Abstract
Rabies is a fatal neurological disease and a persistent global problem. It is spread primarily by domestic dogs but other canid, viverrid (skunks and raccoons) and chiropteran species are considered as the most efficient vectors of the disease. Since dogs are the main perpetuator of rabies, special attention has to be given to bring all the dogs including unauthorized stray dogs under immunization umbrella in order to control rabies. Vaccination is the only way to combat the disease before and after exposure or infection as there is no treatment available once the symptoms have appeared. After the first crude nerve tissue vaccine developed by Pasteur in 1885, a number of rabies vaccines for animal and human use have been developed with varying degree of safety and efficacy over the years. Presently, cell culture based inactivated rabies vaccines are largely used in most of the parts of the world. However, these vaccines are too expensive and unaffordable for vaccination of people and animals in developing countries. The comparatively cheaper inactivated nerve tissues vaccines can cause serious side-effects such as autoimmune encephalomyelitis in inoculated animals and production has been discontinued in several countries. Although attenuated live vaccines can efficiently elicit a protective immune response with a smaller amount of virus, they sometimes can cause rabies in the inoculated animals by its residual virulence or pathogenic mutation during viral propagation in the body. New-generation rabies vaccines generated by gene manipulation although in experimental stage may be a suitable alternative to overcome the disadvantages of the live attenuated vaccines. So, awareness must be created in general public about the disease and the cell culture based vaccines available in the market should be recommended for wide scale use to prevent and control this emerging and reemerging infectious disease in foreseeable future.
Keywords: Lyssavirus, Rabies virus, Rabies, Vaccination, Zoonoses
Introduction
Rabies, an acute fatal encephalomyelitis remains as one of the most feared and dreadful zoonotic disease in the world. It is the most important viral zoonosis recognized today because of its global distribution, incidence, veterinary and human health costs and extremely high case fatality rate. All the mammals right from a small mouse to a massive elephant are infected with the disease. Rabies is enzootic in both wild and domestic animals and poses a potential threat to human beings. In South East Asian Region (SEAR) member countries, rabies is a serious problem and account for approximately 80% human deaths in the world (1–3). The incidence of rabies is particularly high in Bangladesh, Pakistan and India followed by moderate incidence in Nepal and Myanmar and mild in Bhutan, Thailand and Indonesia (4–6).
Rabies is endemic in the countries where more than 2.5 thousand million people live. It is estimated that each year at least 55,000 people die from rabies and more than 10–12 million people receive post exposure vaccination against this disease (7).
Children aged 5- 15 years are at particular risk. More than 99% of all human deaths from rabies occur in Africa, Asia and South America (8). India alone reports 30,000 deaths annually. The disease is commonly transmitted by the bite of rabid animals usually carnivorous animals. In human beings 90% cases occur due to the bite of rabid dogs and 10% are due to the bite of other animals, aerosol transmission and transplantation of cornea and other organs.
Dogs are presumed to be the main transmitter in India due to high density of dog population. It is estimated that the dog population is around 25 million in India and 3/4th of all human rabies cases occur in villages and the incidence is about five times more in males compared to females (9, 10).The other domestic animals like cat, cattle, horse, sheep, goat, etc may be victims of rabies and transmit it to man (6). In India, Andaman and Nicobar Islands and Lakshadeep are rabies free. The distribution of rabies cases is not uniform and states like Nagaland, Manipur and Sikkim have very low incidence of hydrophobia due to wide dog and human ratio (11–13). In India, rabies infections also prevail in wild animals like wolf, fox, mongoose, jackal, hyenas etc (9).
The frugivorous, insectivorous and vampire bats can feed on blood of man and animals and transmit the disease in parts of Latin America (Brazil, Mexico, Venezuela, Trinidad and Tobago) and USA.
The annual cost of rabies has been estimated to be US $ 583.5 million and livestock losses is in the tune of US $ 12.3 millions in Asia and Africa. Dog rabies is present in 87 countries and accounts for major cause of all human rabies cases. However, many countries like Japan, U.K, Denmark, Sweden, Greece, Ireland, Iceland, Portugal, New Zealand, Australia, Switzerland, Finland, Norway, France, Belgium, etc are rabies free (14, 15).
Rabies has the dubious distinction of having the highest case fatality rate of all known infectious diseases. Rabies can be prevented by administration of potent and efficacious rabies vaccines both in pre and post exposure cases (16). It is evident that pre and post exposure use of cell culture rabies vaccines has dramatically reduced the incidence in certain countries (7). In Thailand, administration of Post Exposure Prophylaxis (PEP) has reduced the human rabies cases by 80% in 15 years (17). Other developing countries such as India, Sri Lanka and Philippines have adopted and promoted the use of economical low dose intradermal anti-rabies vaccination regimen using cell culture rabies vaccine (18). Currently, most of the pet dogs and cats are vaccinated against rabies but rabies infection may occur due to the vaccine failures, immuno-compromised animals, and presence of intercurrent diseases and sometimes from the asymptomatic carriers due to the close association between pets and owners (9).
Although a number of countries in the world are free from the disease or have been successful in eradicating the disease by strict enforcement of the prevention and control strategy and ban on import of animals from disease prone countries, the disease is still endemic in many developing countries including India despite the presence of a number of potent and efficacious immuno-prophylactic agents (13). The reasons might be due to the inability to bring all the susceptible animals under the immunization umbrella, no restrictions of movement of animals, frequent dissemination of virus from wild animals, use of nervous tissue or low quality vaccine, improper immunization, non-maintenance of cold chain, presence of maternally derived antibodies and existence of rabies related viruses (19, 20).
Although, nervous tissue vaccine was used both in animals and humans against rabies, the production of these vaccines have been discontinued as it causes neuro-paralytic complications in some individuals (21). The cell culture based rabies vaccines have been available with improved level of potency and safety for quite a long time. However its use has been precluded due to high cost and restricted availability. These vaccines are of better quality and cause little or no side effects (6, 22). Attenuated virus vaccines efficiently elicit the protective immune response and have been widely used in the past for immunization of domestic animals. However, all of them still had some residual pathogenicity to cause vaccine induced rabies in some species particularly in cats.
New generation rabies vaccines generated by gene manipulation developed in recent years have shown promising and encouraging results and elicited protective immune response in mice and could be better candidate vaccine for proper management and control of rabies in human and animals in near future.
Etiology
Rabies is caused by a number of different strains of the large, bullet shaped with one end rounded or conical and other planar or concave; single stranded negative sense RNA viruses of the genus Lyssavirus, family Rhabdoviridae. The RNA genome of rabies virus encodes 5 proteins: the glycoprotein G is the primary structural component of the surface spikes embedded in the viral envelope and is associated with the smaller M protein. Enclosed by the host cell derived envelope is an infectious viral core of nucleocapsid (N) proteins, thus encapsidating the viral genome and the RNA polymerases. The NS protein is associated with the nucleocapsid (23–25).
With the discovery of rabies related viruses the cross-reactivity of internal antigens (the ribonucleoprotein complex) was used to identify new viruses under the Lyssavirus genus within the Rhabdoviridae family. Virus neutralizing antibodies (VNAbs) which recognize the membrane glycoprotein (G) or MAbs sub-divided the genus into six serotypes whereas the viral nucleoprotein gene (N) sequences delineated 7 genotypes (26, 27). The genetic diversity of representative members of the Lyssavirus genus (rabies and rabies related viruses) using the sequence of the gene encoding transmembrane glycoprotein revealed two major phylogroups. Phylogroup I comprises the worldwide serotype 1 [classical rabies virus and Australian Bat Lyssavirus (ABL)] (28, 29), serotype 4 (Duvenhage virus), serotype 5 [European Bat Lyssavirus 1 (EBL-1)] and serotype 6 [European Bat Lyssavirus 2 (EBL-2)] (30, 31). Phylogroup II comprises the divergent African serotype 2 (Lagos bat virus) and serotype 3 (Mokola virus) (32).
Molecular epidemiology of rabies virus isolates from India has been shown to be belonged to genotype 1 indicating the absence of rabies and Rabies Related Viruses (RRVs) in domestic animals, including dogs (33). Among the Indian rabies virus isolates, >95% nucleotide similarity existed, even though they were from different geographical regions and derived from different hosts including domestic animals and dogs. In India, rabies virus is region specific but not host specific (33).
The existence of a number of genotypes may have important implication in the vaccine production. The rabies virus has been grouped further into street rabies virus and fixed rabies virus. The street rabies virus is derived from one that exists in nature in naturally occurring cases and fixed rabies virus denotes to strains of virus that has been adapted by serial intracerebral passage in rabbits in the laboratory. Fixed rabies virus strains are used in the vaccine production. Fixed rabies virus causes paralytic disease with a relatively short incubation period following intracerebral inoculation and there is absence of Negri bodies and absence of virus in saliva and salivary glands (34).
The rabies virus is stable between pH = 3.0 and pH = 11.0 and may survive for many years at -70 °C or when freeze dried and kept at 0 to 4 °C. It is rapidly inactivated by desiccation, UV and X-ray exposure, sunlight, trypsin, β-ropriolactone, ether and detergents. Quarternary ammonium compound (1:5000), 45–70% alcohol, 1% soap, 5–7% iodine solution kills the rabies virus within one minute (35).
Vaccination
The control of rabies largely depends on the prevention of infection in dogs and cats by vaccination in endemic areas and the control of their movement, including measures of quarantine and vaccination (36). Vaccination is the most effective and economical way to control a disease (37). Although the main threat to humans and to domestic animals is classical rabies virus, it is also important to consider the other members of the genus Lyssavirus that may infect animals (38). The conventional vaccines currently used for the vaccination of humans and domestic and free living animals are derived from fixed type virus of genotype 1 and serotype 1. These vaccines provide excellent protection against classical rabies virus but may not confer good protection against serotype 2, 3, 4 and 6 (5). The level of protection as determined in mice that survived after 28 days appears to depend on the virus strain used in the vaccine e.g. Pasteur Virus (PV) or Pitman-Moore (PM) strain and the genotype of the challenge virus. Generally rabies vaccines based on the PM and LEP strain induced weaker protection against EBLV-1 than the PV strain and few data are available for EBLV-2 (39). In general, the protection level is inversely related to the genetic distance between the new isolates and vaccine strain used (5).
Mass vaccination of dogs remains the main strategy for controlling urban rabies in endemic areas. In order to avoid maternally derived immunity, vaccines are better given when the animal is young but not less than 3 months of age in case of dogs (35). Primary vaccination can be a single injection (live attenuated vaccines) or two inoculations of 1 month apart. After that vaccines are given annually, biannually or triannually to boost their immunity depending on the efficacy of the vaccine (40). The WHO Expert committee of Rabies advises that vaccines prepared from cell cultures should replace the nervous tissue vaccines as soon as possible. BHK21 are the most commonly used continuous cell lines for the production of vaccines for animals (41).
Animal Rabies Vaccine
First generation animal rabies vaccine
In 1885, Louis Pasteur demonstrated that the rabies virus could be attenuated by serial passage in rabbits intracranially. On 6th July 1885, the first human was treated by giving 13 consecutive and an increasingly virulent dose of a dessicated spinal cord suspension from a rabid animal and the boy Joseph Meister was survived. Pasteur's crude vaccine was later modified by Fermi and Semple. In 1927, the First International Rabies Conference recommended that fixed virus for canine rabies vaccines be completely inactivated or attenuated so that they cause no disease in dogs vaccinated either S/C or I/M (19). For the next several decades, all nervous tissue rabies vaccines were inactivated by phenol described by Semple (42). The nervous tissue vaccines currently in use for mass vaccination campaign in Africa, Latin America and Caribbean are produced from rabies virus infected suckling mouse brains or lamb brains. However, nervous tissue vaccines for dogs and other animals often caused post vaccinal nervous symptoms and death in some vaccinated animals (8).
Embryonated chicken eggs were used by Koprowski and Cox for serial passage of the Flury strain. The virus was initially passaged 136 times in 1 day old chicks followed by passage in embryonated chicken egg. At 40th to 50th chicken embryo passage, the rabies virus losses its viscerotropic properties but retained some neurotropic properties and named LEP. It could be safely used in dogs but occasionally caused rabies in young pups, cats and cattle. The LEP was further passaged in embryonated chicken eggs to increase the safety by Koprowski and associates and at 205th passage level it was found to be safe for I/M use in cats, cattle as well as puppies 3 months of age (43).
Parenteral modified live virus vaccine
The Flury and Kelev strains of rabies virus were used to produce chick embryo origin Modified Live Virus (MLV) vaccines. Street Alabama Dufferin (SAD) strains adapted in hamster kidney cells and Evelyn Rokitnicki Abelseth (ERA) strains grown on porcine kidney cells were used to produce tissue culture based modified live vaccine for parenteral use in animals against rabies. These vaccines are used in carnivores including dogs and cats in Asia, Africa and parts of Europe (4, 44). These non adjuvanted vaccines are cheap and use low amount of virus, but elicited longer duration of immunity and good cell mediated immune response. However, there is chance of regaining the virulence property by the vaccine virus over a long time use. Further, the vaccine virus may have the residual pathogenicity to other non-target species of animals (10).
Oral modified live virus vaccine
Modified Live Virus (MLV) vaccines are not recommended for parenteral immunization against rabies in animals as rabies infection can occur as a result of vaccine strain. Several types of MLV oral rabies vaccines have been produced for use in baits for free ranging animals that serve as vectors for the maintenance and transmission of the disease in wildlife (45). SAD B19 and SAD P5/88 vaccines are produced by several cell culture passage of the SAD Berne strain, which is a cell culture adapted derivative of ERA strain, was used extensively in Europe since 1977 and in Canada from 1989 with considerable success (46). Unfortunately the live virus SAD vaccines contained some degree of residual pathogenicity for wild rodents and resulted in partially impaired immune responses in fox cub <8 weeks old born from SAD strain vaccinated vixens and resulting in insufficient protection against rabies (47). The SAD strain used in vaccine has been replaced by SAG-1 and SAG2 (SAD avirulent Gif) strains in the development of vaccines. The SAG (Street Alabama Gif) 2 strain was selected from the SAD Berne strain. The SAG-2, the strain of choice is a double mutants and avirulent following I/C inoculation of immunocompetent mice and protects the mice against challenged with CVS (48). No adverse effects following the oral administration of 10 times the field dose of SAG2 were reported in target species (red fox, dog, raccoon dog and arctic fox) or in non-target species including baboons, different rodent species, two species of cervids, wild boars, badgers, goats, ferrets, hedgehogs and diurnal and nocturnal birds (49). Red foxes, raccoon dogs and dogs were protected from virulent challenge after immunization with single SAG2 bait. No salivary excretion of infective SAG2 virus strain was detected in dogs after vaccination. In bait, SAG2 is either contained in a capsule as a viral suspension or in the bait matrix as freeze dried suspension. The SAG2 strain of rabies virus packaged in chicken head baits has also successfully protected captive African wild dogs against rabies virus challenge (49, 50).
Oral live vaccinia rabies virus glycoprotein recombinant vectored vaccine
A recombinant vaccinia virus vector in which the G gene of the Evelyn-Rokitniki-Abelseth (ERA) strain of rabies virus was inserted into the thymidine kinase region of the vaccinia virus genome has been developed for immunization against rabies (51). The vaccinia-rabies glycoprotein (V-RG) recombinant virus induced a rapid Virus-Neutralizing Antibody (VNA) response in mice, both by subcutaneous inoculation and by oral administration and the animals were protected against a lethal rabies virus challenge (52). Similarly, other pox viruses including the orthopoxvirus of raccoon and avian pox viruses such the fowl pox and canary pox viruses were investigated as possible alternative vectors for expression of rabies virus antigens (53). Some of these investigations reported the role of rabies virus nucleoprotein (N) for protective cellular immune responses in addition to G protein of rabies virus for the induction of VNA. A recombinant vaccinia virus expressing the rabies virus glycoprotein gene (V-RG) has been developed by inserting the cDNA of the glycoprotein gene of the ERA strain into the thymidine kinase gene of the Copenhagen strain of vaccinia virus (54). When administered orally by direct instillation into the oral cavity or in a bait, a dose of 108 TCID50 of VRG elicits virus neutralizing antibodies and confers protective immune response against rabies virus challenge in a number of carnivorous species (red fox, arctic fox, coyote, raccoon, raccoon dog, domestic dog and golden jackals) (55). In the field, VRG vaccine strain is stable above 56 °C whereas the melting point of bait case is >60 °C. Safety studies conducted in over 50 mammalian and 10 avian species (most of them are rabies vectors) have not revealed any residual pathogenicity. More than 75 million doses of VRG have been used to successfully control or reduce wildlife or canine rabies in a variety of animal species such as red foxes (Belgium, France, Israel, Luxemburg and Ukraine), raccoon dogs (Republic of Korea), coyotes, raccoons and grey foxes (Canada and USA) and domestic dogs (Sri Lanka) (43, 49, 54, 56, 57). The vacciniarabies glycoprotein (V-RG) recombinant virus induced a rapid Virus-Neutralizing Antibody (VNA) response in mice, both by subcutaneous inoculation and by oral administration and the animals were protected against a lethal rabies virus challenge (52). Similarly, other poxviruses, including the orthopoxvirus of raccoon and avian pox viruses, such as the fowl pox and canary pox viruses, were investigated as possible alternative vectors for expression of rabies virus antigens (53). The vaccine fulfilled all the criteria such as readily accepted by target species, rabies virus free, thermostable and provides protection against rabies but no risks of developing rabies if ingested and safe for contact with humans. The vaccine was used in USA and France to immunize raccoons, coyotes, wild dogs, fox, jackals etc with high success and by 1995 the vaccine was conditionally licensed in the US for use in Oral Rabies Vaccination (ORV) programs (43, 49, 58). The vaccine can be given in fish meal or poultry based bait which contains 150 mg of Tetracycline HCl as a bone biomarker and a plastic sachet containing 1.8 ml of vaccine. The concept that a live recombinant virus vector might fulfill the rabies vaccine requirements for the next generation was rapidly gaining credibility (59, 60).
Parenteral live pox/adenovirus recombinant vectored vaccine
A canary pox rabies glycoprotein recombinant vaccine has been developed and found to be effective as other poxvirus rabies glycolprotein recombinants. Live canarypox virus that expresses the rabies virus glycoprotein has been licensed in the USA as a parenteral monovalent vaccine for cats and as a combination rabies vaccines for cats with feline panleukopenia virus, feline parvovirus, feline calicivirus vaccines included in the product (51). A recombinant adenovirus vectored vaccine expressing rabies virus glycoprotein was shown to be capable of inducing antibody response in greyhound dogs immunized S/C or I/M. This vaccine holds promise as a rabies virus vaccine for dogs (61, 62).
Parenteral inactivated cell culture vaccine
For preparing inactivated vaccines, rabies virus strain (CVS, PM or PV) is grown in chicken embryo fibroblast, BHK-21 or Vero cells (63, 64). Neonatal mice lack the myelin protein that causes allergic reactions occasionally in animals vaccinated earlier with sheep brain tissue origin killed vaccines. On the other hand, tissue culture based rabies vaccines are less allergenic but more immunogenic (57). Although a number of inactivating agents namely phenol, HCHO, UV light, AEI or other amines have been used for inactivation of rabies virus, BPL is most commonly used. After inactivation, adjuvants namely Al(OH)3, AlPO4, saponin (cattle vaccines) and rarely oil adjuvant are used to improve the immunogenicity of the vaccine (42). The rabies vaccine also is available along with canine distemper, canine adenovirus, feline parvovirus and feline calicivirus for use in cats or FMD for use in cattle, sheep and goat (10).
Post vaccination complications
Due to higher antigenic mass and use of adjuvants inactivated vaccines may sometimes produce local and systemic reactions. The most common symptoms are soreness, lameness, regional lymphadenopathy in the injected limb, focal vasculitis, granulomas and sarcoma. However, the new generation vectored recombinant vaccines produce less allergic or neoplastic reactions (21, 65).
Potency requirement for animal vaccines
Inactivated rabies vaccines for animals should have a potency of 1.0 IU/dose as measured by National Institute of Health (NIH) test or other recognized Pharmacopoeia test (66). The NIH rabies vaccine potency test is used internationally for evaluating the efficacy of inactivated rabies vaccine which is performed in vivo. In NIH test, the potency of anti-rabies vaccine is compared with an international reference vaccine while testing in groups of at least ten Swiss albino mice, aged 3–4 weeks inoculated with two doses at one week apart. Different 5 fold dilutions of both the vaccines are inoculated and compared to determine the dilution at which 50% mice are protected against intracerebral challenge 14 days later. The challenge dose should be in the range of 12–50 mouse intra-cranial LD50 (MIC LD50) in 0.03 ml of the suspension. The PD50 and the IU is calculated in the test vaccine by observing and comparing the protection level of the reference vaccine. The duration of immunity should be at least 1 year (66).
Rabies Vaccine for Humans
Nervous tissue vaccine
The production of rabies vaccine has dramatically changed since Pasteur, who produced and treated the patient with serial injections of increasingly virulent rabies virus infected nerve tissue by drying different time interval. In 1900s Fermi and Semple used phenol to inactivate the rabies virus in nerve tissue and used same suspension for all injections. However, the use of the Fermi vaccines has been discontinued due to presence of residual live fixed rabies virus. The nervous tissue vaccine (Semple) that are presently produced and administered in Africa, Asia and Latin America include vaccines produced from the rabies virus infected brain tissue of sheep, goat and mice (67). Although the production of Semple vaccine was recently stopped in India it is still produced and used in some Asian and African countries even though safer and more efficacious cell culture vaccines are available (7, 9).
Due to post vaccinal reactions associated with Semple rabies vaccine, the need for a safer and more efficacious rabies vaccine was felt to protect humans against rabies. The next development of rabies vaccine was the vaccine derived from suckling mice having less reactogenic property used extensively during 1950s in Latin American countries (19, 68). Suckling mouse brain vaccine is less allergenic because brain tissue is unmyelinated and produced from fixed rabies virus strains originally isolated in Chile (strains 51 and 91 of dog and human origin, respectively) (69). Mice not older than 1 day are injected intracerebrally and brain tissue is harvested approximately 4 days later. The brain tissue infected with rabies virus is diluted to 10% suspension and inactivated with UV light or BPL. The vaccine has a final potency of 1.3 IU/dose. The vaccine is supplied in 1–2 ml vials with a shelf life of 1 year stored at 4 °C (70). The adverse reactions associated with suckling mouse brain vaccines are lower (1:8000) than Semple rabies vaccines (1:142 to 1:7000), but the case fatality rate is higher in affected patients (22% for suckling mouse brain vaccine and 4.8% for Semple vaccines) (21). WHO has recommended that the production of all nervous tissue vaccine should be discontinued and replaced by cell culture rabies vaccine.
Cell culture vaccines
The first non-nervous tissue culture rabies vaccine became available in North America was Duck Embryo Vaccine (DEV).
The vaccine was replaced by the first modern cell culture rabies vaccine, the Human Diploid Cell strain Vaccine (HDCV) in 1978 in USA. The HDCV was safe with high immunogenicity and negligible allergic reactions compared to previous vaccines (71). Further, two other cell culture vaccines namely Purified Vero cell culture Rabies Vaccine (PVRV) and Purified Chicken Embryo Cell Vaccine (PCECV) were developed almost simultaneously and became available around the world by international pharmaceutical companies (18).
Presently PVRV and PCECV are the most widely used cell culture vaccines in millions of patients in the world. A more purified version of Vero cell vaccine known as Chromatographically purified Vero cell rabies vaccine was undergone clinical trial in USA and few Asian countries but not marketed in any country (72).
There are many different types of cell substrates viz., primary cell, diploid cell and continuous cell cultures used for the production of cell culture rabies vaccine. In China and former USSR, primary baby hamster kidney cells are used to produce a rabies vaccine (41). Embryonated eggs are a primary cell culture substrate used to produce 3 different types of rabies vaccine in Europe, Japan and India. Rabies vaccines can also be produced in human diploid cell strain (WI-38 and MRC-5). The Vero cell line derived rabies vaccines are available in Asia, Europe and Latin America (73).
The first widely used cell culture rabies vaccine was developed on the human fetal lung diploid fibroblast cell line WI-38 using Pitman Moore strain of rabies virus by Wiktor and his associates at Wistar Institute of Philadelphia. Human diploid cells have a higher but finite life span than primary cells whereas continuous cell lines have a capacity to multiply indefinitely in vitro (71). The cells used for vaccine production from continuous cell lines may have differences in karyotype from the original cell line and may be obtained from healthy or tumor tissues. The FDA (Food and Drug Administration) Center for Biologics Evaluation and Research (CBER) in USA has guidelines for manufacturers using the continuous cell line mentioning that Vero should have less than or equal to 10 ng of cellular DNA per dose for a parenterally administered vaccine (63, 73).
HDCV was the first cell culture rabies vaccine to be developed and this vaccine dramatically changed the perception of human rabies prevention and the concept of rabies vaccination. This vaccine provided a safe and immunogenic means to protect against rabies in both pre and post exposure cases. It is recommended to give booster every 2 years for people with continued risk of exposure to rabies. Although HDCS produces high serologic titers, but the yields of virus is low. Again, due to high production cost, this vaccine is unaffordable by people in most developing countries of the world where over 90% human deaths occur (71, 72).
To overcome the high cost of HDCV and to make rabies vaccine more affordable to common people, other cell culture rabies vaccines were developed that are less expensive to produce and cause fewer adverse reactions. These vaccines are PCECV developed by Barth and his associates using Flury LEP strain of rabies virus propagated in primary chick fibroblast cells. The virus is inactivated by BPL and purified by zonal centrifugation (74). The production of PCECV on chick embryo fibroblasts permits a high yield of virus compared to human diploid cells.
This vaccine technology was transferred from Marburg, Germany to Ankleshwar, India in the late 1980s and cost of production was reduced substantially while maintaining the production standard recommended by WHO. Another transfer was recently occurred from Switzerland to India is purified duck embryo cell vaccine (11). PVRV is a second generation rabies vaccine that is produced on Vero cells (Vervet monkey origin). PVRV is cultivated on microcarriers in large scale biofermentors thus reducing the production cost compared to HDCV which is produced on a monolayer cell culture. PVRV inactivated by BPL and concentrated by ultracentrifugation is widely used throughout the world including Europe and Latin America but it is not licensed to be used in North America (18, 75).
Production standard
Cell culture and embryonated egg based rabies vaccines are of superior quality compared to nervous tissue vaccines of adult and young animal origin in terms of both safety and efficacy and saved the lives of millions of human victims of animal bites (WHO, 2001). WHO recommends that all cell culture rabies vaccines must contain at least 2.5 IU/dose intramuscularly (I/M) for administration to humans. The vaccines are inactivated with BPL and undergone the safety, sterility, potency and stability testing. The vaccines are highly effective when used both in pre and post exposure cases against rabies (72).
Intradermal administration
Currently there is no cell culture rabies vaccine that is prepackaged for use intradermally either for pre or post exposure vaccination. The currently available cell culture rabies vaccines intended for I/M use do not have a preservative and the risk of contamination is increased if they are used as a ‘multiuse’ vial as the case for intradermally administration. WHO recommends that when rabies vaccines are administered intradermally (I/D) they should be kept at 2–8 °C and used within 6–8 hrs (17). In North America, intradermal administration of human rabies vaccine is sometimes used as pre-exposure immunization but never practiced for post exposure vaccination. However, I/D administration of rabies vaccines have been extensively used during the last two decades in Asia for post exposure and in Europe for pre-exposure vaccination. At present, WHO only recommends three vaccines (HDCS, PCECV and PVRV) for I/D post exposure prophylaxis (18, 63). Although WHO recommends a potency of 2.5 IU/human dose for the intramuscular administration of rabies vaccines, there is no similar specific potency requirement for an I/D dose of a cell culture rabies vaccines. The same vaccine intended for I/M can be safely given at the rate of 0.1 ml I/D from a 1 ml vial of PCECV that contained 2.5 IU/ml. All the vaccines responded well with acceptable titer by day 14. There is no need to increase the potency requirements for I/D use of cell culture rabies vaccine (64, 76, 77).
The standard (Essen) five doses I/M regimen for PET are unaffordable in developing countries. So, two economical ID PET regimens have been recommended for use by the WHO since 1997: an eight site and a two site regimen. The eight site regimen is used with PCECV and HDCV (1.0 ml/ampoule) and consists of eight doses of about 0.1 ml I/D on day 0 (using a whole ampoule) on the arms, thighs, suprascapular and lower abdomen areas; four I/D doses on day 7 on the arms and thighs and one dose on days 28 and 91 also over the deltoid. It requires four visits to the clinics (Figure 1) (64, 77).
Figure 1.
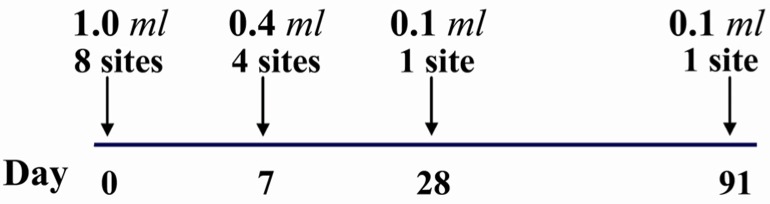
Details of the eight site intradermal rabies post exposure vaccination regimen (PCECV and HDCS)
The two site regimen consists of two I/D doses of 0.2 ml each on day 0, 3, 7 and one 0.2 ml I/D dose on day 28 and 91 for PCEV and HDCV whereas in case of PVRV the dose is 0.1 ml in each occasions (Figure 2) (76). It requires 5 visits to the clinics. Ampoules of vaccines are shared on each occasion whereas sharing is needed on three of the four visits with the eight site regimen. A comparison of these I/D regimens shows that the eight site method induce significantly higher levels of antibody than the two site regimen from day 7 to 1 year. The same amount of antigen is used for the two regimens, a total of less than two ampoules (64).
Figure 2.
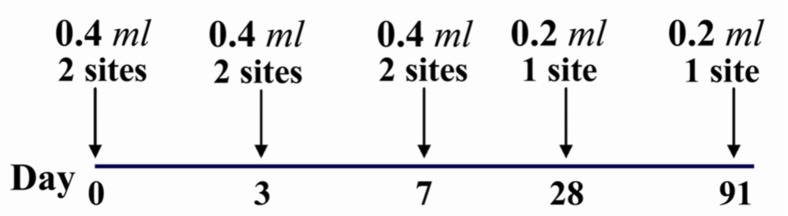
Details of the two site intradermal rabies post exposure vaccination regimen (PCECV and HDCV)
Rabies virus glycoprotein as an immunogenic subunit
Subunit vaccines consisting of the immunogenic component of a specific virus or merely its immuno-reactive portion were considered, for practical purposes, to be a rather futuristic perception of the ultimate in safe (i.e. genome-free) antiviral vaccines. It has been firmly established that rabies virus G protein forms spike projections on the external surface of the virus membrane and is the major antigen responsible for the induction of VN antibodies and for conferring immunity against lethal infection with rabies virus (78). The rosette structures of G protein (haemagglutinin preparation) which consisted solely of polypeptide chains of G protein was fully protective against a lethal challenge infection with rabies virus in mice. The pre-exposure protective and antigenic values of this haemagglutinin preparation were equal to that of inactivated virus vaccine and at least ten times greater than those for the monomeric form of G protein prepared using Triton X-100 (78).
Recombinant adenovirus-vectored vaccines
The G gene coding for the glycoprotein G of rabies virus was inserted into the replication-non-essential E3 locus of the human adenovirus serotype 5 (AdHu5) genome and used as recombinant adenovirus vectored vaccine. The E3 gene locus was deleted to down regulate expression of major histocompatibility complex antigens and protect the adenovirus-infected cells from T-cell-mediated destruction (79). This prototype vaccine tested by parenteral or oral vaccineation in several animal species including mice, dogs, foxes and skunks provided protection against a rabies virus challenge. However, the potential for in vivo replication of this E3-deleted recombinant adenovirus and tendency to cause occasional disease in the vaccinated host posed the question about its safety. Recombinant adenovirus vectored rabies vaccine (recombinant Ad-RG) also capable of inducing high level of anti-rabies virus neutralizing antibodies (VNA) in dogs previously immunized with conventional rabies vaccines. The level of VNA induced following booster immunization with the recombinant Ad-RG was reported to be higher than that of conventional rabies vaccines suggesting that the recombinant Ad-RG vaccine is capable of inducing a strong anamnestic response in dogs (62). Further, activity of recombinant Ad-RG vaccine was not impaired by pre-existing immunity when administered intranasally or orally. With a growing interest at this time to use adenoviruses for gene therapy as well as for vaccination, replication-defective adenovirus vector constructs have been investigated as possible next generation vaccines for rabies (61, 79, 80).
DNA-based rabies vaccines
Another area of innovative rabies vaccine development is that of DNA or gene vaccine. DNA-based vaccines offer new approaches and unique strategies for both prophylaxis and post exposure therapy against rabies. DNA-based vaccines were initially developed as a simple and versatile way to induce a broad spectrum of immune responses (both cellmediated and humoral) when injected directly into the host, compared with conventional vaccines. The most appealing and compelling reason to develop DNA-based rabies vaccines is that plasmids (DNA encoding G gene of rabies virus) are easy to construct and can be produced in large quantity inexpensively. DNA plasmids that carry more than one viral gene component, e.g. G and N genes of different rabies virus strains, or multiple plasmids encoding single expressible genes combined in a ‘plasmid vaccine cocktail’, provide a multivalent vaccine approach for simultaneous induction of immunity to more than ‘one viral pathogen’ (81, 82).
Multiple G genes of rabies and rabies related viruses in one vaccine cocktail would protect individuals not only against rabies but also rabies related viruses at the same time. Further, DNA vaccines not only induce VNA and CD4 + T cells but also cytotoxic CD8 + T cells. The induction of CD8 + T cells are not generally induced by recombinant and synthetic peptides. The first DNA vaccine for rabies developed at Wistar Institute induced long lasting immunity following I/M injection but induction of VNA is usually slower than HDCV. Various parameters such as plasmid doses, route, host species, virus challenge and primary and booster mode of inoculation were studied both as pre-exposure and post exposure formats. However, the results have been encouraging in mouse and some large mammals but not successful in totally protecting in non-human primates following pre-exposure and post exposure vaccination. So, further work needs to be carried out to exploit full potential of DNA vaccines in order to provide fully protective and highly desirable vaccines for animals and humans at low cost (81, 83).
Oral rabies vaccines derived from plants
Plants have also provided new and promising prospects in the process of developing effective, inexpensive and safe production and delivery systems for the next generation of vaccines for rabies. Plant viruses, such as tomato bushy stunt virus and Tobacco Mosaic Virus(TMV) serving as vectors for expression of foreign antigens in plants, have provided a variety of genetically manufactured vaccines from tomatoes and tobacco leaves, respectively. Among the key advantages of plants and plant crops for protein expression, particularly the tobacco plant as a suitable host for plant virus vectored protein expression, is that they represent a biomass of major proportions for recombinant (foreign) protein production. Mice immunized orally or by feeding on antigen producing spinach leaves or parenterally with plant derived rabies virus specific antigen could be protected from a lethal rabies virus challenge. It offers the advantage of safety, low cost, proper glycosylation of antigen, easy oral administration and devoid of several painful injections. It also can generate local and systematic immune response and protection against rabies virus (84).
Pre-exposure Vaccination Schedule in Man
Laboratory personnel working with rabies virus, veterinarians, animal handlers, supporting staff working in the human and veterinary hospitals and wildlife staff are at high risk and should receive the pre-exposure vaccination against rabies. Generally cell culture vaccine of either 1 ml I/M or 0.1 ml I/D are used on days 0, 7 and 28. The serum from the vaccinated individuals should be tested one month after the last dose and if titre is 0.5 IU/ml, booster is recommended otherwise booster doses are administered at intervals of 2 years (7).
Post Exposure Vaccination Schedule in Man
Purified Vero cell vaccine, purified chicken embryo cell vaccines and HDCS vaccines are generally used as prophylactic agents against rabies. Vero cell based vaccine is used at 0.5 ml I/M and other two vaccines are used at 1 ml I/M.
There are two regimens for post exposure vaccination using rabies vaccine that are recommended by WHO as safe and efficacious. The regimens include the Essen regimen where one dose of vaccine is administered I/M in deltoid on each of days 0, 3, 7, 14, and 28. In Zagreb regimen where one dose of vaccine is administered at two sites on day 0 and at one site on days 7 and 21. The 2-1-1 schedule induces an early antibody response and may be particularly effective when post exposure treatment does not include administration of rabies immunoglobulin (Table 1) (7).
Table 1.
WHO recommendation on immunization of humans against rabies
| Vaccine | Pre-exposure immunization | Post exposure immunization |
|---|---|---|
| HDCS | ||
| Yes | Essen regimen (I/M) Zagreb regimen (I/M) 8 site regimen (I/D) |
|
| PCECV | ||
| Yes | Essen regimen (I/M) Zagreb regimen (I/M) 8 site regimen (I/D) |
|
| PVRV | ||
| Yes | Essen regimen (I/M) Zagreb regimen (I/M) 2 site regimen (I/D) |
Passive immunization
It is understood that following vaccination both humoral and cellular immune responses are induced in rabies. A high level of neutralizing antibodies in the sera of vaccinated animals and humans can protect them from lethal infections since antibodies take at least 7–10 days to become effective for virus neutralization. This time is covered by passive immunization with rabies immunoglobulin. The combined serum vaccine therapy is always recommended in exposed persons particularly in severe bite wounds. Purified equine rabies immunoglobulin which occasionally causes adverse reaction is used in most developing countries at 40 IU/kg body weight whereas human rabies immunoglobulin is given at 20 IU/kg body weight (85).
Recent Development in Immunoprophylaxis
Reverse genetics system for generation of infectious c-DNA
In this method plasmid expressing full length anti-genomic RNA (genome plasmid) and three plasmids expressing N, P and L protein of the virus (helper plasmids) are transfected into a cell. The anti-genomic RNA and the proteins form an anti-genomic ribonucleoprotein (RNP) complex. The anti-genomic RNP complex has the same biological activity as occurs in virus infected cells, genomic RNA is synthesized using this anti-genomic RNP as a template, followed by synthesis of mRNA from genomic RNP and expression of viral protein. The assembly of the genomic RNP and other viral proteins as M and G proteins results in generation of an infectious recombinant rabies virus. Using this manipulation system of rabies virus, an attenuated live virus vaccine can be established quickly than conventional cell culture vaccine with change in biological characters such as improved safety and immunogenicity (84).
Insertion of foreign gene into the viral genome
A recombinant rabies virus expressing a proapoptotic protein cytochrome c (SPBN-Cyto c(+) strain) strongly induced apoptosis in infected cells, found to be more attenuated than negative control virus carrying inactivated cytochrome c gene (SPBN-Cyto c(-) strain) and induced protective immune response in mice (86).
Mutation of rabies virus at 333 position of G protein
In rabies virus, the presence of an arginine or lysine residue at position 333 in the G protein is well known for pathogenicity (87, 88). Many recombinant viruses harbouring the G gene from various rabies virus strains with amino acid other than arginine or lysine have been found to be attenuated (55). The deletion of dynein light chain binding site in P protein which is necessary for axonal transport of the virus reduces peripheral infectivity of the recombinant virus in suckling mice (89).
Insertion of additional G gene in the genome
To enhance the immunogenicity of the attenuated vaccine virus, the expression level of G protein in infected cells has been increased by insertion of an additional G gene into the genome (90). Both the G genes contain alteration of amino acid at position 333 position and recombinant rabies virus (SPBNGA-GA strain) produced twice the quantity of G protein in cultured cell compared to virus carrying only a single G gene and that the recombinant virus induced apoptosis more strongly and more efficient protective immunity compared to control one (90).
Recombinant rabies virus lacking the P gene
Recombinant virus lacking the P gene (def-P) has been recovered from the genome plasmid with supplementation of the P protein from helper plasmid. The def-P virus can be produced in cell lines stably expressing P protein. On the other hand, the def-P virus did not effectively grow in normal cells that did not express the P protein. The def-P virus was completely apathogenic for adult and suckling mice inoculated intracerebrally. The def-P virus induced high level VNA and protective immunity sufficient to withstand challenge virus infection in mice. The P protein blocks both type I interferon production and signaling pathway which inhibits host innate immunity. The loss of these functions indef-P may contribute to high immunogenicity (91).
Recombinant rabies virus lacking the M gene
M gene deficient rabies virus (RCHLΔ M) has been developed using gene manipulation system of RC-HL strain used in vaccine production in Japan (92, 93). The M protein is essential for viral assembly and budding (94). The RCHLΔ M strain has been recovered from the genomic plasmid in the presence of helper plasmid coding for M protein. The infectious progeny virus was not detected in a culture supernatant from RCHLΔ M strain infected cells whereas the infectious virus was effectively produced in the supernatant from RC-HL strain infected cells. The BHK21 cell line expressing M protein of RC-HL strain can be used for propagation of RCHLΔ M strain. The RCHLΔ M strain is completely apathogenic for both adult and suckling mice inoculated intracerebrally. The RCHLΔ M induced VNA in adult mice after I/M inoculation or intranasal instillation (95).
The gene manipulation system of rabies virus has opened up the possibility of development of new generation rabies vaccines. However, it is to be carefully investigated to test these vaccines as safety of a recombinant virus with a foreign gene may cause serious side effects in inoculated animals and humans. For example, recombinant virus with a foreign gene or an attenuation related mutation, there is a possibility that the virus will become pathogenic during viral propagation in the body of the inoculated animal due to certain mutation.
Guidelines for Post Exposure Vaccination/Treatment
Rabies is a fatal disease and there is no specific treatment for the disease except management. Patient should be kept in a calm, quiet and isolated room. Analgesics (morphine) and muscle relaxants are prescribed to alleviate pain and muscular spasm.
Rabid animals are potential source of infection as their saliva, vomits, tears, urine and other body fluids contain virus. The attending persons should have pre-exposure vaccination and protective clothing like face masks, gloves, goggles, aprons etc. Immediate treatment of the bite wound includes washing or flushing the wound or area thoroughly with soap and water preferably in running water for 15 min. The next step is to treat the wound with virucidal agents like alcohol, tincture or povidone iodine. The patient may be kept under antibiotic coverage and antitetanus treatment. The recommendation for undergoing post exposure treatment is shown in (Table 2) (96).
Table 2.
WHO guidelines for post-exposure vaccination/treatment
| Category | Type of contact with a suspect or confirmed rabid domestic or wild animal or animal unavailable for observation | Recommendations |
|---|---|---|
| I | Touching or feeding of animals, licks on intact skin | None, if reliable case history is available. |
| II | Nibbling of uncovered skin, minor scratches or abrasions without bleeding. Licks on broken skin | Administer vaccine immediately. Stop treatment if animal remains healthy throughout an observation period of 10 days, or if animal is killed humanely and found to be negative for rabies by appropriate laboratory techniques |
| III | Single or multiple transdermal bites or scratches. Contamination of mucous membrane with saliva (i.e. licks) | Administer rabies immunoglobulin and vaccine immediately. Stop treatment if animal remains healthy throughout an observation period of 10 days, or if animal is killed humanely and found to be negative for rabies by appropriate laboratory techniques |
Immunity
The kinetics of immune responses to rabies virus has been widely studied in experimentally infected mice, vaccinated dogs and humans received post exposure vaccination. But very little information is available on immune responses in naturally infected animals (97). It is not certain whether or not the saliva of the rabid animal plays an immuno-modulating role allowing the virus to elude the host's immune response but salivary gland and brain homogenates have been shown to be immunosuppressive. Experimental studies have shown that the immune response and possible immune suppression are largely influenced by strain, dose and route of inoculation. In mice that resist challenge, virus specific antibodies were first detected 4–6 days after experimental infection reaching peak levels 2 weeks after infection (70). In animal that survive infection the antibody titres remain high, whereas in mice that succumb to lethal experimental infection depletion of B and T cells in the spleen and thymus is noticed. The antigen specific suppression of CMI is mediated by pathogenic Lyssaviuses but not by the non-pathogenic rabies related viruses or inactivated pathogenic rabies viruses (48).
Two types of tests are routinely used for the quantitative assay of humoral immune responses: the virus neutralization test (VNT) and the Rapid Fluorescent Focus Inhibition Test (RFFIT) which uses cell culture (98, 99). High titres of VNT or RFFIT antibodies are indicative of a protective humoral immune response but few animals with very low titres have been shown to resist challenge suggesting the cellular immune responses may play an important role in protection against challenge (88). Experimental infection in nude mice (deficient in T cells) had shown that the cellular arm of immune system is very important. Immunization of man with rabies vaccine has shown that cell mediated immune response as measured by lymphocyte proliferation assay also plays a vital role (57). The cell mediated immune response can be detected for prolonged periods after vaccination. Cytotoxic T lymphocytes and T helper cell specific to epitopes on the viral glycoprotein or ribonucleoprotein of the rabies virus have been detected in the peripheral blood of animals infected with the virus and in vaccinated humans. Mice that are naturally resistant to rabies virus are rendered susceptible by the depletion of CD4+ but CD8+ cell had no effect (100).
Conclusion
Rabies, a dreadful and terrifying disease causing neurological symptoms with high mortality is not only a national but also a global problem. Significant advances have been made by the multi-disciplinary sectors of immunology, vaccinology, molecular virology and epidemiology thus allowing a far greater understanding of rabies virus circulation. Rabies is a disease for which all the necessary remedies exist unlike the situation with many other diseases like dengue, chikungunya etc. It is possible to prevent, control and treat rabies with the safe and effective cell culture produced rabies vaccines or anti-rabies globulins. In spite of this, WHO records more than 55,000 human deaths from rabies each year mostly due to infection by classical rabies virus (genotype 1, serotype 1) from dogs. The number of cases in humans and animals is still believed to be under-estimated due to poor or under reporting in many countries in the world. Continuing molecular epidemiological and surveillance studies are necessary to trace the spillover transmission from reservoir species to non-reservoir animals and humans and also to monitor the emergence of specific rabies strains into new species and geographical area, which to a large extent is often prompted by human activities viz., movement of wildlife and importation of animals.
Bat rabies epidemiology should be more comprehensively explored so that precise risks to the health of humans and domestic carnivores can be identified and effective and efficient disease prevention measures can be applied to those who handle the bats. A further more ambitious goal is to coordinate all efforts from all sectors to increase rabies surveillance programmes particularly in Africa and Asia and thus ultimately to decrease the incidence of rabies in the continents.
Ideally there should be a national reference laboratory for rabies diagnosis with its branches in every state of the country. The destruction of all stray and feral dogs, which is usually very unpopular with the general public and strong opposition from animal activists and animal ethics committee, does not in the long term constitute a realistic method of disease control. This method has been failed in some countries in the world. Pet dogs usually receive rabies vaccinations when they are 3 months old, but very often they become infected before they reach to this age. The stray and unauthorized dogs which are often not brought under the immunization campaign harbour the rabies virus. They act as carriers and transmit the rabies virus to other susceptible animals and humans. It has been reported that vaccination before the age of 3 months is effective, even in an animal with maternal antibodies. Puppies should be vaccinated along with adult dogs during any mass parenteral vaccination campaign. This would help to broaden vaccine coverage and reduce the incidence of rabies in children.
In India, the rabies in animals and humans is a real problem and lot of efforts have been attempted to control the disease. A large number of cases are still reported with a certain percentage of casualties. The currently available cell culture rabies vaccines have been proved to be safe, immunogenic, potent and efficacious when produced and used according to WHO recommendations. The development and proper use of vaccines has undoubtedly enabled millions of human lives to be saved from a dreadful disease. However, the high cost of the vaccines, lack of awareness of public about the usefulness of administering these vaccines for pre and post exposure cases and limited availability in many regions of the world are all factors that prevented the cell culture rabies vaccines to be utilized in full potential to benefit human-kind. However, with the advancement of times and production of vaccines by many manufacturing companies and the use of I/D administration of rabies vaccines, the cost of the vaccines would be reasonable and within the reach to the common people and prevention and control of rabies to a great extent in the near future would be a reality.
References
- 1.Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda ME, et al. Reevaluating the burden of rabies in Africa and Asia. Bull WHO. 2005;83(5):360–368. [PMC free article] [PubMed] [Google Scholar]
- 2.Wilde H, Khawplod P, Khamoltham T, Hemachudha T, Tepsumethanon V, Lumlerdacha B, et al. Rabies control in South and Southeast Asia. Vaccine. 2005;23(17-18):2284–2289. doi: 10.1016/j.vaccine.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Hampson K, Dushoff J, Bingham J, Bruckner G, Ali YH, Andy D. Synchronous cycles of domestic dog rabies in sub-Saharan Africa and the impact of control efforts. Proc Nat Acad Sci. 2007;104(18):7717–7722. doi: 10.1073/pnas.0609122104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamoltham T, Singhsa J, Promsaranee U, Sonthon P, Mathean P, Thinyounyong W. Elimination of human rabies in a canine endemic province in Thailand: five-year programme. Bull WHO. 2003;81(5):375–381. [PMC free article] [PubMed] [Google Scholar]
- 5.Hanlon CA, Kuzmin IV, Blanton JD, Weldon WC, Manangan JS, Rupprecht CE. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 2005;111(1):44–54. doi: 10.1016/j.virusres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS, Ashwath NDH, Abdul Rahman S. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Intl J Infect Dis. 2007;11(1):29–35. doi: 10.1016/j.ijid.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) Geneva: WHO; 2001. Strategies for the control and elimination of rabies in Asia. Report of a WHO interregional consultation. Report of the workshop on rabies control in Asian countries; p. 32. [Google Scholar]
- 8.Cliquet F, Picard-Meyer E. Rabies and rabies-related viruses: a modern perspective on an ancient disease. Rev Sci Tech Off Int Epiz. 2004;23(2):625–642. doi: 10.20506/rst.23.2.1514. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri S. Ecollage; 2005. Rabies prevention and dog population management. India's official dog control policy in context of WHO guidelines. [Google Scholar]
- 10.Hoque M, Islam T, Das SC, Jabeen N, Islam T. An overview of rabies in man and animals. Intas Polivet. 2006;7:388–398. [Google Scholar]
- 11.Sudarshan MK. Assessing burden of rabies in India. WHO sponsored national multi-centric rabies survey. Association for Prevention and Control of Rabies in India. 2004;6:44–45. [Google Scholar]
- 12.Shayam C, Duggal AK, Kamble U, Agarwal AK. Post-exposure prophylaxis for rabies. J Indian Acad Clin Med. 2006;7(1):39–46. [Google Scholar]
- 13.Singh CK, Sandhu BS. Epidemiological investigation of rabies in Punjab. Indian J Anim Sci. 2007;77:653–658. [Google Scholar]
- 14.OIE (World Organization for Animal Health) OIE. Manual of standards for diagnostic tests and vaccines. 5th ed. Paris: OIE; 2004. Rabies; pp. 276–291. [Google Scholar]
- 15.Pastoret PP, Kappeler A, Aubert M. European rabies control and its history. In: King AA, Fooks AR, Aubert M, Wandeler AI, editors. Historical perspective of rabies in Europe and the Mediterranean basin. Paris: OIE; 2004. pp. 337–347. [Google Scholar]
- 16.Chulasugandha P, Khawplod P, Havanond P, Wilde H. Cost comparison of rabies pre-exposure vaccination with post-exposure treatment in Thai children. Vaccine. 2006;24(9):1478–1482. doi: 10.1016/j.vaccine.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 17.Kamoltham T, Khawplod P, Wilde H. Rabies intradermal post-exposure vaccination of humans using reconstituted and stored vaccine. Vaccine. 2002;20(27-28):3272–3276. doi: 10.1016/s0264-410x(02)00299-2. [DOI] [PubMed] [Google Scholar]
- 18.Kamoltham T, Thinyounyong W, Phongchamnaphai P, Phraisuwan P, Khawplod P, Banzhoff A, et al. Pre-exposure rabies vaccination using purified chick embryo cell rabies vaccine intradermally is immunogenic and safe. J Pediat. 2007;151(2):173–177. doi: 10.1016/j.jpeds.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 19.John TJ. An ethical dilemma in rabies immunisation. Vaccine. 1997;15(Suppl 1):S12–15. doi: 10.1016/s0264-410x(96)00316-7. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh TK. Rabies, Proceedings of the IX National conference of pediatric infectious diseases; Chennai, India: 2006. [Google Scholar]
- 21.Fishbein DB, Yenne KM, Dreesen DW, Teplis CF, Mehta N, Briggs DJ. Risk factors for systemic hypersensitivity reactions after booster vaccination with human-diploid cell rabies vaccine-a nationwide prospective study. Vaccine. 1993;11:1390–1394. doi: 10.1016/0264-410x(93)90167-v. [DOI] [PubMed] [Google Scholar]
- 22.Dietzschold B, Faber M, Schnell MJ. New approaches to the prevention and eradication of rabies. Exp Rev Vac. 2003;2(3):399–406. doi: 10.1586/14760584.2.3.399. [DOI] [PubMed] [Google Scholar]
- 23.Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Veterinary Virology. 3rd ed. New York: Academic Press; 1999. [Google Scholar]
- 24.Pringle CR. The order Mononegavirales. Arch Virol. 1991;117:137–140. [PubMed] [Google Scholar]
- 25.Nandi S. Molecular basis of rabies virus virulence with special reference to wildlife; Global meet on Veterinary Public Health and Symposium on “New Horizons in Food Security with special reference to Veterinary Public Health and Hygiene Evolving Strategies with Global Perspectives” and 8th Convention of APHV held at Lucknow; 2008. Nov 19-21, pp. 196–204. [Google Scholar]
- 26.Amengual B, Whitby JE, King A, Cobo S, Bourhy H. Evolution of European bat lyssavirus. J Gen Virol. 1997;78:2319–2328. doi: 10.1099/0022-1317-78-9-2319. [DOI] [PubMed] [Google Scholar]
- 27.Luo M, Green TJ, Zhang X, Tsao J, Qiu Sh. Conserved characteristics of the rhabdovirus nucleoprotein. Virus Res. 2007;129(2):246–251. doi: 10.1016/j.virusres.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gould AR, Kattenbelt JA, Gumley SG, Lunt RA. Characterization of an Australian bat lyssavirus variant isolated from an insectivorous bat. Virus Res. 2002;89(1):1–28. doi: 10.1016/s0168-1702(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt KJ, Twin J, Davis P, Holmes EC, Smith GA, Smith IL, et al. A molecular epidemiological study of Australian bat lyssavirus. J Gen Virol. 2003;84(2):485–496. doi: 10.1099/vir.0.18652-0. [DOI] [PubMed] [Google Scholar]
- 30.Fekadu M, Nesby SL, Shaddock H, Schumacher CL, Linhart SB, Sanderlin DW. Immunogenicity, efficacy and safety of an oral rabies vaccine (SAG-2) in dogs. Vaccine. 1996;14(6):465–468. doi: 10.1016/0264-410x(95)00244-u. [DOI] [PubMed] [Google Scholar]
- 31.Fooks AR, Brookes SM, Johnson N, Mc Elhinney LM, Hutson AM. European bat lyssaviruses: an emerging zoonosis. Epidem Infect. 2003;131(3):1029–1039. doi: 10.1017/s0950268803001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nandi S, Audarya SD, Suresh I, Chauhan RS. Emergence of Rabies and Rabies related Viruses- a Real Challenge. National Symposium on “Prospective Role of Veterinary Public Health in Integrated Rural Development; Bhubaneswar, Orissa: College of Veterinary Science and Animal Husbandry; 2006. Dec 7-8, pp. 83–89. [Google Scholar]
- 33.Nagarajan T, Mohanasubramanian B, Seshagiri EV, Nagendrakumar SB, Saseendranath MR, Satyanarayana ML, et al. Molecular epidemiology of rabies virus isolates in India. J Clin Microbiol. 2006;44(9):3218–3224. doi: 10.1128/JCM.00801-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton EC, Burns DK, Opatowsky MJ, El-Feky WH, Fischbach B, Melton L, et al. Rabies encephalomyelitis: clinical, neuro-radiological and pathological findings in 4 transplant recipients. Arch Neurol. 2005;62(6):873–882. doi: 10.1001/archneur.62.6.873. [DOI] [PubMed] [Google Scholar]
- 35.Nandi S, Maiti SK. When should your pets get vaccination against rabies. Asian Livestock (FAO) 1995;20(4):46–48. [Google Scholar]
- 36.Slater MR. The role of veterinary epidemiology in the study of free-roaming dogs and cats. Prev Vet Med. 2001;48(4):273–286. doi: 10.1016/s0167-5877(00)00201-4. [DOI] [PubMed] [Google Scholar]
- 37.Schneider MC, Belotto A, Ade MP, Hendrickx S, Leanes LF, de Freitas Rodrigues MJ, et al. Current status of human rabies transmitted by dogs in Latin America. Cad Sade Pblica. 2007;23(9):2049–2063. doi: 10.1590/s0102-311x2007000900013. [DOI] [PubMed] [Google Scholar]
- 38.Kumar M, Nandi S, Manohar M, Chauhan RS. Epidemiology and immunoprophylaxis of rabies in dogs. Pet India. 2009;15(8):18–22. [Google Scholar]
- 39.Wunderli PS, Dreesen DW, Miller TJ, Baer GM. Effect of heterogeneity of rabies virus strain and challenge route on efficacy of inactivated rabies vaccines in mice. American J Vet Res. 2003;64(4):499–505. doi: 10.2460/ajvr.2003.64.499. [DOI] [PubMed] [Google Scholar]
- 40.Hanlon CA, Kuzmin I, Blanton J, Manangan J, Murphy SM, Rupprecht CE. Proceedings of 14th International conference on Rabies in the Americas. 2003. Efficacy of biologics against newly described lyssaviruses; pp. 55–56. [Google Scholar]
- 41.Kallel H, Jouini A, Majoul S, Rourou S. Evaluation of various serum and animal protein free media for the production of a veterinary rabies vaccine in BHK-21 cells. J Biotechnol. 2002;95(3):195–204. doi: 10.1016/s0168-1656(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 42.Nandi S, Goel AC, Pandey KD. Immunogenic response to partially purified rabies virus as measured by ELISA. Indian J Virol. 1990;6(1-2):89–92. [Google Scholar]
- 43.Pastoret IP, Brochier B. Epidemiology and elimination of rabies in Western Europe. Vet J. 1998;156(2):83–90. doi: 10.1016/s1090-0233(05)80034-6. [DOI] [PubMed] [Google Scholar]
- 44.Madhusudana SN, Anand NP, Shamsundar R. Economical multi site intradermal regimen with purified chick embryo cell vaccine (Rabipur) prevents rabies in people bitten by confirmed rabid animals. Intl J Infect Dis. 2002;6(3):210–214. doi: 10.1016/s1201-9712(02)90113-x. [DOI] [PubMed] [Google Scholar]
- 45.Artois M, Cliquet F, Barrat J, Schumacher CL. Effectiveness of SAG1 oral vaccine for the long-term protection of red foxes (Vulpes vulpes) against rabies. Vet Rec. 1997;140(3):57–59. doi: 10.1136/vr.140.3.57. [DOI] [PubMed] [Google Scholar]
- 46.Dietzschold B, Schnell MJ. New approaches to the development of live attenuated rabies vaccines. Hybrid Hybridomics. 2002;21(2):129–134. doi: 10.1089/153685902317401735. [DOI] [PubMed] [Google Scholar]
- 47.Vos A, Neubert A, Aylan O, Schuster P, Pommerening E, Muller T, et al. An update on safety studies of SAD B19 rabies virus vaccine in target and non-target species. Epidemiol Infect. 1999;123:165–175. doi: 10.1017/s0950268899002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuffereau C, Leblois H, Benejean J, Coulon P, Lafay F, Flamand A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 1989;172(1):206–212. doi: 10.1016/0042-6822(89)90122-0. [DOI] [PubMed] [Google Scholar]
- 49.Cliquet F, Gurbuxami JP, Pradhan HK, Pattnaik B, Patil SS, Regnault A, et al. Safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007;25(17):3409–3418. doi: 10.1016/j.vaccine.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 50.Hammami S, Schumacher C, Cliquet F, Tlatli A, Aubert A, Aubert M. Vaccination of Tunisian dogs with the lyophilised SAG 2 oral rabies vaccine incorporated into the DBL 2 dog bait. Vet Res. 1999;30(6):607–613. [PubMed] [Google Scholar]
- 51.Kieny MP, Lathe R, Drillien R, Spehner D, Skory S, Schmitt D, et al. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1990;312:163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- 52.Rupprecht CE, Gibbons RV. Prophylaxis against Rabies. New England J Med. 2004;351(25):2626–2635. doi: 10.1056/NEJMcp042140. [DOI] [PubMed] [Google Scholar]
- 53.Lodmell DL, Ewalt LC. Rabies vaccination: comparison of neutralizing antibody responses after priming and boosting with different combinations of DNA, inactivated virus, or recombinant vaccinia virus vaccines. Vaccine. 2000;18(22):2394–2398. doi: 10.1016/s0264-410x(00)00005-0. [DOI] [PubMed] [Google Scholar]
- 54.Hanlon CA, Niezgoda M, Hamir AN, Schumacher C, Koprowski H, Rupprecht CE. First North American field release of a vaccinia-rabies glycolprotein recombinant virus. J Wildlife Dis. 1998;34(2):228–239. doi: 10.7589/0090-3558-34.2.228. [DOI] [PubMed] [Google Scholar]
- 55.Morimoto K, McGettigan JP, Foley HD, Hooper DC, Dietzschold B, Schnell MJ. Genetic engineering of live rabies vaccines. Vaccine. 2001;19(25-26):3543–3551. doi: 10.1016/s0264-410x(01)00064-0. [DOI] [PubMed] [Google Scholar]
- 56.Cleaveland S. Epidemiology and control of rabies. The growing problem of rabies in Africa. Transact Royal Soc Trop Med Hyg. 1998;92(2):131–134. doi: 10.1016/s0035-9203(98)90718-0. [DOI] [PubMed] [Google Scholar]
- 57.Woldehiwet Z. Rabies: recent developments. Res Vet Sci. 2002;73(1):17–25. doi: 10.1016/s0034-5288(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 58.Blanton JD, Hanlon CA, Rupprecht CE. Rabies surveillance in the United States during 2006. J American Vet Med Assoc. 2007;231(4):540–556. doi: 10.2460/javma.231.4.540. [DOI] [PubMed] [Google Scholar]
- 59.Lodmell DL, Ray NB, Ewalt LC. Gene gun particle-mediated vaccination with plasmid DNA confers protective immunity against rabies virus infection. Vaccine. 1998;16(2-3):115–118. doi: 10.1016/s0264-410x(97)88325-9. [DOI] [PubMed] [Google Scholar]
- 60.Masson E, Bruyere-Masson V, Vuillaume P, Lemoyne S, Aubert M. Rabies oral vaccination of foxes during the summer with the VRG vaccine bait. Vet Res. 1999;30(6):595–605. [PubMed] [Google Scholar]
- 61.Vos A, Neubert A, Pommerening E, Muller T, Dohner L, Neubert L, et al. Immunogenicity of an E1-deleted recombinant human adenovirus against rabies by different routes of administration. J Gen Virol. 2001;82:2191–2197. doi: 10.1099/0022-1317-82-9-2191. [DOI] [PubMed] [Google Scholar]
- 62.Hu RL, Zhang SF, Fooks AR, Yuan H, Liu Y, Li H, et al. Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microb Infect. 2006;8(4):1090–1097. doi: 10.1016/j.micinf.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Briggs DJ, Banzhoff A, Nicolay U, Sirikwin S, Dumavibhat B, Tongswas S, et al. Antibody response of patients after post-exposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified vero cell rabies vaccine. Bull WHO. 2000;78(5):693–698. [PMC free article] [PubMed] [Google Scholar]
- 64.Madhusudana SN, Sanjay TV, Mahendra BJ, Sudarshan MK, Narayana DH, Giri A, et al. Comparison of safety and immunogenicity of purified chick embryo cell rabies vaccine (PCECV) and purified vero cell rabies vaccine (PVRV) using the Thai Red Cross intradermal regimen at a dose of 0.1ml. HumVaccin. 2006;2(5):200–204. doi: 10.4161/hv.2.5.3197. [DOI] [PubMed] [Google Scholar]
- 65.Dreesen DW, Fishbein DB, Kemp DT, Brown J. Two-year comparative trial on the immunogenicity and adverse effects of purified chick embryo cell rabies vaccine for pre-exposure immunization. Vaccine. 1989;7(5):379–400. doi: 10.1016/0264-410x(89)90152-7. [DOI] [PubMed] [Google Scholar]
- 66.Meslin FX, Kaplan MM, Koprowski H. World Health Organization Laboratory techniques in Rabies. 4th ed. Geneva, Switzerland: WHO; 1996. [Google Scholar]
- 67.Dressen DW. A global review of rabies vaccines for human use. Vaccine. 1997;15:S2–6. doi: 10.1016/s0264-410x(96)00314-3. [DOI] [PubMed] [Google Scholar]
- 68.Council of Europe (COR) 6th ed. Strasbourg (Germany): Council of Europe; 2007. European Pharmacopoeia Commission. Rabies vaccine (inactivated) for veterinary use; pp. 3375–3377. [Google Scholar]
- 69.Dreesen DW, Hanlon CA. Current recommendations for the prophylaxis and treatment of rabies. Drugs. 1998;56(5):801–809. doi: 10.2165/00003495-199856050-00005. [DOI] [PubMed] [Google Scholar]
- 70.Lodmell DL, Ewalt LC. Rabies cell culture vaccines reconstituted and stored at 40°C for 1 year prior to use protect mice against rabies virus. Vaccine. 2004;22(25-26):3237–3239. doi: 10.1016/j.vaccine.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 71.Warrell MJ, Suntharasamai P, Sinhaseni A, Phanfung R, Vincent-Falquet JC, Bunnag D, et al. An economical regimen of human diploid cell strain anti-rabies vaccine for post-exposure prophylaxis. Lancet. 1983;2(8345):301–304. doi: 10.1016/s0140-6736(83)90288-x. [DOI] [PubMed] [Google Scholar]
- 72.Wilde H, Hemachudha T. How far can the antigen content of tissue culture rabies vaccine be reduced safely? Vaccine. 2006;24(10):1489. doi: 10.1016/j.vaccine.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Jaiiaroensup W, Lang J, Thipkong P, Wimalaratne O, Samranwataya P, Saikasem A, et al. Safety and efficacy of purified vero cell rabies vaccine given intramuscularly and intradermally (results of a prospective randomized trial) Vaccine. 1998;16(16):1559–1562. doi: 10.1016/s0264-410x(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 74.Ramanna BC, Reddy GS, Srinivassan VA. An out-break of rabies in cattle and use of tissue culture rabies vaccine during the outbreak. J Comm Dis. 1991;23(4):283–285. [PubMed] [Google Scholar]
- 75.Khawplod P, Wilde H, Tantawichien T, Limu-sanno S, Tantawichien T, Mitmoonpitak C, et al. Potency, sterility and immunogenicity of rabies tissue culture vaccine after reconstitution and refrigerated storage for 1 week. Vaccine. 2002;20(17-18):2240–2242. doi: 10.1016/s0264-410x(02)00111-1. [DOI] [PubMed] [Google Scholar]
- 76.Quiambao BP, Dimaano EM, Ambas C, Davis R, Banzhoff A, Malerzcyk C. Reducing the cost of post-exposure rabies prophylaxis: efficacy of 0.1ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine. 2005;23(14):1709–1714. doi: 10.1016/j.vaccine.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 77.Sudarshan MK, Madhusudana SN, Mahendra BJ, Narayana DHA, Giri MSA, Popova O, et al. Evaluation of a new five-injection, two-site, intradermal schedule for purified chick embryo cell rabies vaccine: A randomized, open-label, active-controlled trial in healthy adult volunteers in India. Curr Ther Res. 2005;66(4):323–334. doi: 10.1016/j.curtheres.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox JH, Dietzschold B, Schneider G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang ZQ, Spitalnik S, Tran M, Wunner WH, Cheng J, Ertl HC. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199(1):132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 80.Hu R, Liu Y, Zhang S, Zhang F, Fooks AR. Experimental immunization of cats with a recombinant rabies-canine adenovirus vaccine elicits a long-lasting neutralizing antibody response against rabies. Vaccine. 2007;25(29):5301–5307. doi: 10.1016/j.vaccine.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 81.Osorio JE, Tomlinson CC, Frank RS, Haanes EJ, Rushlow K, Haynes JR, et al. Immunization of dogs and cats with a DNA vaccine against rabies virus. Vaccine. 1999;17(9-10):1109–1116. doi: 10.1016/s0264-410x(98)00328-4. [DOI] [PubMed] [Google Scholar]
- 82.Perrin P, Jacob Y, Aguilar-Setien A, Loza-Rubio E, Jallet C, Desmezieres E, et al. Immunization of dogs with a DNA vaccine induces protection against rabies virus. Vaccine. 2000;18(5):479–486. doi: 10.1016/s0264-410x(99)00247-9. [DOI] [PubMed] [Google Scholar]
- 83.Bahloul C, Taieb D, Diouani MF, Ahmed SB, Chtourou Y, B'chir BI, et al. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 2006;24(8):1063–1072. doi: 10.1016/j.vaccine.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 84.Sugiyamaa M, Ito N. Control of rabies: Epidemiology of rabies in Asia and development of new-generation vaccines for rabies. Comp Immunol Microbiol Infect Dis. 2007;30(5-6):273–286. doi: 10.1016/j.cimid.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Xiang Z, Pasquiru S, Ertl HCJ. Effect of passive immunization or maternally transferred immunity on the antibody response to a genetic vaccine to rabies virus. J Virol. 1998;72(3):1790–1796. doi: 10.1128/jvi.72.3.1790-1796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faber M, Bette M, Preuss MA, Pulmanausahakul R, Rehnelt J, Schnell MJ, et al. Overexpression of tumor necrosis factor alpha by a recombinant rabies virus attenuates replication in neurons and prevents lethal infection in mice. J Virol. 2005;79(24):15405–15416. doi: 10.1128/JVI.79.24.15405-15416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dietzschold B, Wunner WH, Wiktor TJ, Lopes AD, Lafon M, Smith CL, et al. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA. 1983;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seif I, Coulon P, Rollin PE, Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mebatsion T. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J Virol. 2001;75(23):11496–11502. doi: 10.1128/JVI.75.23.11496-11502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faber M, Pulmanausahakul R, Hodawadekar SS, Spitsin S, McGettigan JP, Schnell MJ, et al. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J Virol. 2002;76(7):3374–3381. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brzzka K, Finke S, Conzelmann KK. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol. 2005;79(12):7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ito N, Takayama M, Yamada K, Hosokawa J, Sugiyama M, Minamoto N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol Immunol. 2003;47:613–617. doi: 10.1111/j.1348-0421.2003.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 93.Ito N, Takayama M, Yamada K, Sugiyama M, Minamoto N. Rescue of rabies virus from cloned cDNA and identification of the pathogenicity-related gene: glycoprotein gene is associated with virulence for adult mice. J Virol. 2001;75(19):9121–9128. doi: 10.1128/JVI.75.19.9121-9128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mebatsion T, Weiland F, Conzelmann KK. Matrix protein of rabies virus is responsible for the assembly and budding of bullet shaped particles and interacts with the transmembrane spike glycoprotein G. J Virol. 2007;73(1):242–250. doi: 10.1128/jvi.73.1.242-250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ito N, Sugiyama M, Yamada K, Shimizu K, Takayama M, Hosokawa J. Characterization of M gene-deficient rabies virus with advantages of effective immunization and safety as a vaccine strain. Microbiol Immunol. 2005;49:971–979. doi: 10.1111/j.1348-0421.2005.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 96.WHO. Geneva; 2004. Expert Consultation on Rabies. (WHO Technical Report Series-931) [Google Scholar]
- 97.World Health Organization. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus neutralizing antibody. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in Rabies. 4th ed. Geneva: WHO; 1996. pp. 181–192. [Google Scholar]
- 98.Mansfield KL, Burr PD, Snodgrass DR, Sayers R, Fooks AR. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet Rec. 2004;154(14):423–426. doi: 10.1136/vr.154.14.423. [DOI] [PubMed] [Google Scholar]
- 99.Moore SM, Ricke TA, Davis RD, Briggs DJ. The influence of homologous versus heterologous challenge virus strains on the serological test results of rabies neutralization assays. Biologicals. 2005;33(4):269–276. doi: 10.1016/j.biologicals.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Perry LL, Lodmell DL. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol. 1991;65(7):3429–3434. doi: 10.1128/jvi.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


