Abstract
Hepatitis B virus (HBV) infection is the 10th leading cause of death worldwide. The most important diagnostic and screening marker for HBV infection is Hepatitis B surface antigen (HBsAg), and the most widely used HBsAg screening test is Enzyme-linked Immunosorbent Assay (ELISA). In this study, an ELISA assay has been developed for detection of HBsAg using two novel monoclonal antibodies (mAb) as capture layer and a polyclonal biotinylated Ab as detector phase. We evaluated the sensitivity, specificity, detection limit, seroconversion time, positive and negative predictive values and reproducibility of our assay with standard panels and different serum samples. The results were compared with a well established commercial kit. Both assays showed similar detection limit values of 0.5 to 0.7 ng/ml and the same seroconversion periods of 42 and 65 days for “ad” and “ay” serotypes of HBsAg, respectively. Sensitivity and specificity of the assay were 98.98% and 99.6%, respectively. The positive and negative predictive values of our assay were also calculated as 99.49% and 99.2%, respectively. Analysis of reproducibility of the present assay demonstrated 3.96% and 5.85% intra-and inter-assay coefficient of variations, respectively, which were less than those obtained by the commercial kit. There was a highly significant correlation between our designed assay and the commercial ELISA kit (p < 0.0001, r = 0.957). Altogether, our results indicate that the designed assay is comparable to the commercial kit in terms of sensitivity, specificity, positive and negative predictive values and reproducibility and could be employed for diagnosis of HBV infection in blood samples.
Keywords: ELISA, Hepatitis B, Hepatitis B surface antigens, Monoclonal antibody
Introduction
Hepatitis B virus (HBV) infection is a global health issue and it is estimated that approximately 360 million people are chronic carriers of the HBV (1, 2). HBV has a double stranded DNA encoding for P, X, core and surface proteins (3). In the course of infection, an array of these proteins may be detected in the blood. Amongst these proteins, Hepatitis B surface antigen (HBsAg) is the earliest indicator of infection which may identify infected people before appearance of clinical symptoms (4). During the recovery period, HBsAg disappears from the blood; however, this protein remains positive in chronic carriers (5, 6). Hence, HBsAg detection is the most important approach to determine chronic and acute HBV infection.
Antibodies against HBV proteins are other immunological markers of infection, of which anti hepatitis B core antigen (anti-HBc) appears shortly after HBsAg and is an important marker of past or present HBV infection (7).
Both serological and molecular screening tests are employed for the diagnosis and monitoring of HBV infection (8). Although molecular Nucleic Acid Testing (NAT) is preferable in terms of its simplicity, rapidness and sensitivity (9, 10); yet for blood screening and diagnosis, especially in some cases of occult HBV (11, 12) that NAT may miss some positive samples, there is a room to improve serological assays (13). The ability of NAT to decrease window period is controversial (14, 15). It is also shown that NAT sensitivity is affected by viral load and pool size and it is too costly to test individual samples by NAT (16). Even by using individual HBV NAT, serological screening may be necessary to prevent some HBV transmission through blood transfusion (9, 16).
Among all HBsAg assays, enzyme-linked immunosorbent assays (ELISA) are most frequently used because of their sensitivity and cost-effectiveness (17). In the blood banks of all developed countries screening for hepatitis B virus (HBV) using enzyme immunoassay (EIA) is mandatory (18).
Recent studies have revealed that quantitative monitoring of serum HBsAg may be used as a novel tool for the assessment of antiviral therapy efficacy, because there is a correlation between concentration of HBsAg and HBV-DNA (19–21). Seroconversion period is one of the most important features of the HBsAg assays. This period has been estimated to be 35 days by individual sample NAT, 41 days by minipool NAT, and 50 days by highly sensitive HBsAg immunoassays (22).
The other important characteristic of the ELISA assays which needs continuous improvement is their capability to detect mutant HBV isolates by employment of new monoclonal antibodies (mAbs) specific for HBsAg (22). In the present study, a sensitive ELISA was developed by incorporating two novel mAbs which have recently been shown to detect a variety of mutant HBsAgs from all major subtypes (23).
Materials and Methods
Antibody production and conjugation
Nine murine monoclonal antibodies (mAbs) and rabbit polyclonal antibodies against recombinant adw subtype of HBsAg (Heberbiovac, Cuba) were produced, purified, characterized, conjugated with Horseradish Peroxidase (HRP) and biotinylated as previously described (23, 24).
Reactivity pattern of mAbs with three different HBsAg subtypes
To study interaction between ayw, adr and adw subtypes of HBsAg with mAbs raised against adw subtype, an indirect ELISA was performed. Polystyrene plates (Maxisorp, Nunc, Denmark) were coated in triplicate with 1 µg/ml of rHBsAg diluted in phosphate buffered saline (PBS, 0.15 M, pH=7.2) for 90min at 37 °C. Three wells were coated with 1µg/ml of Bovine Serum Albumin (BSA, Sigma, USA) as negative control.
After washing with PBS containing 0.05% Tween 20 (Sigma, USA) and blocking with PBS containing 3% skim milk, 10 µg/ml of mAbs were added to the plate followed by washing steps. Appropriate dilution of Horseradish Peroxidase (HRP)-conjugated sheep anti-mouse Ig (prepared in our lab) was then added and reaction revealed with O-phenylendiamine dihydrocloride (OPD, Sigma) as substrate. HRP-conjugated sheep anti mouse was added to wells coated with rHBsAg (with out mAbs) to determine background reaction. Finally, the reaction was stopped with 20% H2SO4 and Optical Density (OD) measured by a multiscan ELISA reader (Organon Teknika, Boxtel, The Netherlands) at 492 nm.
Design of ELISA for determination of HBsAg
Polystyrene microtiter plates were coated in triplicate with 100 µl of 10 µg/ml of unlabelled mAbs diluted in PBS for 90 min at 37 °C. Three wells were coated with 3% skim milk as negative control. After washing with PBS and blocking with PBS containing 3% skim milk, different dilutions of purified rHBsAg or serum samples were added to the plates followed by washing steps. Appropriate dilutions of biotinylated anti-HBsAg mAbs or polyclonal antibodies were then added. After washing, appropriate dilution of HRP-conjugated Strepavidin (Sigma, USA) was sub-sequently added and the reaction revealed using 3,3’,5,5’-Tetramethyl Benzidine (TMB, Sigma, USA) as substrate. HRP-conjugated Strepavidin was added to wells coated with unlabelled mAbs (without rHBsAg or serum samples) to account for background reactivity. Finally, the reaction was stopped with 20% H2SO4 and OD measured at 450 nm.
Determination of the detection limit of the assay
Detection limit of the designed assay was evaluated using decreasing concentrations of three serotypes of purified HBsAg (“ayw”, “adr” and “adw”). A standard sensitivity panel (PHA 807, BBI Co, USA) was also employed to determine sensitivity of our designed ELISA assay. Two standard seroconversion panels (PHM 912 and PHM 916, BBI Co, USA) were used to determine the earliest seroconversion time which could be identified by this assay. Samples included in the three standard panels were undiluted sera collected at different time points from subjects infected with “ay” or “ad” HBs serotype at early stage of infection.
Assay validation and statistical analysis
Specificity, sensitivity, positive predictive value and negative predictitive value of the designed ELISA assay were determined using three collections of human sera including 198 HBsAg positive, 250 HBsAg negative and 100 unselected samples collected from the Iranian Blood Transfusion Organization and referral medical labs, respectively. Seropositivity for HBsAg was approved by a commercial ELISA kit employed as a gold standard (Hepanostika; bioMerieux, France). Intra- and inter-assay variations were determined using two pooled HBsAg positive sera. Then, Intra- and inter-assay variations were calculated using the formula:
where CV= coefficient of variation, SD= standard deviation and X=mean. Correlation between the results of the designed assay and the commercial kit was determined by Spearman's correlation test and values of less than 0.05 were considered significant.
Results
Reactivity pattern of HBsAg –specific mAbs with HBsAg serotypes
Nine HBsAg-specific mAbs were selected and evaluated in this study. Specificity and immunochemical characteristics of the selected mAbs have previously been described (23, 24). The results of reactivity pattern of these mAbs with “adw”, “ayw” and “adr” serotypes are summarized in Table 1.
Table 1.
Reactivity pattern of the selected mAbs with different HBsAg serotypes
| Monoclonal antibody | Antibody isotype | Antibody affinity (M−1) | HBsAg serotype | ||
|---|---|---|---|---|---|
|
| |||||
| adw | ayw | adr | |||
| 34D7/S3 | IgG1 | 4.87×108 | 1.76 | 0.93 | 0.95 |
| 33D7/S4 | IgG1 | 3.38×108 | 1.88 | 1 | 1 |
| 38F3/S6 | IgG1 | 2.73×108 | 1.47 | 0.79 | 1 |
| 37C5/S7 | IgG1 | 4.2×108 | 1.41 | 0.95 | 1.1 |
| 35C6/S11 | IgG1 | 2.87×108 | 1.64 | 1 | 1.2 |
| 40B9/S13 | IgG1 | 2.82 ×108 | 1.38 | 0.35 | 0.32 |
| 31D4/H12 | IgG1 | 1.5×108 | 0.97 | 0.83 | 0.93 |
| 48A4/H14 | IgG1 | 6.6×108 | 1.9 | 0.29 | 0.25 |
| 35G9/S15A | IgG1 | 1.77×108 | 1.85 | 1 | 1 |
| NE3 | IgG1 | 1.6 | 0.76 | 0.56 | |
| NE5 | IgG2a | 1.4 | 0.5 | 0.74 | |
The results represent OD values
NE3 and NE5 are commercial anti-HBs mAbs recognizing the “a” determinant (IbtGMbh aspenhouster, Germany) which have been employed as control
Selection of capture and detection mAbs
To identify the best combinations of mAbs to be used as capture and detector pair in our designed ELISA assay, we checked mAbs which had high reactivity to rHBsAg as solid phase (unlabelled capture) and liquid phase (biotynilated detector) Abs pairwise (data not presented). Based on our results, “34D7/S3” and “33D7/S4” were selected as suitable candidates for detection and “37C5/S7” and “35C6/S11” as capture mAbs.
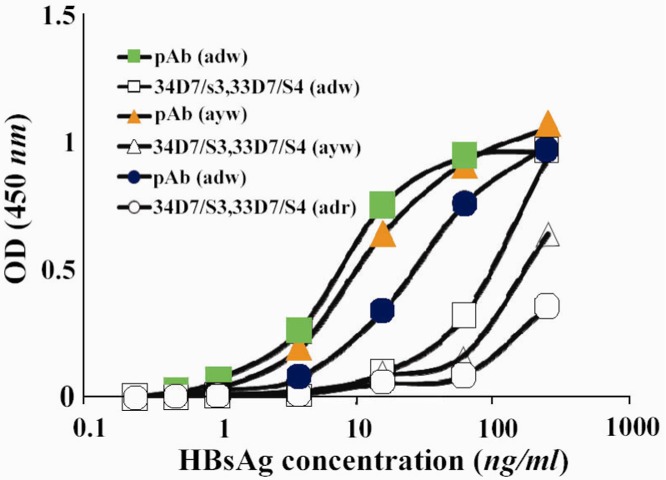
Reactivity of the two selected detector mAbs was compared with purified polyclonal anti-HBsAg antibodies (pAbs). Appropriate concentrations of biotynilated “34D7/S3” and “33D7/S4” mAbs were mixed and compared with pre-selected concentration of the biotinilated pAb. As illustrated in Figure 1, biotinilated anti-HBsAg pAb displayed substantially better reactivity than the combination of the two selected mAbs. Thus, biotynilated pAb was subsequently employed as detector probe in our designed ELISA assay.
Figure 1.
Comparison between reactivity of a combination of the two selected mAbs and polyclonal antibody as detector phase in the designed ELISA assay pAb: polyclonal antibody; mAb: monoclonal antibodies (a combination of 34D7/S3 and 33D7/S4 mAbs)
Detection limit of the designed ELISA assay
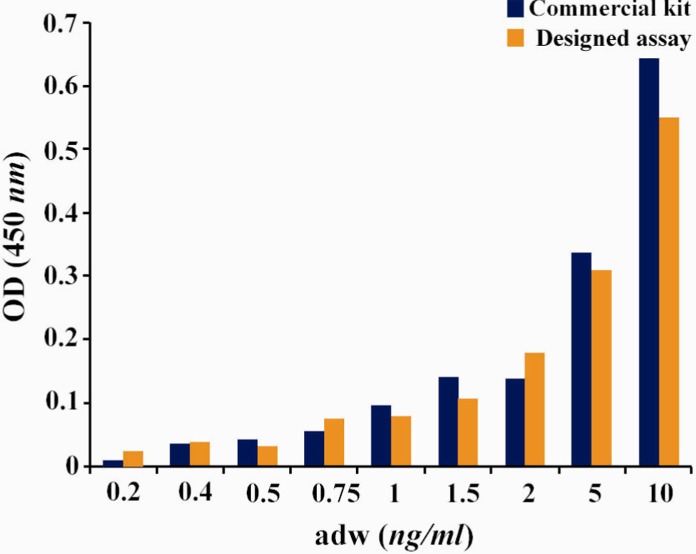
Using this assay, down to 0.5 ng/ml of HBsAg was detectable (Figure 2). Using the standard BBI sensitivity panel, this value was 0.7 and 0.5 ng/ml for “ad” and “ay” subtypes of HBsAg, respectively (Table 2). These results are comparable to the commercial ELISA kit employed in this study.
Figure 2.
Comparison of detection limit of the designed assay and commercial kit
Table 2.
Determination of detection limit of the designed ELISA assay using the standard BBI sensitivity panel
| Sensitivity panel | HBsAg Subtype | Concentration (ng/ml) | Designed assay (OD) | Commercial kit (OD) |
|---|---|---|---|---|
| PHA 807-01 | ad | 3.1 | 0.28 | 0.45 |
| 2 | ad | 1.5 | 0.27 | 0.35 |
| 3 | ad | 1 | 0.24 | 0.21 |
| 4 | ad | 0.9 | 0.21 | 0.19 |
| 5 | ad | 0.7 | 0.19 | 0.16 |
| 6 | ad | 0.5 | 0.2 | 0.15 |
| 7 | ad | 0.4 | 0.18 | 0.14 |
| 8 | ad | 0.3 | 0.18 | 0.15 |
| 9 | ad | 0.2 | 0.18 | 0.13 |
| 10 | ad | 0.1 | 0.18 | 0.13 |
| 11 | ay | 2.6 | 0.3 | 0.33 |
| 12 | ay | 1.3 | 0.28 | 0.23 |
| 13 | ay | 0.9 | 0.24 | 0.2 |
| 14 | ay | 0.8 | 0.22 | 0.19 |
| 15 | ay | 0.6 | 0.21 | 0.16 |
| 16 | ay | 0.5 | 0.2 | 0.16 |
| 17 | ay | 0.4 | 0.19 | 0.15 |
| 18 | ay | 0.3 | 0.19 | 0.12 |
| 19 | ay | 0.2 | 0.18 | 0.14 |
| 20 | ay | 0.1 | 0.19 | 0.11 |
| 21 | Neg | 0 | 0.15 | 0.1 |
Samples with OD≥ mean of triplicate Neg (sample NO.21)+0.05 were considered reactive
Determination of seroconversion time
Two BBI standard seroconversion panels were used to determine the earliest serocon-version time identifiable by the designed assay. Infection with “ad” and “ay” serotypes of HBV could be identified as early as 42 and 65 days post infection, respectively (Table 3). Similar results were obtained by the commercial kit.
Table 3.
Determination of the seroconvertion time of HBV infection by the designed ELISA assay using two BBI standard seroconvertion panels
| Seroconvertion panels | HBs Ag subtype | Cocentration (ng/ml) | Post infection (Day) | Designed assay (s/c) | Commercial kit (s/c) |
|---|---|---|---|---|---|
| PHM 912-01 | ad | <0.01 | 0 | 0.8 | 0.7 |
| 2 | ad | <0.1 | 5 | 0.9 | 1 |
| 3 | ad | <0.1 | 7 | 0.8 | 0.9 |
| 4 | ad | <0.1 | 12 | 0.8 | 0.6 |
| 5 | ad | <0.1 | 17 | 0.8 | 0.5 |
| 6 | ad | <0.1 | 20 | 0.8 | 0.4 |
| 7 | ad | 0.1 | 24 | 0.8 | 0.5 |
| 8 | ad | >2.6 | 42 | 1.5 | 3.6 |
| 9 | ad | >2.6 | 47 | 2.5 | 10.7 |
| PHM 916-01 | ay | <0.1 | 0 | 0.6 | 0.6 |
| 2 | ay | <0.1 | 7 | 0.8 | 0.7 |
| 3 | ay | <0.1 | 41 | 0.9 | 0.6 |
| 4 | ay | <0.1 | 43 | 0.8 | 0.8 |
| 5 | ay | <0.1 | 48 | 0.8 | 0.8 |
| 6 | ay | <0.1 | 50 | 0.8 | 0.7 |
| 7 | ay | <0.1 | 55 | 0.8 | 0.8 |
| 8 | ay | <0.1 | 57 | 0.8 | 0.8 |
| 9 | ay | 0.2 | 62 | 0.8 | 0.7 |
| 10 | ay | 0.5 | 65 | 1.2 | 1.2 |
| 11 | ay | 2.3 | 69 | 1.34 | 2.2 |
Samples with signal-to cutoff (s/c)>1.0 were considered reactive
Specificity and sensitivity of the assay
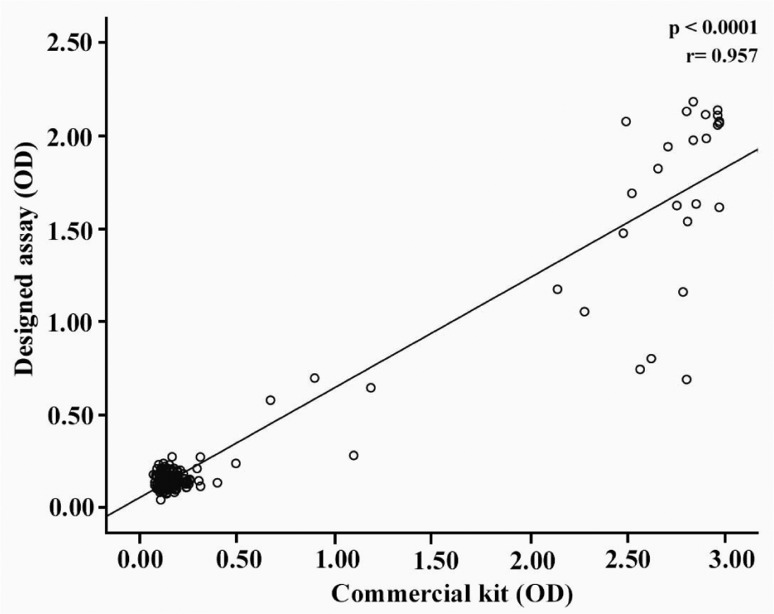
Specificity and sensitivity of the designed assay were determined using two panels of positive (n=198) and negative (n=250) serum samples and a panel of unselected samples (n=100). The results are presented in Table 4. Based on these results, sensitivity and specificity of our designed assay was found to be 98.98% and 99.6%, respectively. The positive and negative predictive values of the assay were also calculated as 99.49% and 99.2%, respectively. A highly significant correlation was observed between the designed assay and the commercial kit (p<0.0001) (Figure 3).
Table 4.
Reactivity of the designed ELISA assay with different panels of serum samples
| Samples | Designed assay | Commercial kit | ||
|---|---|---|---|---|
|
| ||||
| Positive | Negative | Positive | Negative | |
| Positive panel (n=198) | 196 | 2 | 198 | 0 |
| Negative panel (n=250) | 1 | 249 | 0 | 250 |
| Unselected panel (n=100) | 7 | 93 | 7 | 93 |
Unselected panel refers to serum samples collected from different referral hepatitis diagnostic laboratories without prior information about their hepatitis B status. Positive and negative panels were collected from the Iranian Blood Transfusion Organization based on reactivity with the commercial ELISA kit which was employed as a gold standard
Figure 3.
Correlation between the results obtained by the designed ELISA assay and commercial kit
Assay reproducibility
Assay variation was checked using two serum samples with different concentrations of HBsAg. Each sample was run eight times to calculate the Coefficient of Variation (CV). Intra-assay and inter-assay CV were found to be 3.96% and 5.85% for the designed assay and 4.5% and 9.29% for the commercial kit, respectively.
Discussion
Improvement of immunoassays for detection of viral infections, particularly HBV infection, has always been considered as a health priority, due to emergence of mutant isolates and the need to early diagnosis of the disease. Both requirements are achieved by employment of new mAbs with improved affinity and specificity. In the present study, novel HBsAg-specific mAbs were employed to develop a sensitive sandwich ELISA for detection of HBsAg. Different combinations of the mAb which had high reactivity with rHBsAg (Table 1) were applied to select the best capture and detector pairs.
Our results showed that “37C5/S7” and “35C6/S11” were the most active capture mAbs and “34D7/S3” and “33D7/S4” were the best detector mAbs (data not presented). Interestingly, the two capture mAbs have already been shown to recognize linear epitopes within the “a” region of HBsAg, making them more suitable targets for detection due to their stability (23, 24).
Using this combination of mAbs gave a detection limit of about 4-15.6 ng/ml, employing different serotypes of HBsAg (Figure 1). In comparison to the detection limit recommended by the Food and Drug Administration of USA (FDA) (0.5 ng/ml) (23), the sensitivity of the developed ELISA was not acceptable. Thus, we prepared affinity purified HBsAg-specific pAb and used the biotinylated pAb instead of the mAbs, as detector phase.
This strategy enables recognition of several epitopes on the antigen surface by the pAb which would minimizes the possibility of Ag escape from detection system. Consequently, “37C5/S7” and “35C6/S11” were selected as capture and biotinylated pAb as detector antibodies.
In addition to the capture and detector antibodies, selection of suitable blocking agents plays an important role in terms of assay sensitivity and specificity. To optimize an ELISA, background signals should be minimized while specific signals must be maximized. Antigen-antibody reaction is site-directed and blocker agents cause more effective site-directed interactions, by inhibiting non-specific interactions (25). Our results showed that the best blocking agent was 3% skim milk (data not presented). For increasing specific signals, TMB instead of OPD was used as substrate, because OPD has less sensitivity and is carcinogenic (26). These strategies improved the detection limit of our designed assay to 0.7 and 0.5 ng/ml for “ad” and “ay” subtypes of HBsAg, respectively. This detection limit is comparable to many approved commercial kits (27).
Seroconversion occurs after a subclinical incubation period required for establishment of viral infection. Detection of Ag or Ab sero-conversion is the most important tool for specific diagnosis of the infection. Therefore, reducing the seroconversion period is a vital aspect in designing diagnostic kits. Early detection of seroconversion is accompanied with reducing the risk of infection transmission by blood or blood components. Our designed ELISA could detect a seroconversion period of 42 and 65 days for “ad” and “ay” sero-types, respectively.
Comparison of this seroconversion time with those detected by other licensed commercial HBsAg assays shows comparable results. Bernard Weber and colleagues reported that “Auszyme Monoclonal B” and “IMx HBsAg” assays had the same seroconversion time i.e. 42 days for “ad” serotype (28). Concurrent screening of large panels of positive and negative serum samples by our designed assay and the commercial kit revealed sensitivity and specificity of 98.98% and 99.6%, respectively, for our assay. Specificity and sensitivity of our assay is not only comparable to other enzyme immunoassays, but it is also comparable to the more sensitive chemiluminescence immunoassays. In a comparative study by Ismail et al, specificity and sensitivity of “Ortho/ECi” assay were 100% and 97.4%, respectively. These parameters for Abbott assay were 99.7% and 96.8%, respectively (29).
Analysis of reproducibility of the designed assay demonstrated 3.96% and 5.85% intra- and inter-assay Coefficient of Variations (CV), respectively, which were less than those obtained by the commercial kit. These CV values are very similar to those reported for HBsAg-based ELISA assay (30). The positive and negative predictive values of the designed assay were also calculated as 99.49% and 99.2%, respectively, which are similar to those reported elsewhere (31).
Conclusion
In conclusion, the parameters validated for our designed ELISA assay, including detection limit, sensitivity, specificity, reproducibility, positive and negative predictive values as well as the highly significant correlation observed between this assay and the commercial ELISA kit (r= 0.957, p < 0.0001, Figure 3) indicate that this ELISA assay might be suitable for HBsAg detection and diagnosis of HBV infection.
Acknowledgement
This study was supported in part by a grant from Tehran University of Medical Sciences
References
- 1.Chen DS. Toward elimination and eradication of hepatitis B. J Gastroenterol Hepatol. 2010;25(1):19–25. doi: 10.1111/j.1440-1746.2009.06165.x. [DOI] [PubMed] [Google Scholar]
- 2.Glebe D. Recent advances in hepatitis B virus research: A German point of view. World J Gastroenterol. 2007;13(1):8–13. doi: 10.3748/wjg.v13.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokosuka O, Arai M. Molecular biology of hepatitis B virus: effect of nucleotide substitutions on the clinical features of chronic hepatitis B. Med Mol Morphol. 2006;39(3):113–120. doi: 10.1007/s00795-006-0328-5. [DOI] [PubMed] [Google Scholar]
- 4.Ganem D, Prince AM. Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 5.Hatzakis A, Magiorkinis E, Haida C. HBV virological assessment. J Hepatol. 2006;44(1):S71–S76. doi: 10.1016/j.jhep.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: An update for clinicians. Mayo Clinic Proc. 2007;82(8):967–975. doi: 10.4065/82.8.967. [DOI] [PubMed] [Google Scholar]
- 7.Urbani S, Fagnoni F, Missale G, Franchini M. The role of anti-core antibody response in the detection of occult hepatitis B virus infection. Clin Chem Lab Med. 2010;48(1):23–29. doi: 10.1515/CCLM.2010.002. [DOI] [PubMed] [Google Scholar]
- 8.Pawlotsky JM. Virologic techniques for the diagnosis and monitoring of hepatitis B. Gastroenterol Clin Biol. 2008;32(1 Pt 2):S56–S63. doi: 10.1016/S0399-8320(08)73266-4. [DOI] [PubMed] [Google Scholar]
- 9.Kuhns MC, Busch MP. New strategies for blood donor screening for hepatitis B virus - Nucleic acid testing versus immunoassay methods. Mol Diag Ther. 2006;10(2):77–91. doi: 10.1007/BF03256447. [DOI] [PubMed] [Google Scholar]
- 10.Niederhauser C, Mansouri Taleghani B, Graziani M, Stolz M, Tinguely C, Schneider P. Blood donor screening: how to decrease the risk of transfusion-transmitted hepatitis B virus? Swiss Med Wkly. 2008;138(9-10):134–141. doi: 10.4414/smw.2008.12001. [DOI] [PubMed] [Google Scholar]
- 11.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46(1):160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Vernet G. Molecular diagnostics in virology. J Clin Virol. 2004;31(4):239–247. doi: 10.1016/j.jcv.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer R. The ADVIA Centaur (R) infectious disease assays: a technical review. J Clin Virol. 2004;30(1):S1–S5. doi: 10.1016/j.jcv.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, et al. Comparative sensitivity of HBVNATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43(6):788–798. doi: 10.1046/j.1537-2995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa A, Gotanda Y, Itabashi M, Minegishi K, Kanemitsu K, Nishioka K. HBV NAT positive blood donors in the early and late stages of HBV infection: analyses of the window period and kinetics of HBV DNA. Vox Sang. 2005;88(2):77–86. doi: 10.1111/j.1423-0410.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 16.Sato S, Ohhashi W, Ihara H, Sakaya S, Kato T, Ikeda H. Comparison of the sensitivity of NAT using pooled donor samples for HBV and that of a serologic HBsAg assay. Transfusion. 2001;41(9):1107–1113. doi: 10.1046/j.1537-2995.2001.41091107.x. [DOI] [PubMed] [Google Scholar]
- 17.Shanmugham R, Thirumeni N, Rao VS, Pitta V, Kasthuri S, Singanallur NB, et al. Immunocapture enzyme-linked immunosorbent assay for assessment of in vitro potency of recombinant hepatitis B vaccines. Clin Vaccine Immunol. 2010;17(8):1252–1260. doi: 10.1128/CVI.00192-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novack L, Sarov B, Goldman-Levi R, Yahalom V, Safi J, Soliman H, et al. Impact of pooling on accuracy of hepatitis B virus surface antigen screening of blood donations. Trans R Soc Trop Med Hyg. 2008;102(8):787–792. doi: 10.1016/j.trstmh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Borgniet O, Parvaz P, Bouix C, Chevallier P, Trepo C, Andre P, et al. Clearance of serum HBsAg and anti-HBs seroconversion following antiviral therapy for chronic hepatitis B. J Med Virol. 2009;81(8):1336–1342. doi: 10.1002/jmv.21519. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Kim SJ, Ahn SH, Lee J, Park Y, Kim HS. Correlation between quantitative serum HBsAg and HBV DNA test in Korean patients who showed high level of HbsAg. J Clin Pathol. 2010;63(11):1027–1031. doi: 10.1136/jcp.2010.078832. [DOI] [PubMed] [Google Scholar]
- 21.Galli C, Orlandini E, Penzo L, Badiale R, Caltran G, Valverde S, et al. What is the role of serology for the study of chronic hepatitis B virus infection in the age of molecular biology? J Med Virol. 2008;80(6):974–979. doi: 10.1002/jmv.21179. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara N, Kusano O, Sugamata Y, Itoh T, Mizuii M, Tanaka J, et al. A novel hepatitis B virus surface antigen immunoassay as sensitive as hepatitis B virus nucleic acid testing in detecting early infection. Transfusion. 2009;49(3):585–595. doi: 10.1111/j.1537-2995.2008.02026.x. [DOI] [PubMed] [Google Scholar]
- 23.Roohi A, Yazdani Y, Khoshnoodi J, Jazayeri SM, Carman WF, Chamankhah M, et al. Differential reactivity of mouse monoclonal anti-HBs antibodies with recombinant mutant HBs antigens. World J Gastroenterol. 2006;12(33):5368–5374. doi: 10.3748/wjg.v12.i33.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roohi A, Khoshnoodi J, Zarnani AH, Shokri F. Epitope mapping of recombinant hepatitis B surface antigen by murine monoclonal antibodies. Hybridoma. 2005;24(2):71–77. doi: 10.1089/hyb.2005.24.71. [DOI] [PubMed] [Google Scholar]
- 25.Growther J. New Jersey: Human Press Inc; 2010. The ELISA guidebook. [Google Scholar]
- 26.Pekary AE, Turner LF, Jr, Hershman JM. New immunoenzymatic assay for human thyrotropin compared with two radioimmunoassays. Clin Chem. 1986;32(3):511–514. [PubMed] [Google Scholar]
- 27.Yang J, Kim JH, Kim Y. Comparison of nine different qualitative HBsAg assay kits. Korean J Lab Med. 2010;30(2):178–184. doi: 10.3343/kjlm.2010.30.2.178. [DOI] [PubMed] [Google Scholar]
- 28.Weber B, Bayer A, Kirch P, Schluter V, Schlieper D, Melchior W. Improved detection of hepatitis B virus surface antigen by a new rapid automated assay. J Clin Microbiol. 1999;37(8):2639–2647. doi: 10.1128/jcm.37.8.2639-2647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismail N, Fish GE, Smith MB. Laboratory evaluation of a fully automated chemiluminescence immunoassay for rapid detection of HBsAg, antibodes to HBsAg, and antibodies to hepatitis C virus. J Clin Microbiol. 2004;42(2):610–617. doi: 10.1128/JCM.42.2.610-617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leyva A, Franco A, Gonzalez T, Sanchez JC, Lopez I, Geada D, et al. A rapid and sensitive ELISA to quantify an HBsAg specific monoclonal antibody and a plant-derived antibody during their downstream purification process. Biologicals. 2007;35(1):19–25. doi: 10.1016/j.biologicals.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Toplikar E, Carlomagno A, Rojkin LF, Gariglio R, Lorenzo LE. Development of an enzyme Immuno-assay for the detection of Hepatitis-B surface antigen employing monoclonal antibodies. J Clin Lab Anal. 1993;7(6):324–328. doi: 10.1002/jcla.1860070605. [DOI] [PubMed] [Google Scholar]