Abstract
Studies on biomedical applications of nanoparticles are growing with a rapid pace. In medicine, nanoparticles may be the solution for multi-drug-resistance which is still a major drawback in chemotherapy of cancer. In the present study, we investigated the potential cytotoxic effect of silver nanoparticles (Ag NPs) and silver ions (Ag+) in both parent and tamoxifen-resistant T47D cells in presence and absence of tamoxifen. Ag NPs were synthesized (< 28 nm) and MTT assay was carried out. The associated IC50 values were found to be: 6.31 µg/ml for Ag NPs/parent cells, 37.06 µg/ml for Ag NPs/tamoxifen-resistant cells, 33.06 µg/ml for Ag+/parent cells and 10.10 µg/ml for Ag+/resistant cells. As a separate experiment, the effect of subinhibitory concentrations of Ag NPs and Ag+ on the proliferation of tamoxifen-resistant cells was evaluated at non-toxic concentrations of tamoxifen. Our results suggested that in non-cytotoxic concentrations of silver nanomaterials and tamoxifen, the combinations of Ag+-tamoxifen and Ag NPs-tamoxifen are still cytotoxic. This finding may be of great potential benefit in chemotherapy of breast cancer; since much lower doses of tamoxifen may be needed to produce the same cytotoxic effect and side effects will be reduced.
Keywords: Breast neoplasms, Chemotherapy, Cytotoxicity, Nanoparticles, Tamoxifen
Introduction
The past few years witnessed an increasing attention towards rapid development in every aspect of what is called “nanotechnology”. Nanotechnology is briefly termed as “The ability to fabricate, characterize, and manipulate artificial structures, whose features are controlled at the nanometer level” (1). This may involve design, synthesis and preparation of nanoparticles to create products with novel properties (2–4). In recent years, there has been much interest in the application of nano-materials for biological purposes. These materials include nanoparticles (5–7), nanotubes (8), nanowires (9), and quantum dots (10). Nevertheless, the biomedical applications of nano-materials as cytotoxic or antimicrobial agents, biosensors and, drug carriers are growing with a rapid pace (11–13).
Despite many efforts, multi drug resistance is still considered as a major drawback in chemotherapy of cancer which has been the subject of exhaustive experiments in this area (14). The cellular mechanisms underlying this phenomenon are well-discussed before (15). It is also well-known that one of the main mechanisms underlying drug resistance is based on the expression of MDR-1 gene which leads to the formation of membrane-bound ABC transporter proteins, such as P-glycoproteins (16, 17). Alternative mechanisms include detoxification processes by cytochrome-P450 metabolism enzymes and glutathione, cellular repair mechanisms which repair drug-induced DNA damages, and alteration of apoptotic signaling pathways, so that cells could resist drug-induced apoptosis.
Tamoxifen is a frequently-prescribed medication, widely used in all stages of breast cancer (18). However, tamoxifen-resistance is still a major problem which limits its application in chemotherapy of breast cancer (19, 20). In order to reduce tamoxifen-resistance, prodigious efforts have been made on combination therapies of tamoxifen and different compounds, such as gap junctional activator (21). The combination therapy of drugs and nano-particles is promising since it may improve the penetration of drugs into tumor cells, enhances their ability of tumor targeting, and reduces their side effects (22). Recently, the cytotoxic effect of tamoxifen-loaded polymeric nanoparticles was investigated by Amiji and co-workers (23).
The biological applications of silver nano-particles (Ag NPs) have been widely studied; antimicrobial properties in particular (24–27). Ag NPs are known to be cytotoxic to both normal and cancer cells in mammals (28) and the modes of interactions of Ag NPs have been investigated in different prokaryotic and eukaryotic systems (29–31). The cytotoxic effects of silver ions (Ag+) have been reported in different cell lines as well (32, 33). However, no comparative experiment has been yet carried out on cytotoxic effects of the combination of silver nanomaterials and tamoxifen on cancer cell lines. In addition, along with promising cytotoxic effects of silver nanomaterials, much concern has lately been raised about the safety issue of these compounds due to undesirable toxic effects of Ag NPs on both human health and environment (34, 35).
This study represents a comparative in vitro evaluation of the effect of Ag NPs and Ag+ on the viability of two cell subtypes; human T47D breast cancer cell line and tamoxifen resistant T47D cell line. Also, the cytotoxic effect of subinhibitory concentrations of Ag NPs and Ag+ on tamoxifen-resistant cells both in presence and absence of tamoxifen was investigated.
Materials and Methods
Materials
RPMI-1640 and fetal bovine serum were purchased from Gibco (United States); 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT), tamoxifen, penicillin, and streptomycin from Sigma (United Kingdom); and dextrose, silver nitrate (AgNO3), sodium hydroxide (NaOH), hydrogen chloride (HCl) and isopropanol from Merck (Germany).
Synthesis of Ag NPs
The chemical reduction of an aqueous solution of AgNO3 is one of the most widely-used methods for synthesis of Ag colloids (36). An aqueous solution containing 0.5 mM Ag+ was prepared by adding 22.5 mg of dextrose to 100 ml of AgNO3 solution (0.5 mM). This solution was then alkalized using 20 µl of 0.1 N NaOH and treated in a microwave oven for 60 sec to induce the reduction of metal ions. The reduction of the Ag+ by dextrose in the solution was monitored by sampling the aqueous component (2 ml) and then measuring the ultraviolet-visible (UV-Vis) spectra of the solutions on a UV-Vis double-beam automatic-scanning spectrophotometer (Labomed, Inc., Spectro UV-Vis Double PC, Model UVD-2950), operated at 2 nm resolution. Furthermore, Ag NPs were characterized by Transmission Electron Microscopy (TEM) (Phillips CM200 field emission gun) and Energy-Dispersive Spectroscopy (EDS). For this propose, an aqueous suspension containing the Ag NPs was dispersed ultrasonically, and a drop of the suspension was placed on carbon-coated copper TEM grids and dried under an IR lamp. Micrographs were obtained using a TEM operated at an accelerating voltage of 80 kV.
Cell line and culture conditions
The T47D human breast cancer cell line (ATCC HTB-133, United States) was purchased from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). The subline of the tamoxifen-resistant T47D human breast cancer cell line was a gift from the Molecular Research Laboratory, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tehran University of Medical Sciences (Tehran, Iran) (37). Parent cancer cell line was maintained in RPMI-1640 culture medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin in a 5% carbon dioxide (CO2) cell incubator at 37 °C. The tamoxifen-resistant cancer cell line was cultured in the described medium supplemented with 1 µM of tamoxifen and maintained under the same conditions of parent cancer cells.
Cytotoxicity assay
MTT-based assay was performed according to a previously-described method (38) by preliminarily seeding of 45,000 cancer cells in a 100 -µl growth medium in the presence of increasing concentrations of Ag NPs and Ag+ (15, 20, 30, 40, 45, and 50 µg/ml) in 96-well plates, individually and subsequent incubation of cells at 37 °C in 5% CO2 for 48 hr. The cells were thereafter treated with 25 µl of MTT (5 mg/ml) and incubated at 37 °C for 4hr. Nontreated cells were used as control. After dissolving formazan crystals in 0.04 N HCl in isopropanol, 96-well plates were read in a microplate reader (Dynatech Laboratories, Inc, United States) at 570 nm. Each experiment was repeated three times and the MTT assay was performed in triplicate for each experiment. Cytotoxicity was calculated as the percentage of viable cells at different concentrations of samples relative to the control (untreated) cells. Also, the half maximal Inhibitory Concentration (IC50) was calculated as the concentration required inhibiting the growth of tumor cells in culture by 50% compared to the untreated cells.
As a separate experiment, the combination effect of Ag NPs and Ag+ was studied both in presence and absence of tamoxifen, in tamoxifen-resistant T47D cells. Subinhibitory concentrations of Ag NPs (2.5 µg/ml) and Ag+ (1.5 µg/ml) were determined by a preliminary MTT assay on tested cell lines. The effects of these samples were investigated at the concentrations mentioned above on the proliferation of tamoxifen-resistant T47D cells grown in both tamoxifen-containing (1 µM) and tamoxifen-free culture media and the proliferation of cells in this culture media was evaluated at different incubation time intervals (20, 40, 60, 80, and 100 hrs), using the same MTT assay, described before.
Statistical analyses
The differences in cell cytotoxicity were compared using the Student's t-test. The p-values < 0.05 were considered significant.
Results
Synthesis of silver nanoparticles and their characterization
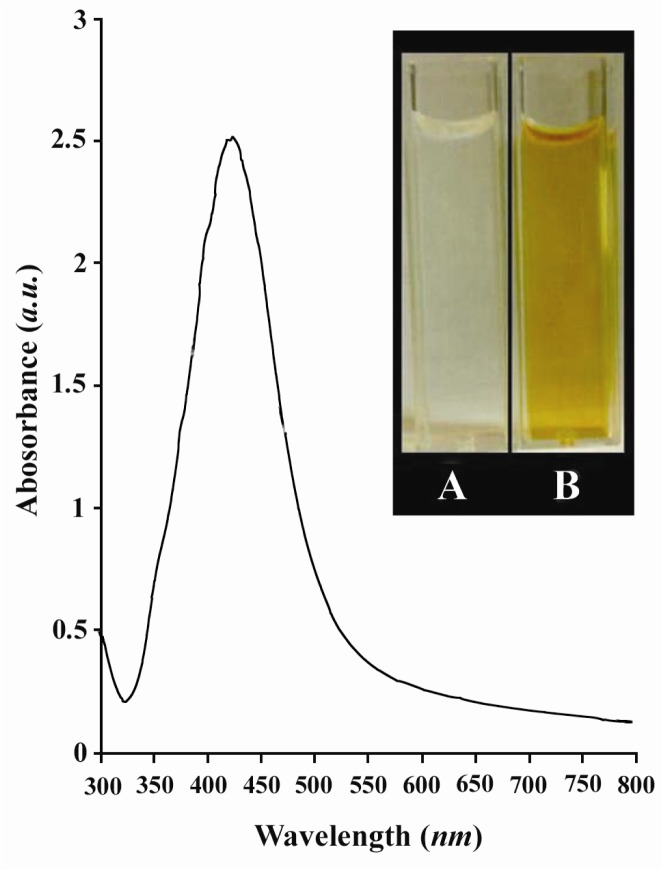
In this study, the Ag NPs were synthesized using the chemical reduction method described earlier (36). The synthesized Ag NPs were subsequently characterized by UV-Vis spectroscopy (Figure 1). It is worth mentioning that the technique outlined above, proved to be very useful in analysis of nano-particles. As illustrated in Figure 1, the strong absorption band with a maxima located at 420 nm was caused by the formation of Ag NPs produced by the dextrose. This peak is attributed to the surface plasmon phenomenon which is well identified in various metal nanoparticles with sizes ranging from 2 nm to 100 nm (39, 40). The inset to Figure 1 is a photograph of as-prepared Ag colloids.
Figure 1.
UV-Vis spectrum of as-prepared Ag NPs synthesized by chemical reduction of Ag+ by dextrose. The inset is A) a photograph of the reaction mixture before reduction and B) after reduction
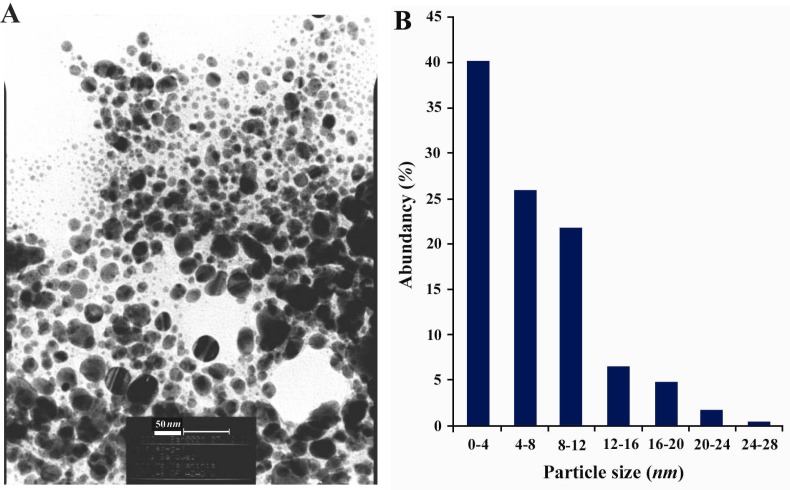
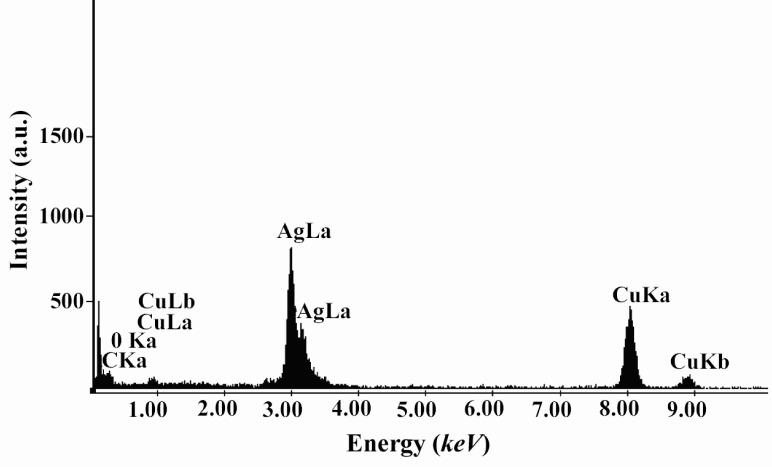
Figure 2A, shows representative TEM image recorded from the drop-coated film of the Ag NPs synthesized by treating the Ag NPs solution with the dextrose in a microwave oven. At least 200 randomly selected nanoparticles from TEM image were used to illustrate a particle size distribution histogram. The particle-size histogram of Ag NPs produced by dextrose (Figure 2, on the right) indicates that Ag NPs vary in size from 1 nm to 28 nm. Most Ag NPs (approximately 90%) obtained by this method varied in their size from about 1 nm to almost 12 nm. EDS analysis of the Ag NPs confirmed the presence of an elemental Ag signal in the sample (Figure 3). Ag nanocrystallites displayed an optical absorption band with a peak at 3 keV, which is typical for the absorption of metallic Ag nanocrystallites (copper and carbon peaks existed due to the grid used for TEM imaging) (30).
Figure 2.
A) TEM recorded from a small region of a drop-coated film of AgNO3 solution treated with dextrose for 60 sec in a microwave oven (the scale bars correspond to 50 nm). B) The related particle-size distribution histogram was obtained after counting 400 individual particles
Figure 3.
EDS spectra of prepared Ag NPs. Ag x-ray emission peaks are labeled. The strong signals from the atoms in the nanoparticles shown in the spectrum confirm the reduction of Ag+ to Ag NPs
Cytotoxicity assay of silver nanoparticles and its combination with tamoxifen
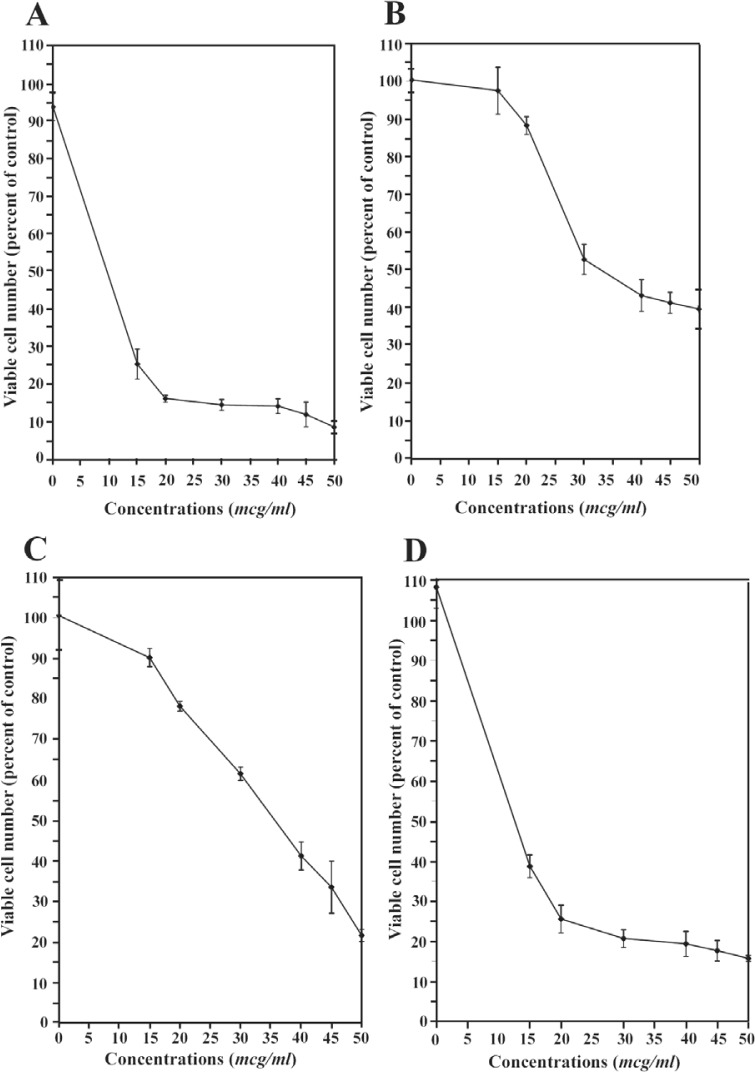
The cytotoxic effect of Ag in both forms of Ag+ and Ag NPs was evaluated in vitro in both parent and tamoxifen-resistant T47D human breast cancer cell lines at different concentrations of 15, 20, 30, 40, 45, and 50 µg/ml. As indicated in Figure 4, both parent and tamoxifen-resistant cells shows different patterns of dose-dependent responses in presence of metallic and ionic forms of silver nanomaterials. In detail, at the low concentration of 15 µg/ml Ag NP, a significant cytotoxic effect was observed which reduced the percentage of viable cells from 100% to 25%, followed by a moderate decrease from 25% at the concentration of 15 µg/ml Ag NPs, to less than 10% at 15 µg/ml concentration (Figure 4A). However, in the tamoxifen-resistant T47D cells, no more than 10% decrease in the proportion of viable cells was observed at concentrations below 30 µg/ml of Ag NPs, at which, the percentage of viable cells slowly decreased from 50% to 40% (Figure 4B).
Figure 4.
The effects of Ag+ and Ag NPs on the cells: A) Ag NPs on parent T47D cells, B) Ag NPs on the 1×10−6 M tamoxifen-resistant T47D cells, C) Ag+ on the parent T47D cells and D) Ag+ on the 1×10−6 M tamoxifen-resistant T47D cells (n = 6)
Figures 4C and 4D resemble the behaviors of parent and tamoxifen-resistant cells in presence of ionic – instead of metallic – form of silver. In the presence of Ag+, the viability of parent cells decreased in an almost linear manner, from 90% at 15 µg/ml concentration to about 20% at the maximal 50 µg/ml concentration (Figure 4C), whilst an essentially different pattern was observed for tamoxifen-resistant T47D cells. The proportion of viable tamoxifen-resistant cells fell down to 40 percent at the meager concentration of 15 µg/ml and mildly decreased from 40 to about 17% at 50 µg/ml concentration (Figure 4D).
The IC50 values associated with Ag NPs and Ag+ in both cell lines are shown in Table 1. The concentrations needed to produce 50% cell death were 37.06 µg/ml for the Ag NPs and 10.1 µg/ml for the Ag+ on the tamoxifen-resistant T47D cell lines, whereas 6.31 -µg/ml and 33.06 -µg/ml concetrations of Ag NPs and Ag+ produced the same effect on parent T47D cells (Table 1).
Table 1.
The inhibitory potential of the Ag NPs and Ag+ on the viability of parent T47D human breast cancer cells and its tamoxifen-resistant cell subline (TamR) (sample size = 6, repetitions = 3, calculated p < 0.05)a
| Cell line | IC50 value for cytotoxicity of Ag NPs (µg/ml) | IC50 value for cytotoxicity of Ag+ (µg/ml) |
|---|---|---|
| Parent T47D cells | 6.31±0.52 | 33.06±0.66 |
| TamR-T47D cells | 37.06±0.69 | 10.10±0.48 |
IC50 was calculated using Sigmaplot software. IC 50 for parent T47D cells and tamoxifen resistant T47D cell sub line were obtained > 2.5 µM. Tamoxifen was used at concentration of 1 µM in all experiments
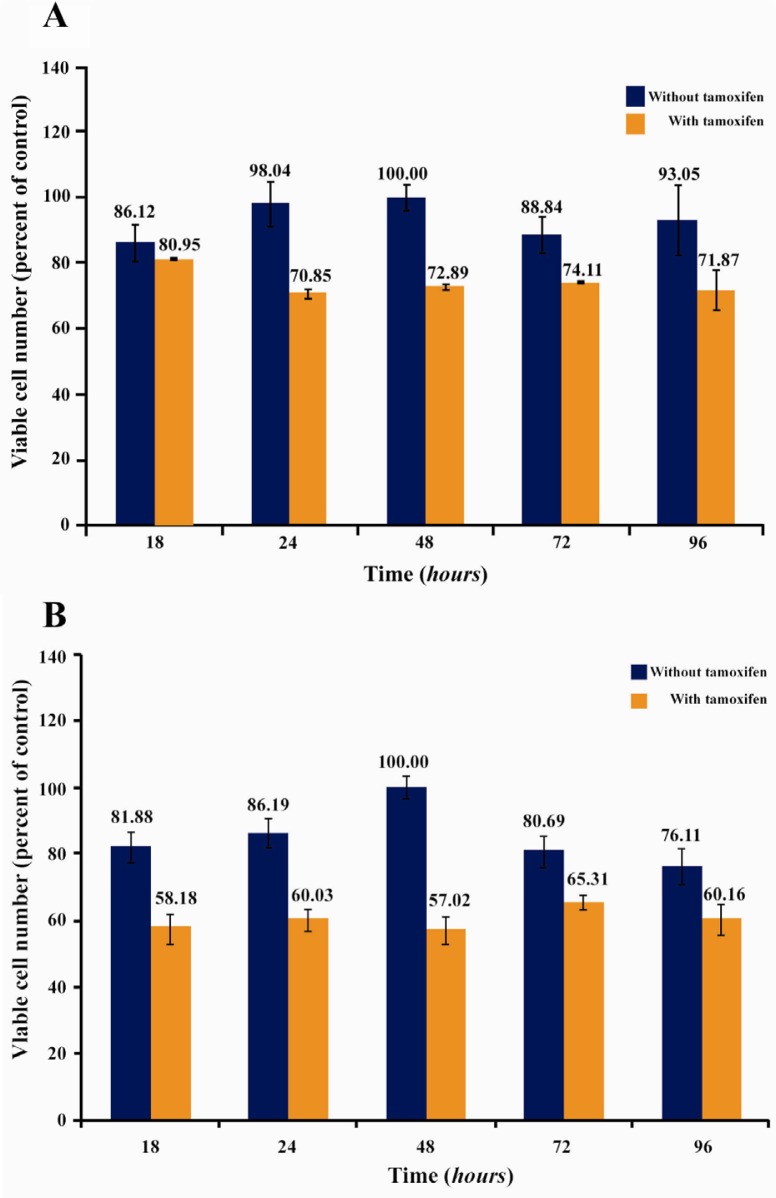
In a separate experiment, the effect of subinhibitory concentrations of Ag NPs (2.5 µg/ml) and Ag+ (1.5 µg/ml) on the prolif-eration of tamoxifen-resistant T47D human breast cancer cells was investigated both in presence and absence of sub inhibitory con-centration of tamoxifen (1 µM). Figure 5 shows the effect of Ag NPs (Figure 5A) and Ag+ (Figure 5B) on the proliferation of tamoxifen-resistant T47D cells in the presence and absence of tamoxifen (1 µM).
Figure 5.
The combined effect of subinhibitory concentrations of Ag NPs (A) and Ag+ (B) with tamoxifen (1 µM) on tamoxifen-resistant T47D human breast cancer cells
According to Figures 5A and 5B, a mild cytotoxicity occurred in a tamoxifen-free culture medium in the presence of silver; both as Ag NPs and Ag+, and these samples did not show any cytotoxicity at their subinhibitory concentrations at 48 hr after incubation. In contrast, in the presence of tamoxifen (1 µM), the antiproliferative effect of both silver-contained nanomaterials (Ag NPs and Ag+) on tamoxifen-resistant T47D cells enhanced (Figures 5A and 5B).
It is notable that the subinhibitory concentrations of samples (2.5 µg/ml for Ag NPs, 1.5 µg/ml for Ag+, and 1 µM for tamoxifen) were chosen to guarantee that the further cytotoxic effect was due to the combination effect of silver nanomaterials and tamoxifen, and not to the cytotoxic effect of each sample, alone. Therefore, the resultant cytotoxic effect in Figures 5A and 5B is only attributable to the tamoxifen-Ag combination which induce significant cytotoxicity in resistant T47D cells.
Discussion
The antiproliferative effect of Ag NPs and Ag+ is evident (41–44). Furthermore, the interaction of Ag NPs and Ag+ with certain proteins has been the subject of extensive studies in recent years. In this study, antiproliferative effect of silver in both ionic (Ag+) and metallic (Ag NPs) forms on parent and tamoxifen-resistant cells was investigated. Cells were observed to exhibit different responses after treatment with silver nanomaterials. The associate IC50 values as indicated in Table 1, were 6.31 µg/ml for parent cells in presence of AgNPs, 37.06 µg/ml for tamoxifen-resistant cells in presence of Ag NPs, 33.06 µg/ml for parent cells in presence of Ag+, and 10 µg/ml for tamoxifen-resistant cells in presence of Ag+.
The results primarily imply that silver, either in ionic or metallic forms, have cytotoxic effects on both parent T47D and tamoxifen-resistant cells. Moreover, it appears from Table 1 that parent cells were more susceptible to Ag NPs, while in reverse, tamoxifen-resistant cells were more susceptible to Ag+ rather than Ag NPs.
In parent T47D cells, the reason for enhanced cytotoxicity of Ag NPs (6.31 µg/ml) compared with Ag+ (33.06 µg/ml) may be due to the relative stability of Ag NPs which facilitates their subsequent penetration and survival in tumor cells. Therefore, since Ag+ possess low stability and more reactivity, it is likely that silver ions undergo further deionization and other unwanted reactions before reaching tumor cells.
On the other hand, the condition is not the same in tamoxifen-resistant cells due to numerous substantial differences between parent and resistant cells. As mentioned earlier, several mechanisms are involved in multi drug resistance of cells, among which, the pumping activity of ABC transporters such as P-glycoproteins, located in the cell membrane, is of particular importance. Therefore, assuming membrane glycoproteins as a major obstacle for the internalization of silver-based nanomaterials into resistant cells, it is probable that Ag+ may has easily penetrated into tamoxifen-resistant cells, while Ag NPs have been pumped out of the cells by P-glycoproteins. In other words, although nanomaterials have been shown to easily pass through cell membranes (45), the active reflux of Ag NPs from cells may hinder its maintenance in cells, thereby limiting its cytotoxic effect from IC50=6.31 µg/ml in parent cells to IC50=37.6 µg/ml in tamoxifen-resistant cells.
However, after internalization of Ag NPs and Ag+ into cells (which resembles the situation in parent cells), the cytotoxic effects of Ag NPs is more than that of Ag+, probably due to the fact that Ag NPs may interfere with the proper functioning of cellular proteins and induce subsequent changes in cellular chemistry; as proposed previously by Rogers et al. (42). This hypothesis is also in good agreement with experiments of Zolghadri and co-workers (46) where they demonstrated that Ag NPs provide a relatively high hydrophobicity inside bovine hemoglobin which causes a transition from alpha helixes to beta sheets and leads to partial unfolding and aggregation of the protein (46). Other experiments suggest that Ag NPs are likely to interact with thiolrich enzymes (47). Therefore, it sounds possible that once penetrated into cells, silver nano-particles may attack functional proteins of cells which results in partial unfolding and aggregation of proteins as it is the case in the bovine hemoglobin.
Figure 5 is a graphic illustration of the cytotoxic effects of subinhibitory concentrations of Ag NPs (A) and Ag+ (B) in presence and absence of subinhibitory concentrations of tamoxifen in tamoxifen-resistance cells. It should be pointed out that the tamoxifen concentration of 1 µM was chosen to guarantee that the effect produced was due to the combination and not to the effect of the tamoxifen itself. So the effect observed in this condition could be due to the inorganic materials–tamoxifen combination. The bar graph clearly suggests that at their subinhibitory concentrations, both Ag NPs and Ag+ possess insignificant cytotoxicity. Interestingly, however, the combination of subinhibitory concentration of tamoxifen and non-toxic concentration of Ag NPs and Ag+ induced significant cytotoxic effect in tamoxifen-resistant cells. This result is potentially promising because it suggests that by using negligible non-cytotoxic amounts of silver nanomaterials, tamoxifen may still be considered as a potent chemotherapic agent in tamoxifen-resistant cells.
Another result which can be deduced from Figure 5 is that the combination effect of Ag+-tamoxifen is considerably more potent than that of Ag NPs-tamoxifen. A number of possibilities may be addressed to explain this phenomenon. First and the foremost is that Ag+ is able to form more stable bonds via making complexes with nitrogen group in tamoxifen. The remarkable ability of Ag+ to form coordination networks is well-known (48, 49). Moreover, it was previously shown by Shukla and Pitre that tamoxifen forms complex with both metallic and ionic forms of Fe III and the complex is more potent, compared to the pure drug (50).
Taking this finding into consideration, it sounds likely that tamoxifen may become chaleted by silver atoms too. However, the Ag+-tamoxifen complex is more potent than Ag NPs-tamoxifen possibly because Ag+ is able to stabilize the system through forming network coordinations by placing between the tamoxifen molecules and making specific bridges. This hypothesis is inspired from recent studies of Ilker et al. (48), in which they proposed the same interaction among Ag+ and nitrogen-containing-cytosine molecules which literally increases the stability of the parallel motif of the duplex DNA. One can not conceal the recent global attention towards discovering novel biomedical applications for metal-based nanoparticles in both diagnosis and treatment of cancer (51, 52). The importance of using nanoparticles as a new generation of drug carriers is also evident and well-discussed before (53–56). However, the toxicity of metal-based nanoparticles is a major health and environmental issue in development of novel drug delivery systems (57) which has raised many fundamental criticisms in this area (34).
Conclusion
In this study, for the first time we investtigated the cytotoxicity of Ag+ and Ag NPs in parent and tamoxifen-resistant T47D human breast cancer cell lines in comparison. We demonstrated that parent and resistant cells show different responses to the presence of Ag+ and Ag NPs. Furthermore, we demonstrated that at non-cytotoxic concentrations of Ag+, Ag NPs, and tamoxifen, the combination of Ag+-tamoxifen and Ag NPs-tamoxifen is still cytotoxic to tamoxifen-resistance cells. Our result may be of great potential benefit in chemotherapy of breast cancer, since with such an approach, much lower doses of tamoxifen may produce the same cytotoxic effect with less unwanted side effects. The response of tamoxifen-resistant cells improves as well. Also, by using nanoparticles in non-toxic concentrations, this approach is much concerned about potential hazards of prevalent use of metallic nanoparticles. However, much effort still remains to be done in this area.
As future experiments, we suggest evaluation of silver nanoparticles coated with biologically-compatible polymers and evaluation of their combination effect with tamoxifen. We also recommend experiments on the cyto-toxic effects of Ag NPs and Ag NPs –tamoxifen combination in relation to their size as the size of Ag NPs is shown to be determinant in its antiproliferative activity (47, 57). Finally, our results suggest that with the aid of metal-based nanoparticles, conditional chemotherapic agents may have even broader range of applications in future.
Acknowledgement
This work was financially supported by a grant (No: 85-04-33-4778) from the Deputy of Research, Tehran University of Medical Sciences, Tehran, Iran. Also the support of the Iranian Nanotechnology Initiative is also gratefully acknowledged.
References
- 1.Parak WJ, Manna L, Simmel FC, Gerion D, Alivisatos P. Quantum dots. In: Schmid G, editor. Nano-particles: from theory to application. Weinheim: Wiley-VCH; 2004. pp. 4–49. [Google Scholar]
- 2.Mohanraj VJ, Chen Y. Nanoparticles; a review. Trop J Pharm Res. 2006;5(1):561–573. [Google Scholar]
- 3.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10(3):507–517. [Google Scholar]
- 4.Henglein A. Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J Phys Chem. 1993;97(21):5457–5471. [Google Scholar]
- 5.Panacek A, Kvitek L, Prucek-Kolar RM, Vecerova R, Pizurova N, Sharma VK, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110(33):16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 6.Dubey M, Bhadauria S, Kushwah BS. Green synthesis of nanosilver particles from extract of eucalyptus hybrida (saeda) leaf. Dig J Nanomater Bios. 2009;4(3):537–543. [Google Scholar]
- 7.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Edn. 2007;18(3):241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefania P, Oumarou S, Philippe P, L'Hocine Y. Applications of carbon nanotubes-based bio-materials in biomedical nanotechnology. J Nanosci Nanotechnol. 2006;6(7):1883–1904. doi: 10.1166/jnn.2006.197. [DOI] [PubMed] [Google Scholar]
- 9.Law M, Goldberger J, Yang P. Semiconductor nanowires and nanotubes. Annu Rev Mater Res. 2004;34:83–122. [Google Scholar]
- 10.Rhyner MN, Smith AM, Gao X, Mao H, Yang L, Nie S. Quantum dots and multifunctional nano-particles: new contrast agents for tumor imaging. Nanomedicine. 2006;1(2):209–217. doi: 10.2217/17435889.1.2.209. [DOI] [PubMed] [Google Scholar]
- 11.Sinha V, Trehan A. Biodegradable microspheres as protein delivery. J Control Release. 2003;90(3):261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 12.Nam JA, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301(5641):1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 13.Sondi I, Sondi B. Silver nanoparticles as anti-microbial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interf Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 15.Harris AL, Hochhauser D. Mechanisms of multi-drug resistance in cancer treatment. Acta Oncol. 1992;31:205–213. doi: 10.3109/02841869209088904. [DOI] [PubMed] [Google Scholar]
- 16.Di Niclantonio F, Mercer SJ, Knight LA, Gabriel FG, Whitehouse PA, Sharma S, et al. Cancer cell adaptation to chemotherapy. BMC Cancer. 2005;5:78. doi: 10.1186/1471-2407-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganasia-Leymarie V, Bischoff P, Bergerat J P, Holl V. Signal transduction pathways of taxanes-induced apoptosis. Curr Med Chem Anti-Cancer Agents. 2003;3(4):291–306. doi: 10.2174/1568011033482422. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Faller DV, Wang S. HIC1 regulates tumor cell responses to endocrine therapies. Mol Endocrinol. 2009;23(12):2075–2085. doi: 10.1210/me.2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010;119(2):283–293. doi: 10.1007/s10549-009-0345-x. [DOI] [PubMed] [Google Scholar]
- 20.Giordano C, Cui Y, Barone I, Ando S, Mancini MA, Berno V, et al. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;119(1):71–85. doi: 10.1007/s10549-009-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunjan G, Duy H, Annelise N T. Combinational treatment of gap junctional activator and tamoxifen in breast cancer cells. Anticancer Drugs. 2010;21(1):77–88. doi: 10.1097/CAD.0b013e328333d557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yezhelyev AV, Gao X, Xing Y, Al-Hajj A, Nie S, O'Regan R M. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7(8):657–667. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]
- 23.Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(ε-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005;293(1-2):261–270. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu N, Raj D T, Gowrik Y, Siddiqua A, Innocent JP. Synthesis of silver photo nanoparticles and their antibacterial efficacy. Dig J Nanomater Bios. 2010;5:185–189. [Google Scholar]
- 25.Kim JS, Kuk E, Yu KN, Kim J, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomed: Nanotechnol Biol Med. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Raja ASM, Thailagavathi G, Kannaian T. Synthesis of spray dried polyvinyl pyrrolidone coated silver nanopowder and its application on wool and cotton for microbial resistance. Indian J Fiber & Textile Res. 2010;35(1):59–64. [Google Scholar]
- 27.Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activitiesm. Adv Colloid Interface Sci. 2009;145(1-2):83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In vitro. 2005;19(7):975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri J B, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):23–46. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 30.Schrand AM, Braydich-Stolle LK, Schlager JJ, Dai L, Hussain SM. Can silver nanoparticles be useful as potential biological labels? Nanotechnology. 2008;19(23):235104. doi: 10.1088/0957-4484/19/23/235104. [DOI] [PubMed] [Google Scholar]
- 31.Braydich-Stolle LK, Hussain SM, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88(2):412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon VKM, Burd A. In vitro cytotoxicity of silver: implication for clinical wound care. Burns. 2004;30(2):140–147. doi: 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Wataha JC, Lockwood PE, Schedle A. Effect of silver, copper, mercury and nickel on cellular proliferation during extended, low-dose exposures. J Biomed Mater Res. 2000;52(2):360–364. doi: 10.1002/1097-4636(200011)52:2<360::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Panyala NR, Pena-Mendez EM, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? J App Biomed. 2008;6:117–129. [Google Scholar]
- 35.Liu Z W, Ren G G, Zhang T, Yang Z. Action potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal CA1 neurons by silver nanoparticles. Toxicology. 2009;264(3):179–184. doi: 10.1016/j.tox.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Raveendran P, Fu J, Wallen SL. A simple and ‘‘green’’ method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006;8:34–38. [Google Scholar]
- 37.Emami-Forushania A, Azizi E, Zeinali S, Ostad SN. Cross-resistance to vincristin and etoposide in a sub line of the human breast cancer T47D cells selected for adriamycin-resistance. Iranian J Pharm Res. 2004;3(2):103–107. [Google Scholar]
- 38.Freshney R I. Culture of Animal Cells; a Manual of Basic Techniques. 2nd ed. New Jersey: Wiley-VCH Publications; 2000. [Google Scholar]
- 39.Sastry M, Mayya K S, Bandyopadhyay K. pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf A: Physicochem Eng Asp. 1997;127(1-3):221–228. [Google Scholar]
- 40.Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Physics B. 2001;299(1-2):142–146. [Google Scholar]
- 41.Okamoto I, Iwamoto K, Watanabe Y, Miyake Y, Ono A. Metal-ion selectivity of chemically modified uracil pairs in DNA duplexes. Chem Int Ed. 2009;48(9):1648–1651. doi: 10.1002/anie.200804952. [DOI] [PubMed] [Google Scholar]
- 42.Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett. 2008;3(4):129–133. [Google Scholar]
- 43.Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharm. 2008;233(3):404–410. doi: 10.1016/j.taap.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Rahman F, Wang J, Patterson TA, Saini UT, Robinson B, Newport GD, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187(1):15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Gopinath P, Gogoi SK, Chattopadhayay A, Gosh SS. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. J Nano-technology. 2008;19(7):075104. doi: 10.1088/0957-4484/19/7/075104. [DOI] [PubMed] [Google Scholar]
- 46.Zolghadri S, Sabouri AA, Golestani A, Divsalar A, Rezaei-Zarchi S, Moosavi-Movahedi AA. Interaction between silver nanoparticle and bovine hemoglobin at different temperatures. J Nanopart Res. 2009;11(7):1751–1758. [Google Scholar]
- 47.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 48.Ilker I, Yeşilel OZ, Günay G, Büyükgüngör O. Dinuclear and polynuclear silver (I) saccharinate complexes with 1,3-diaminopropane and N-methylethylenediamine constructed from Ag … C interactions. J Organomet Chem. 2009;694(26):4178–4184. [Google Scholar]
- 49.Jin CM, Chen ZF, Mei HF, Shi XK. Ag (I) coordination polymers with flexible bis-imidazole ligands: 2D interwoven structure and wavy layer network based on silver–silver interactions. J Mol Struct. 2009;921(1-3):58–62. [Google Scholar]
- 50.Shulka J, Pitre KS. Role of bio-metal Fe (III) in anticancer behavior of tamoxifen. Indian J Exp Biol. 1999;37(5):429–433. [PubMed] [Google Scholar]
- 51.Galanzha EI, Shashkov EV, Kelly T, Kim J, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumor cells. Nat Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agasti SS, Chompoosor A, You C, Ghosh P, Kim CK, Rotello VM. Photoregulated release of caged anticancer drugs from gold nanoparticles. J Am Chem Soc. 2009;131(16):5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lok C, Ho C, Chen R, He Q, Yu WY, Sun H, et al. Silver nanoparticles: partial oxidation and anti-bacterial activities. J Biol Inorg Chem. 2007;12(4):527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 54.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Res Crit Care. 2005;172:1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napier ME, DeSimone JM. Nanoparticle drug delivery platform. Polym Rev. 2007;47(3):321–327. [Google Scholar]
- 56.Williams P S, Carpino F, Zborowski M. Magnetic nanoparticle drug carriers and their study by quadrupole magnetic field-flow fractionation. Mol Pharmaceut. 2009;6(5):1290–1306. doi: 10.1021/mp900018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karlsson HL, Cronholm P, Gustafsson J, Möller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21(9):1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]