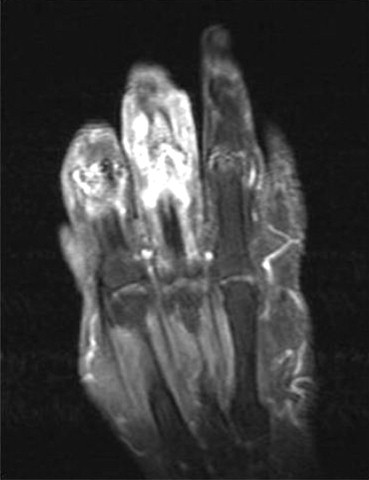
A 78 years old female patient, under treatment for RA and other co-morbidities, who developed pain and joint swollen. Coronal T1-weighted (844/21) with fat suppression sequence after gadolinium (Dotarem) administration shows two infective focus at index (digitus secundus) and middle (digitus tertius) fingers with important soft tissue c.e.
Figure 7.
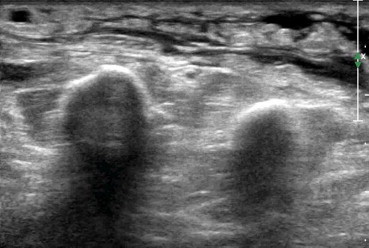
Soft tissue edema. US shows diffuse edema with hypoechoic aspects of soft tissue.
We choose to not perform a p-value evaluation because of the small sample size available and because the intention was to report our experience as a high volume but not musculoskeletal and rheumatologic-oriented structure.
Conclusions
The choice to revise our experience in differentiate RA reactivation and infective complication in patients with clinical reacutization of symptoms of undetermined origin, who underwent MR and US imaging of hand and wrist in our structure, springs up from two studies.
The first is Graif's [14] analysis of difference between septic and non-septic joint in naïve patients (without pre-existing diagnosis of inflammatory arthritis), which provided us with the methodological background; the second is Kherani's case report [9], which focalized the importance and toughness of differentiate RA reactivation and infective complication in patients with pre-existing articular disease.
Hand and wrist localization were considered because of a great number of articles and reviews [4,15] analyzing RA and septic manifestation at this site, and also because our everyday practice was consistent.
A semi-quantitative scale was introduced to reduce one of Graif's work bias: the high prevalence of alteration in both groups directly correlated with MR high sensitivity [13] (one of its intrinsic characteristic). Hence only manifestations of severe grade were retained for subsequent analysis, trying to enhance the difference between our two study groups.
Our results suggest that joint effusion and soft tissue edema are markers suggestive for superimposed SA and that MRI is more sensitive in their evaluation. Although US is less sensitive than MRI, the former is important in guiding invasive procedure and evaluating patients that cannot undergo MRI.
Also, contrast medium administration's importance is evident because contrast medium enhances the difference between the two groups.
Our study has several important limitations: absence of significance analysis, lacks in true MR criteria (e.g. for synovial thickening), and suffers inter-observer variation of several signs.
Accepting these limitations we can conclude that some MR signs seems to be more useful than US findings in differentiate RA reactivation and infective complications, and that contrast medium administration is an important tool to enhance the ability of radiologist to develop a diagnostic hypothesis.
Although we believe this first report will be important to pave the way to develop a methodological background, this experience needs to be enlarged in order to obtain statistical significance.
Conflict of interest
The authors have no conflict of interest to disclose.
Appendix. ASupplementary material
References
- 1.Gabriel S.E., Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11(3):229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott D.L., Wolfe F., Huizinga T.W. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 3.Sommer O.J., Kladosek A., Weiler V., Czembirek H., Boeck M., Stiskal M. Rheumatoid arthritis: a practical guide to state-of-the-art imaging, image interpretation, and clinical implications. Radiographics. 2005 Mar-Apr;25(2):381–398. doi: 10.1148/rg.252045111. [DOI] [PubMed] [Google Scholar]
- 4.Brahee D.D., Pierre-Jerome C., Kettner N.W. Clinical and radiological manifestations of the rheumatoid wrist. A comprehensive review. J Manipulative Physiol Ther. 2003 Jun;26(5):323–329. doi: 10.1016/S0161-4754(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 5.Oliver J.E., Silman A.J. What epidemiology has told us about risk factors and aetiopathogenesis in rheumatic diseases. Arthritis Res Ther. 2009;11:223–235. doi: 10.1186/ar2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku I.A., Imboden J.B., Hsue P.Y., Ganz P. Rheumatoid arthritis: model of systemic inflammation driving atherosclerosis. Circ J. 2009;73:977–985. doi: 10.1253/circj.cj-09-0274. [DOI] [PubMed] [Google Scholar]
- 7.Doran M.F., Crowson C.S., Pond G.R., O'Fallon W.M., Gabriel S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002 Sep;46(9):2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 8.Doran M.F., Crowson C.S., Pond G.R., O'Fallon W.M., Gabriel S.E. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002 Sep;46(9):2294–2300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 9.Kherani R.B., Shojania K. Septic arthritis in patients with pre-existing inflammatory arthritis. CMAJ. 2007 May 22;176(11):1605–1608. doi: 10.1503/cmaj.050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.van Tuyl L.H., Vlad S.C., Felson D.T., Wells G., Boers M. Defining remission in rheumatoid arthritis: results of an initial American College of rheumatology/European League Against Rheumatism consensus conference. Arthritis Rheum. 2009;61:704–710. doi: 10.1002/art.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi S., Martinoli C., de Gautard R., Gaignot C. Ultrasound of the digital flexor system: normal and pathological findings. J Ultrasound. 2007;10:85–92. doi: 10.1016/j.jus.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taouli B., Zaim S., Peterfy C.G., Lynch J.A., Stork A., Guermazi A. Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. AJR Am J Roentgenol. 2004 Apr;182(4):937–943. doi: 10.2214/ajr.182.4.1820937. [DOI] [PubMed] [Google Scholar]
- 14.Graif M., Schweitzer M.E., Deely D., Matteucci T. The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol. 1999;28:616–620. doi: 10.1007/s002560050562. [DOI] [PubMed] [Google Scholar]
- 15.Cimmino M.A., Bountis C., Silvestri E., Garlaschi G., Accardo S. An appraisal of magnetic resonance imaging of the wrist in rheumatoid arthritis. Semin Arthritis Rheum. 2000 Dec;30(3):180–195. doi: 10.1053/sarh.2000.9204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


