Abstract
In this study TSGA10 has been demonstrated as a testis-specific human gene that encodes a protein localized in sperm-tail and conserved in ciliary structure. Further investigations showed TSGA10 signalling and expression during embryogenesis, brain development and some malignancies including brain tumors. Given the role of this protein in neuronal development and in certain tumors, it could potentially serve as a diagnostic marker and therapeutic target in brain tumors. Therefore, using immunohistochemistry, we evaluated the localization of TSGA10 in different regions of brain, and its pattern/level of expression in tissue microarray (Cybrdi) containing human brain tumors and normal brain. In rat specimens, TSGA10 was mainly expressed in subventricular zone, hippocampus and granular layer of cerebellum of the brain. The antibody also stained the diverse and different types of human brain cancers. The TSGA10 was strongly over-expressed in glioblastoma and astrocytoma when compared to normal human brain. The expression of TSGA10 was also confirmed in astrocyte derived from a human astroctyoma cell line by immunocytochemistry. This study indicates that TSGA10 can be used as an immunohistochemical marker for human neuroglia and astrocyte cells and is over-expressed in brain tumors.
Keywords: Astrocytes, Brain neoplasms, Immunohistochemistry, Neuron-glia, TSGA10 protein
Introduction
TSGA10 has been described as a 82-kDa protein expressed in testis and during developmental stage of spermatogenesis and embryiogenesis (1). It has already been indicated that full-length TSGA10 is post-translational modified, and the processed N-terminus 27-kDa mature TSGA10 is located in the Fibrous Sheath (FS) of sperm tail (2). However, the C-terminal 55-kDa part of TSGA10 accumulates in the midpiece of spermatozoa, where it co-localizes with HIF-1a, and expressed during brain development (3), and in some solid and nonsolid tumors as a testis-cancer antigen.
The solid tumors include melanoma, pancreatic adenocarcinoma, hepatocellular carcinoma (4) and cutaneous lumphoma (5), ovarian leiomyosarcoma, germ cell tumor and gastric adenocarcinoma (derived ESTs). The non-solid tumors include Acute Lymphocytic Leukemia (ALL) and Acute Myeloplastic Leukemia (AML) (6, 7).
The TSGA10 interacts directly with hypoxia inducible factor-1-alpha (3) and contributes to the ciliary structure; thus it may behave such as VHL. In this study, we have shown the over-expression of TSGA10 in brain tumors, and our eventual goal is to determine its role in brain tumorigenesis.
Materials and Methods
Immunohistochemistry
To study the TSGA10 expression and localization, immunohistochemistry was performed in paraffin embedded brain tissue sections (2 µ) including adult rat brain and a high-density tissue microarray of human brain tumors utilizing TSGA10 antisera. For generating anti-TSGA10 antibodies, peptides derived from the TSGA10 N-and C-terminal regions (aa 206-219 and 676-689, respectively) which were also highly conserved in human, rat and mouse. The peptides were synthesized and conjugated to keyhole limpet hemocyanin and used to immunize two rabbits. The resultant antisera thus contained antibodies directed against both the TSGA10 N-and C-terminal peptides.
The tissue microarray (Cybrdi) contained largely consecutive invasive brain tumor and eight normal brain tissues samples. The specificity of the polyclonal rabbit anti-TSGA10 antibody has previously been defined and characterized (1). The TSGA10 protein expression was evaluated both semiquantitatively (0, 1+, 2+, 3+, 4+, 5+ scoring) and qualitatively (specificity and nonspecific background). The most reliable results (98.2% concordance; 0.9% of background) were obtained using a 1:800 primary antibody (10 µg/ml TSGA10 antibody) dilution. Specimens were incubated with horse-radish peroxidase goat anti-rabbit IgG (SantaCruz), and stained with DAB kits (Vetor). Mayer's hematoxylin (Sigma) was used as a counterstain. An irrelevant rabbit IgG antibody was used as an isotype control in all cases to demonstrate that staining was specific for TSGA10.
Cell culture, immunocytochemistry, and direct fluorescent staining
To study localization of TSGA10 in astrocytes, a cell line was derived from a human astrocytoma (garde III, WHO). And the astrocyte cell line (A735) cultured in Dulbecco's Modified Eagle's Medium (DMEM, high glucose) and incubated at 37 °C, 5% CO2. The astrocytes were seeded on sterilized glass cover slips treated with poly-L-lysine (Sigma) in 6-well plates.
A day after seeding, cells were fixed with methanol in -20 °C for 10 min and incubated with primary TSGA10 rabbit polyclonal antibody diluted 1:50 (v/v) in blocking solution for 1 hr at 37 °C and washed in PBS. After washing, they were incubated for 1 hr at 37 °C with the relevant secondary antibody (FITC-conjugated goat anti-rabbit IgG; Jackson Immunoresearch Laboratory, Inc., West Grove, PA) in blocking solution and washed in PBS before staining with DAPI. Cover slips were mounted on glass slides with Permount SP15-100 (Fisher Scientific, Edmonton, AB, Canada), and analyzed by fluorescence microscopy (Zeiss Vision, Mannheim, Germany).
Western blot
Twenty µg of total protein was separated after extraction from cultured cells by discontinueous 10% SDS-PAGE at 100 volts for one hr using a Mini-Protean III electrophoresis apparatus (Bio-Rad, Hercules, CA). Following electrophoresis, the gel was equilibrated in transfer buffer for 15 min and then was transferred to nitrocellulose membrane at 30 volts overnight at 4°C. Post transfer, the membrane was subjected to blocking per Western Breeze Chemiluminescent immunodetection protocol (Invitrogen, Carlsbad, CA) for 30 min at room temperature. Western blotting was performed using a 1:500 dilution of the polyclonal antibody against TSGA10 (1) and 1:10000 dilution of the monoclonal antibody against β-actin proteins for 2 hrs at room temperature.
After primary antibody probing, the membrane was washed four times and then incubated with anti-rabbit IgG horse-raddish peroxidase conjugated secondary antibody for 1 hr at room temperature, and washed with TBS (1% Tween 20 in PBS) for three times. The TSGA10 and β-actin expressions were detected by ECL enhanced chemiluminescence. Following this development, the films were scanned and the images saved in JPEG format.
Results
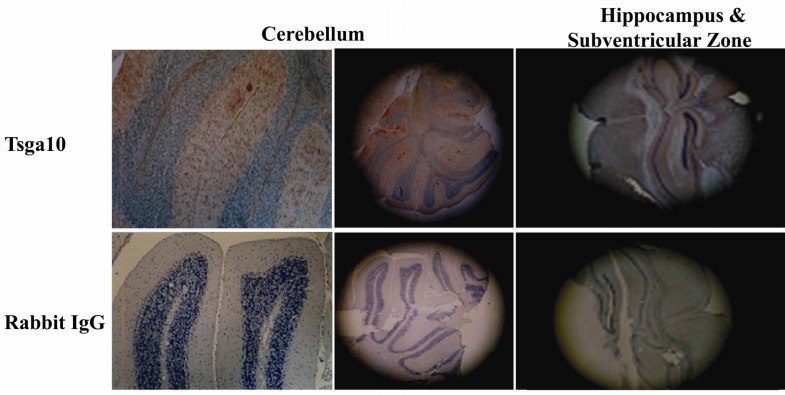
It has been shown that TSGA10 is expressed in some particular regions of normal brain. The brain regions in which TSGA10 is mainly expressed include the granular layer of cerebellum, hippocampus and subventricular zone (Figure 1). This pattern of TSGA10 expression in the brain suggested a possible high expression of TSGA10 in dividing astrocyte and/or glia cells.
Figure 1.
TSGA10 expression in rat brain
TSGA10 is expressed in different regions of rat brain including granular layer of cerebellum, hippocampus and Subventricular zone (SVZ). These brain zones are well known as the glia-rich regions of brain
This study has demonstrated TSGA10 over-expression in different pathologic types of human brain tumors with a significant difference compared to normal brain. The signals were scored via a point system by two different reviewers and final scores were an average of points in different samples by different reviewers.
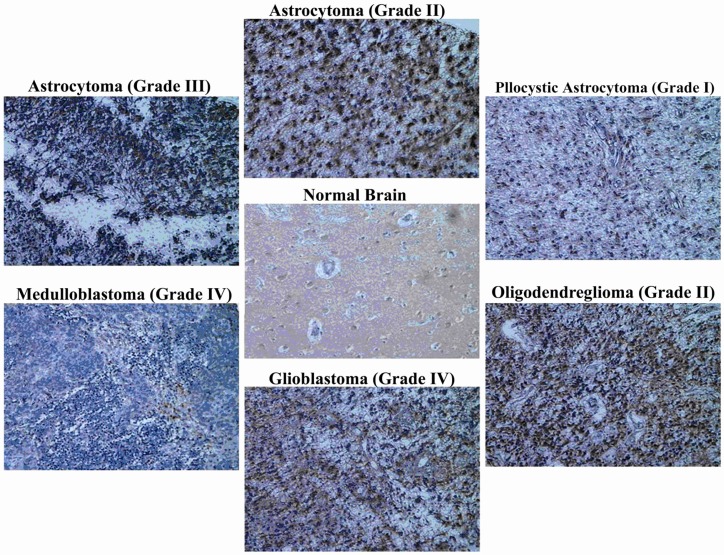
The brain tumors include glioblastoma, astrocytoma (pilocytic, grades II and III), oligodendroglioma, and medulloblastoma with highest TSGA10 over-expression in grade II oligodendrogliom (average 5+ in three samples) followed by glioblastoma (Table 1, Figure 2).
Table 1.
TSGA10 protein expression in 63 human brain samples
| No. | Pathologic Diagnosis | TSGA10 expression level (Score) |
|---|---|---|
| 8 | Normal Brain | 1 |
| 3 | Medulloblastoma | 3 |
| 12 | Oligodendroglioma | 3.4 |
| 3 | - Grade II | 5 |
| 9 | - Grade III | 3 |
| 7 | Astrocytoma (Grade III) | 3.9 |
| 9 | Astrocytoma (Grade II) | 4 |
| 3 | Pilocytic Astrocytoma | 4 |
| 21 | Glioblastoma | 4.2 |
Figure 2.
TSGA10 is expressed in human normal brain and over-expressed in human brain tumors
By immunohistochemistry and utilizing polyclonal antibody (raised in rabbit) and Cybrdi tissue microarray, TSGA10 expression is shown in normal brain, and in different brain tumors in human. TSGA10 expression shows a significant increase in human brain tumors compared to normal brain. “Glioblastoma” followed by “pilocytic astrocytoma” and “astrocytoma (grade II)” show the highest expression of TSGA10 protein and “medulloblastoma” the lowest among the brain tumors. (Zeiss microscope, x40)
Sixty three samples (dots) have been provided in the tissue array slide with nine different histopathologic diagnoses including 8 normal brains (1 + ), 3 medulloblastoma (3 + ), 12 oligodendroglioma (3 grade II:5+, and 9 grade III:3 + ), 19 astrocytoma (9 grade II:4+ and 7 grade III:3.9+, and 3 pilocytic:4 + ), and 21 glioblastoma (4.2 + ). Medulloblastoma and oligodendroglioma (grade III) generally show a lower expression of TSGA10 protein compared to other tumors. Although TSGA10 protein seems to be expressed more in grades II compared to grade III, this difference is minimal in astrocytoma whereas maximal in oligodendroglioma. Meanwhile, the highest expression of TSGA10 is observed in grade II oligodendroglioma. However, glioblastoma identifies maximum TSGA10 expression as a histopathologic entity among brain tumors (Table 1).
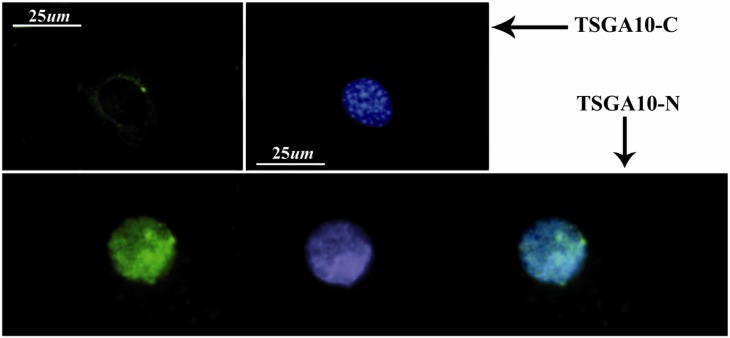
To investigate a possible expression of TSGA10 in an astrocyte, a cell line which was derived from a known human astrocytoma (grade III, WHO, as a gift) selected and endogenous TSGA10 expression was shown within the cells. Using immunostaining and IF methods, the antibody against the C-terminal of TSGA10 could localize the protein as a single spot around the nucleus, and as several spots within the nucleus, respectively (Figure 3). We also found a significant increase in The TSGA10 expression in this human cell line which was derived from astrocytoma, utilizing protein analysis. Western blot shows a 82 kDa TSGA10 band which was more intense in above-mentioned cell line (astrocyte derived from grade III astrocytoma) than the astrocyte cell line (A735), as verified by scanning densitometry normalized to β-actin (Figure 4).
Figure 3.
TSGA10 localization in astrocyte
In immunocytochemistry, TSGA10 antibody could detect the protein expression and localize the C-terminus (top panel) of the TSGA10 protein as a single perinuclear spot in the culture of astrocyte which is derived from human astrocytoma (WHO, grade III). However, the N-terminus of the protein (button panel) is expressed within the nucleus as several spots. DAPI used for the nuclei staining and FITC-conjugated (green) secondary antibody was used in IF
Figure 4.
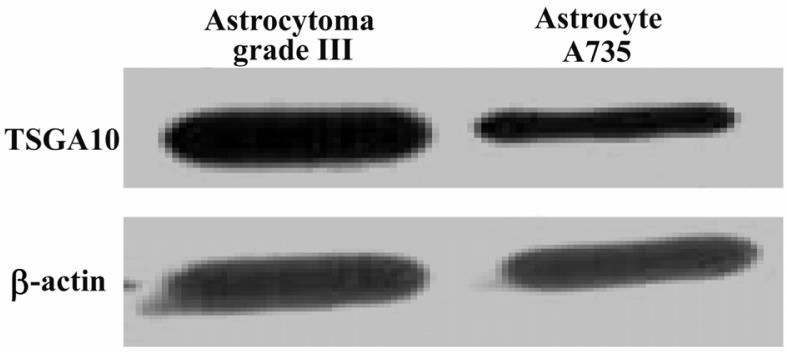
TSGA10 protein expression in astrocytes
Western blots for TSGA10 expression in astrocytes derived from a grade III astrocytoma (left panel) and an astrocyte cell line (735), in which its expression normalized to β-actin. TSGA10 protein levels in grade III astrocytoma is significantly increased compared to the astrocyte cell line (735).
Discussion
It has already been shown that TSGA10 protein has a post-translational modification, resulting in an N-terminus 27-kDa and a C-terminus 55-kDa components (1). Although the C-terminus TSGA10 may be associated with an organelle such as centrosome which is consistent with its role in cell division and proliferation however a further study in tumor biology is necessary to confirm a precise and specific function of each component.
In this study, we have demonstrated an over-expression of TSGA10 in brain tumors with a significant difference between normal brain and malignant cells. This is consistent with the previously reported over-expression in some other tumors (4-6; BLAST/NCBI ESTs). The TSGA10 (C-terminus) also interacts with HIF-1-alpha (3) and contributes to ciliary-centrosomal structure (1). The results of this study along with prior molecular findings on the TSGA10, may address a crucial role in regulating brain tumorigenesis, apoptosis and hypoxia pathways. These findings on the TSGA10 molecular behavior and expression also remind a similar function to VHL tumor suppresser protein.
Acknowledgement
Authors would like to thank Prof. Sue Povey (UCL, London, UK) for providing the cell line astrocyte which was derived from a known human astrocytoma (grade III, WHO).
References
- 1.Behnam B, Modarressi MH, Conti V, Taylor KE, Puliti A, Wolfe J. Expression of Tsga10 sperm tail protein in embryogenesis and neural development: from cilium to cell division. Biochem Biophys Res Commun. 2006;344(4):1102–1110. doi: 10.1016/j.bbrc.2006.03.240. [DOI] [PubMed] [Google Scholar]
- 2.Modarressi MH, Behnam B, Cheng M, Taylor KE, Wolfe J, van der Hoorn FA. Tsga10 encodes a 65-kilodalton protein that is processed to the 27-kilodalton fibrous sheath protein. Biol Reprod. 2004;70(3):608–615. doi: 10.1095/biolreprod.103.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagele S, Behnam B, Borter E, Wolfe J, Paasch U, Lukashev D, et al. TSGA10 prevents nuclear local-ization of the hypoxia inducible factor (HIF)-1 alpha. FEBS Lett. 2006;580(15):3731–3738. doi: 10.1016/j.febslet.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka R, Ono T, Sato S, Nakada T, Koizumi F, Hasegawa K, et al. Over-expression of the testis-specific gene TSGA10 in cancers and its immunogenicity. Microbiol Immunol. 2004;48(4):339–345. doi: 10.1111/j.1348-0421.2004.tb03515.x. [DOI] [PubMed] [Google Scholar]
- 5.Theinert SM, Pronest MM, Peris K, Sterry W, Walden P. Identification of the testis-specific protein 10 (TSGA10) as serologically defined tumour associated antigen in primary cutaneous T-cell lymphoma. Br J Dermatol. 2005;153(3):639–641. doi: 10.1111/j.1365-2133.2005.06669.x. [DOI] [PubMed] [Google Scholar]
- 6.Mobasheri MB, Modarressi MH, Shabani M, Asgarian H, Sharifian RA, Vossough P, et al. Expression of the testis-specific gene, TSGA10, in Iranian patients with acute lymphoblastic leukemia (ALL) Leuk Res. 2006;30(7):883–889. doi: 10.1016/j.leukres.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Mobasheri MB, Jahanzad I, Mohagheghi MA, Aarabi M, Farzan S, Modarressi MH. Expression of two testis-specific genes, TSGA10 and SYCP3, in different cancers regarding to their pathological features. Cancer Detect Prev. 2007;31(4):296–302. doi: 10.1016/j.cdp.2007.05.002. [DOI] [PubMed] [Google Scholar]