Abstract
Methanolic extract of whole plant of Amaranthus viridis L (MEAV), was screened for antinociceptive activity using acetic acid induced writhing test, hot plate test and tail immersion test in mice. In a similar way a screening exercise was carried out to determine the antipyretic potential of the extract using yeast induced pyrexia method in rats. Administration of the extracts was applied to both laboratory animals at the doses of 200 and 400 mg/kg body weight, respectively. The results of the statistical analysis showed that MEAV had significant (p<0.01) dose dependent antinociceptive and antipyretic properties at 200 and 400 mg/kg. Hence present investigation reveals the antinociceptive and antipyretic activities of methanolic extract of Amaranthus viridis.
Keywords: Analgesics, Animals, Dose response relationship, Methanol, Plant extracts
Introduction
Amaranthus viridis L (A. viridis Amaran-thaceae), commonly called ‘Chilaka Thota-Kura’ in Telugu, has been used in Indian and Nepalese traditional system to reduce labour pain and act an antipyretic (1, 2). The Negritos of the Philippines apply the bruised leaves directly to eczema, psoriasis and rashes etc (3). Other traditional uses range from an anti-inflammatory agent of the urinary tract, venereal diseases vermifuge, diuretic, anti-rheumatic, antiulcer, analgesic, antiemetic, laxative, improvement of appetite, antileprotic, treatment of respiratory and eye problems, to treatment of asthma (1, 4–11).
Furthermore, the plant possesses antiproliferative and antifungal lactin properties as well as ribosome inactivating protein, β-carotene (12–14) and antiviral activities (15). In addition the whole plant possesses analgesic and antipyretic properties and is used for the treatment of pain and fever respectively in traditional systems of medicine (16). However, there is not enough scientific reports to support these supposed analgesic and antipyretic activities. This has prompted us to conduct the studies to ascertain the authenticity of these important claims of traditional potency.
Materials and Methods
Collection and extraction of plant material
Fresh plant material of A. viridis was collected from surroundings of Chickballapur, Karnataka in the month of May 2009. The plant material was identified and authenticated by Dr. Rajan, Department of Botany, Government Arts College, Ootcamund, Tamilnadu.
A voucher specimen (SKVCP 13) was deposited in college herbarium. Plant material was washed with water to remove dirt and shade dried for one week. The dried material was powdered by using grinder and passed through 10-mesh sieve. The coarsely powdered material was extracted with methanol by using soxhlet apparatus. The extract was later evaporated to dryness under reduced pressure and the residue was preserved for future use.
Preliminary phytochemical screening
The methanol extract of A. viridis was screened for the presence of various phytoconstituents like steroids, alkaloids, glycolsides, flavonoids, carbohydrates, proteins and phenolic compounds (17).
Animal models
Male Swiss albino mice weighing 20-25 g were acclimatized to the experimental room at temperature 23±2°C, controlled humidity conditions (50-55%) and 12 hr light and 12 hr dark cycle. A maximum of two animals were kept in a polypropylene cage and fed with standard food pellets (Kamadenu Enterprises, Bangalore) and water ad libitum.
Acute toxicity studies
Methanol extracts of A. viridis was studied for acute oral toxicity as per revised OECD (Organization for Economic Cooperation and Development) guidelines No. 423. The extract was devoid of any toxicity in rats when given in doses up to 2000 mg/kg by oral route. Hence, in our study 200 and 400 mg/kg doses of extract were dissolved in 0.1% Carboxy Methyl Cellulose (CMC) and used for the study (18).
Antinociceptive activity
Acetic acid-induced writhing test: This test was done using the method described by Collier et al (19). Muscle contractions were induced in mice by intra peritoneal injection of 0.6% solution of acetic acid (10 ml/kg). Thirty minutes before this administration the animals were treated with diclofenac sodium (50 mg/kg), MEAV orally at doses of (200 and 400 mg/kg) and 0.1% CMC (5 ml/kg). Immediately after administration of acetic acid, the animals were placed in glass cages, and the number of ‘stretching’ per animal was recorded during the following 15 min.
Writhing movement was accepted as contraction of the abdominal muscles accompanied by stretching of hind limbs. There was significant reduction in the number of writhes by drug treatments as compared to vehicle treated animals. This was considered a positive analgesic response and the percentage inhibition of writhing was calculated (19).
Hot plate method: The hot plate test described by Eddy and Leimback (1953) was used. The mice were first treated with different doses of MEAV (200 and 400 mg/kg orally). One hour after this administration the animals were placed on a hot plate maintained at 55±1.0 °C. A cut-off period of 15 sec was considered as maximal latency to avoid injury to the paws. The time taken by the animals to lick the hind paw or jump out of the place was taken as the reaction time and was measured at 0,30,60 and 120 mins. Morphine (5 mg/kg) was used as a reference drug (20).
Tail immersion: Tail immersion was conducted as described by Aydin et al (21). This involved immersing extreme 3cm of the rat's tail in a water bath containing water maintained at a temperature of 55±0.5°C. Within a few minutes, the rats reacted by withdrawing the tail. The reaction time was measured at 0, 30,60,120,180,240 and 300 mins.
The test groups were given MEAV (200 and 400 mg/kg), morphine (5mg/kg) and 0.1% CMC in water were administered orally (21).
Screening for antipyretic activity
The antipyretic activity of MEAV was evaluated using Brewer's yeast-induced pyrexia in rats as described by Loux et al (22). Fever was induced by administering 20 ml/kg of 20% aqueous suspension of Brewer's yeast in normal saline subcutaneously. The MEAV (200 and 400 mg/kg, orally) was administered orally, paracetamol (150 mg/kg, orally) was used as reference drug and control group received distilled water. Rectal temperature was determined by thermal probe Eliab themistor thermometer at 1,2,3,4,5 and 6 hrs after test extract/reference drug administration (22).
Statistical analysis
Data were recorded as mean±SEM. The statistical significance of differences between groups was determined by analysis of variance (ANOVA), followed by Dunnett's test for multiple comparisons among groups. Differences of p<0.05 were considered statistically significant.
Results
The present study was conducted to assess the antinociceptive and antipyretic properties of methanolic extract of A. viridis. The methods selected were chemical nociception in the test model of acetic acid-induced writhing and thermal nociception hot plate and tail immersion test. These methods were selected to evaluate both centrally and peripherally mediated effects of MEAV. The acetic acid induced abdominal constriction is believed to show the involvement of peripheral mechanisms, whereas the hot plate and tail immersion tests are believed to do same by central mechanisms (23).
The results of the present study demonstrated that MEAV-possessed antinociceptive activity is evident in all the nociceptive models, suggesting the involvement of both central and peripherally mediated activities.
In acetic acid-induced abdominal constriction test, the results showed that the MEAV (200 and 400 mg/kg) potently and significantly reduced the number of abdominal writhing in a dose dependent manner with 51.01% and 64.73% of inhibition, respectively as compared to control animals (Table 1). The positive control group treated with diclofenac sodium (50 mg/kg) also showed significant reduction in the number of writhes (72.66%).
Table 1.
Effect of methanolic extract of Amaranthus viridis (MEAV) on acetic acid induced writhing test in mice
| Treatment | Dose (mg/kg) | Number of writhes | % inhibition |
|---|---|---|---|
| Control | – | 57.166±1.66 | – |
| Diclofenac sodium | 50 | 15.5±0.18 | 72.88 |
| MEAV | |||
| 200 | 28±0.89 | 51.07 | |
| 400 | 20.16±0.10 | 64.73 |
Values are in mean ±SEM; (n = 6)
It has been postulated that acetic acid acts indirectly by inducing the release of endogenous mediators, such as PGE2 (prostaglandin E2) and PGE2α in peritoneal fluids as well as lipooxygenase products, which stimulate the nociceptive neurons sensitive to NSAIDs (18, 24). Therefore, the results of the acetic acid-induced writhing strongly suggests that the mechanism of this extract may be linked partly to inhibition of lipooxygenase and/or cyclo-oxygenase in peripheral tissues, reducing in PGE2 synthesis and interfering with the mechanism of transduction in primary afferent nociceptor (Table 1).
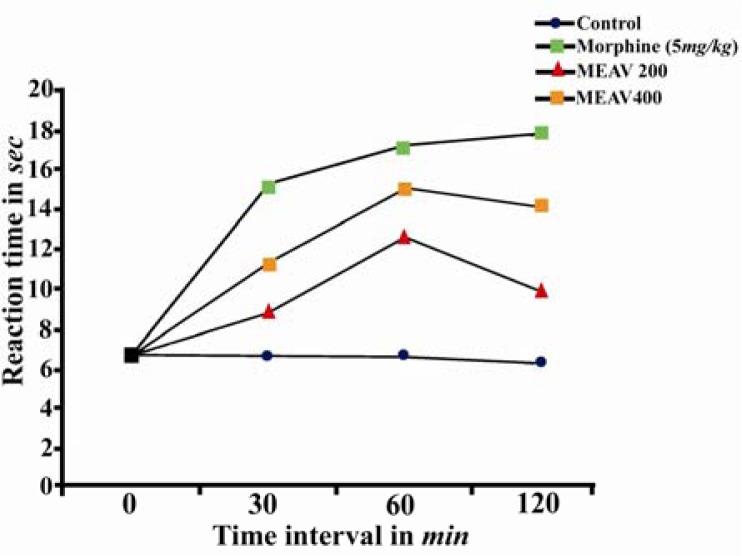
The central analgesic effect of the MEAV may be supported by the results recorded in the hot plate and tail immersion tests, a selective method used to screen centrally acting opiate analgesic drugs (23). It was demonstrated that oral administration of MEAV (200 and 400 mg/kg) significantly prolonged the latency time to the heat stimulus (Table 2, Figure. 1). This effect began early at 30 mins after administration of MEAV and persisted until the following 120 mins. As expected, morphine (5 mg/kg) significantly increased the latency time to the nociceptive response compared with control group.
Table 2.
Effect of methanolic extract of Amaranthus viridis (MEAV) on tail immersion test in mice
| Treatment | Dose (mg/kg) | Post treatment reaction time (seconds) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 min | 30 min | 60 min | 120 min | 180 min | 240 min | 300 min | |||
| Control | 2.31±0.08 | 2.48±0.07 | 2.43±0.09 | 2.31±0.056 | 2.31±0.056 | 2.34±0.06 | 2.3±0.03 | 2.3±0.06 | |
| Morphine | 5 | 2.45±0.095 | 6.0±0.15** | 7.8±0.13** | 9.8±0.19** | 8.2±0.15** | 3.8±0.06** | 3±0.014 | |
| MEAV | |||||||||
| 200 | 2.45±0.12 | 4.62±0.17* | 6.0±0.07** | 6.2±0.07** | 5.6±.0.148** | 2.8±0.12* | 2.4±0.15 | ||
| 400 | 2.33±0.06 | 5.23±0.12** | 7.48±0.12** | 8.05±0.14** | 5.7±0.15** | 3.14±0.08** | 2.67±0.08 | ||
Values are in mean ±SEM; (n = 6)
p<0.05
p<0.01 vs control
Figure 1.
Effect of methanolic extract of Amaranthus viridis (MEAV) on hot plate test in mice
Fever may be a result of infection or one of the sequelae of tissue damage, inflammation, graft rejection, or other disease states. Antipyretics are drugs, which reduce the elevated body temperature. Regulation of body temperature requires a delicate balance between production and loss of heat, and the hypothalamus regulates the set point at which body temperature is maintained. In fever this set point elevates and a drug like paracetamol does not influence body temperature when it is elevated by the factors such as exercise or increase in ambient temperature (25).
The results obtained (Table 3) revealed that MEAV showed significant (p<0.05) antipyretic activity at all doses tested. The MEAV at 400 mg/kg showed antipyretic activity after 19 hrs of administration of Brewer's yeast and continued until the end of the experiment, while 200 mg/kg dose showed reduction in temperature after 22 hrs of administration of Brewer's yeast extending up to 23 hrs. Therefore, the MEAV possesses a significant antipyretic effect in yeast-induced elevation of body temperature in rats and this may be attributed to the anti-inflammatory properties of the plant.
Table 3.
Effect of methanolic extract of Amaranthus viridis (MEAV) on yeast induced pyrexia
| Treatment | Dose (mg/kg) | Rectal temperature (°C) after yeast injection | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 0hr | 19hr | 20hr | 21hr | 22hr | 23hr | 24hr | ||
| Control | 37.39±0.03 | 39.16±0.02 | 39.2±0.15 | 39.2±0.15 | 39.2±0.15 | 39.05±0.18 | 38.58±0.21 | |
| Paracetamol | 150 | 36.93±0.41 | 38.6±0.56 | 37.33±0.21** | 37.33±0.31** | 37.52±0.17** | 37.41±0.2** | 37.26±0.16** |
| MEAV | ||||||||
| 200 | 37.26±0.17 | 39.1±0.18 | 38.5±0.34 | 38.35±0.335* | 38.28±0.17* | 38.12±0.21* | 38.1±0.106 | |
| 400 | 37.4±0.17 | 38.78±0.45 | 37.86±0.42* | 37.63±0.22** | 37.45±0.19** | 37.57±0.20** | 37.4±0.17** | |
Values are in mean ±SEM; (n = 6)
p<0.05
p<0.01 vs control
Discussion
Preliminary phytochemical study indicated the presence of alkaloids, steroids, glycosides, flavonoids, phenolic compounds, terpenoids, proteins and carbohydrates which might be responsible for the antinociceptive and antipyretic effects of the MEAV.
Flavonoids and phenolic compounds have been reported to have multiple biological effects such as anti-oxidant activity (26), antinociceptive activity in vivo (27, 28), anti inflammatory action (29, 30), inhibition of platelet aggregation (31), inhibition of mast cell histamine release (32) and inhibitory action on arachidonic acid metabolism as demonstrated by in vitro and in vivo tests (33).
Acknowledgement
The authors wish to thank Sri K.V. Naveen Kiran, Chairman, Sri K.V. College of Pharmacy, Chickballapur, Karnataka (India), for providing the research facilities to carry out this work successfully.
References
- 1.Kirtikar KR, Basu BD. Indian Medicinal Plants. In: Kirtikar KR, Basu BD, editors. Dehra Dun. 2nd ed. Vol. 3. India: International book distributors; 1987. pp. 2061–2062. [Google Scholar]
- 2.Turin M. Ethnobotonical notes on Thangmi plant names and their medicinal and ritual uses. CNAS. 2003;30(1):19–52. [Google Scholar]
- 3.Quisumbing E. Manila: Bureau of Printing; 1951. Medicinal plants of the Philippines, Department of Agriculture and Natural Resources; pp. 298–351. [Google Scholar]
- 4.Council of Scientific and Industrial Research (CSIR) Publications and Information Directorate. The Wealth of India; A Dictionary of Indian raw materials and industrial products; New Delhi, India: 1988. p. 221. [Google Scholar]
- 5.Agra MF, Baracho GS, Nurit K, Basilio IJLD, Coelho VPM. Medicinal and poisonous diversity of the flora of “Cariri Paraibano” Brazil. J Ethno-pharmacol. 2007;111(2):283–395. doi: 10.1016/j.jep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 6.De Fatima Agra M, Silva KN, Basilio IJLD, De Freitas PF, Filho JMB. Survey of medicinal plants used in the region northeast of Brazil. Braz J Pharmacognosy. 2008;18(3):472–508. [Google Scholar]
- 7.Sher H, Khan ZD. Resource utilization for economic development and folk medicine among the tribal people. Observation from Northern part of Pakistan. Pak J Plant Science. 2006;12(2):149–162. [Google Scholar]
- 8.Quershi SJ, Khan MA, Ahmed M. A survey of useful medicinal plants of Abbottabad, in Northern Pakistan. Trakia J Sci. 2008;6(4):39–51. [Google Scholar]
- 9.Dar MEI. Ethnobotonical uses of plants of Lawat district Muzaffarabad Azad Jammu and Kashmir. Asian J Plant Sci. 2003;2(9):680–682. [Google Scholar]
- 10.Arshad M, Khan QUA. Ethnobotonical study of some medicinal plants of Rawal Town. Pak J Biol Sci. 2000;3(8):1245–1246. [Google Scholar]
- 11.Muhammad S, Amusa NA. The important food crops and medicinal plants of north-western Nigeria. Res J Agric Biol Sci. 2005;1(3):254–260. [Google Scholar]
- 12.Kaur N, Dhuna V, Kamboja SS, Agrewala JN, Singh J. A novel antiproliferative and antifungal lactin from Amaranthus viridis Linn seeds. Protein Pept Lett. 2006;13(9):897–905. doi: 10.2174/092986606778256153. [DOI] [PubMed] [Google Scholar]
- 13.Kwon SY, An CS, Liu JR, Pack KH. A Ribosome inactivating protein from Amaranthus viridis. Biosci Biotechnol Biochem. 1997;61(9):1613–1614. doi: 10.1271/bbb.61.1613. [DOI] [PubMed] [Google Scholar]
- 14.Sena LP, Vanderjagt DJ, Rivera C, Tsin ATC, Muhamadu I, Mahamadou O, et al. Analysis of nutritional components of eight famine foods of the Republic of Nigeria. Plant Foods Hum Nutr. 1998;52(1):17–30. doi: 10.1023/a:1008010009170. [DOI] [PubMed] [Google Scholar]
- 15.Obi RK, Iroagba II, Ojiako OA. Virucidal potential of some edible Nigerian vegetables. Afr J Biotechnol. 2006;5(19):1785–1788. [Google Scholar]
- 16.Yusuf M, Chowdhury JU, Wahab MA, Begum J. Chittagong Bangladesh Council for Science and Industrial Research (BCSIR) 1994. Medicinal plants of Bangladesh. [Google Scholar]
- 17.Kokate CK. Preliminary phytochemical analysis. In: Kokate CK, editor. Practical Pharmacognosy. 1st ed. New Delhi: Vallabh Prakashan; 1986. p. 111. [Google Scholar]
- 18.Organization for Economic Cooperation and development (OECD) Guideline 423 for testing chemicals: Paris; 2001. pp. 1–14. [Google Scholar]
- 19.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br Jr Pharmacol Chemther. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddy NB, Leimback D. Synthetic analgesic. II. Dithienylbutenyl and dithienybutylamines. J Pharmacol ExpTher. 1953;107:385–402. [PubMed] [Google Scholar]
- 21.Aydin S, Demir T, Ozturk Y, Baser KHC. Analgesic activity of Nepeta italica L. Phytother Res. 1999;13(1):20–23. doi: 10.1002/(SICI)1099-1573(199902)13:1<20::AID-PTR380>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Loux JJ, De Palma PD, Yankell SL. Antipyretic testing of aspirin in rats. Toxicol Appl Pharmacol. 1972;22(4):672–675. doi: 10.1016/0041-008x(72)90295-5. [DOI] [PubMed] [Google Scholar]
- 23.Abbott FV, Melzack R. Brainstem lesions dissociate neural mechanisms of morphine analgesia in different kinds of pain. Brain Res. 1982;251(1):149–155. doi: 10.1016/0006-8993(82)91282-3. [DOI] [PubMed] [Google Scholar]
- 24.Deraedt R, Jouquey S, Delevallée F, Flahaut M. Release of prostaglandin E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61(1):17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 25.Brunton L, Lazo J, Parker K. 11th ed. New York: McGraw-Hill; 1996. Goodman & Gilman's: The pharmacological basis of therapeutics; pp. 959–975. [Google Scholar]
- 26.Bors W, Saran M. Radical scavenging by flavonoid antioxidant. Free Radic Res. 1987;2(4-6):289–294. doi: 10.3109/10715768709065294. [DOI] [PubMed] [Google Scholar]
- 27.Delorme P, Jay M, Ferry S. Anti-inflammatory and analgesic activity from roots of Angelica pubescens. Planta Medica. 1995;61(1):2–8. doi: 10.1055/s-2006-957987. [DOI] [PubMed] [Google Scholar]
- 28.Mills S, Bone K. Principles and practice of Phytotherapy. Edinburgh: Churchill Livingstone. 2000:23–24,31-34,229-231. [Google Scholar]
- 29.Moreira AS, Spitzer V, Schapoval EES, Schenkel EP. Anti-inflammatory activity of extracts and fractions from the leaves of Gochnatia polymorpha. Phytother Res. 2000;14(8):638–640. doi: 10.1002/1099-1573(200012)14:8<638::aid-ptr681>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Rao ChV, Ojha SK, Amresh G, Mehrotra S, Pushpangadan P. Analgesic, anti-inflammatory and antiulcerogenic activities of unripe fruit of Aegle marmelos. Acta Pharmaceutica Turcica. 2003;45:85–91. [Google Scholar]
- 31.Van Wauve JP, Goosens JG. Arabinolactan and dextran induced ear inflammation in mice: differential inhibition of H1-antihistamines, 5HT-serotonin antagonist and lipoxygenase blockers. Agents Actions. 1989;28:78–82. doi: 10.1007/BF02022984. [DOI] [PubMed] [Google Scholar]
- 32.Amresh G, Reddy GD, Rao ChV, Singh PN. Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol. 2007;110(3):526–531. doi: 10.1016/j.jep.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Amresh G, Zeashan H, Rao ChV. Prostaglandin mediated anti-inflammatory and analgesic activity of Cissampelos pareira. Acta Pharmaceutica Sci. 2007;49:153–160. [Google Scholar]