Abstract
Collagens are the most abundant proteins in the human body. Their main function is to provide structural and mechanical support for the tissues, but they are also involved in a number of other biological functions including cell attachment, migration and differentiation. Collagens and gelatins are widely used in pharmaceutical and medical applications. Every year, more than 50,000 tons of collagen and gelatin are used in medical applications. These materials may have some viral and prion impurity and/or stimulate allergic response in human body. Therefore, scientists have produced human collagen in recombinant systems. In this study we have constructed two yeast shuttle vectors containing human procollagen genes expression cassette for expression in yeast. Total RNA was extracted from human skin fibroblast cell line, and cDNA synthesis was done by oligo dt. Then gene fragments were amplified from the cDNA with the necessary changes by Polymerase Chain Reaction (PCR). Finally they were cloned in yeast vector pPICZαA containing regulatory sequences for expressing and secreting the polypeptide product. Two yeast shuttle vectors containing human COL1A1 and COL1A2 expression cassettes were created. Final constructs were confirmed by enzymatic digestion, PCR of desired fragment and sequencing. The yeast shuttle vectors containing human COL1A1 and COL1A2 can be transferred into the yeast in the later stages to determine the scale of expression.
Keywords: Collagen, Fibroblasts, Yeasts
Introduction
Collagens are the most abundant protein in body, which support most of the tissues and form the cells (1). Collagen has high elasticity and is capable of forming connective tissues. Collagen is the major component in extra-cellular matrix of cartilage, tendons, bone and skin (2, 3). Individual characteristic of connective tissues are because of collagen structure, function and its correlation with other molecules (4). Collagen is essential for healthy and firm skin, so decrease in quantity of collagen in ageing skin leads to loss of elasticity. Collagen tightens the blood vessels and plays a role in tissue development (2).
Collagen molecules consist of three polypeptide chains, called α chains that are coiled around each other into a triple helix. These chains differ for each collagen type, collagen type I contains two α1 and one α2 chains (5). Distinct characteristic of collagen polypeptide chains is repeating triplet sequence Gly-X-Y, in which the X position amino acid is often proline and Y position amino acid is often 4-hydroxyproline (6). The collagen superfamily in vertebrates consists of at least 28 proteins encoded by 43 specific genes (7–9).
Collagen is widely used in medical and pharmaceutical applications such as cell scaffolds in tissue engineering, biomaterials in drug delivery, implants, hemostats, wound dressings, vascular graft coatings, capsules and tablets. Moreover, collagen has been used in cosmetic surgery, reconstructive bone surgery, dentistry, orthopedics and composition of artificial skin. In addition, denaturated and partially degraded collagen, gelatin, plays an important role in setting the weight and appetite reducing as a protein, and gelatin is also used in photography and as a gelling in food industry (10–14).
Sources of collagens and gelatins for these applications were extracted from animals, mainly from bovine and porcine skin. The use of animal derived collagen and gelatin comprise several concerns. These extracted proteins have the potential to cause immune reactions and may also carry disease causing contaminants such as viruses or prions (8, 9). Production of recombinant human collagen and gelatin could overcome this problem. Recombinant production systems are provided to generate engineered collagen products for different purposes (8).
Several expression systems have been tested in producing human recombinant collagens such as transfected mammalian cells, insect cells, yeast, Escherichia coli (E. coli), transgenic tobacco, mice, silkworms and barley (15–17). These experiences showed that yeast expression system is the best system for producing of collagen. Yeasts are single-celled microorganisms that are easy to manipulate and culture and are suitable for genetic research as a eukaryotic organism (15, 16).
Among the yeast systems, Pichia pastoris (P.pastoris) is highly successful for the production of a wide variety of recombinant proteins. Several types of collagen have been produced in P.pastoris (8). Since 1984, more than 300 proteins such as Hepatitis B surface antigen, Bovine herpes virus and FMD viral proteins have been successfully expressed in P.pastoris. Among these proteins, several types of collagen proteins are expressed with similar characteristics to human collagen (18, 19).
More than 50,000 metric tons of collagen and gelatin are used in research and medical applications annually (2). Considering the strategic importance of collagen in health and pharmaceutical industries, we embarked on recombinant production of collagen for the first time in Iran. In this study, the cDNA encoding COL1A1 and COL1A2 genes were cloned and the recombinant plasmids were analysed by restriction digestion and sequencing.
Materials and Methods
Vector design
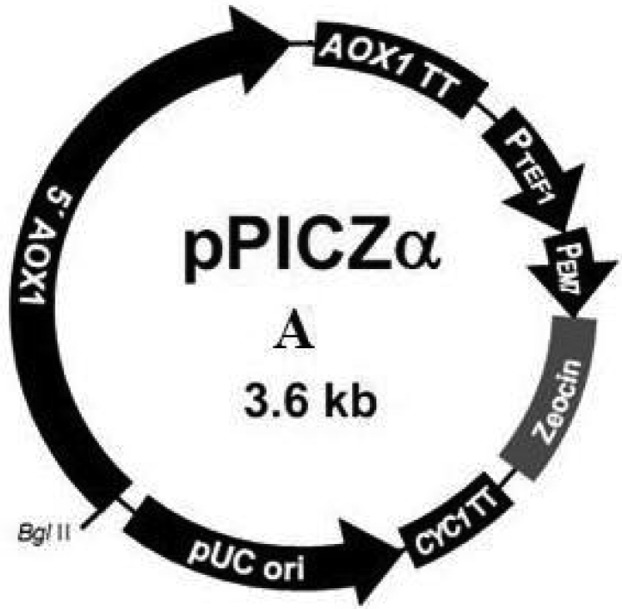
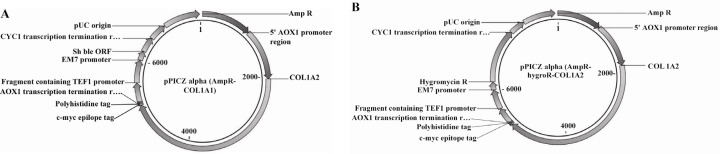
According to the objective of this study, pPICZαA vector was chosen as the final expression vector obtained from Recombinant Gene Bank of Iran (Pasteur Institute of Iran). This vector has the alcohol oxidase strong promoter. The AOX1 promoter has been utilized for production of heterologous protein in P.pastoris. Several copies of pPICZαA vector (Figure 1) are introduced into yeast genome, so it will have high expression of heterologous protein.
Figure 1.
pPICZα vector map
According to this vector, the restriction enzymes and primers were designed. To replace the collagen gene in frame, multiple cloning sites of vector was exchanged. In addition to Zeocin resistant gene, the Ampicillin resistant gene was also introduced into vector as a cheap bacterial selection marker. In order to generate a second construct, Pichia expression vector was modified by replacing Zeocin resistant gene with Hygromycin resistant gene. Gene Runner software was used to evaluate these gene fragments.
Fibroblast cultures
Human Skin Fibroblast Cell line (HSF) was purchased from National Cell Bank of Iran (NCBI). The cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, U.S.A.) supplemented with 10% fetal bovine serum (FBS) (Gibco, U.S.A.), 1% p/s (100 U/ml penicillin, 100 µg/ml streptomycin) and was incubated at 37°C in 5% CO2 incubator. Cultured cells were passaged using trypsin containing EDTA (Gibco, U.S.A.).
Total RNA extracted from fibroblast cells
Total RNA was isolated from fibroblasts using TRIZOL reagent (Gibco BRL). Fifty µl total RNA was extracted from 105 cells by trizol according to the manufacturer's instruction.
cDNA synthesis
Total cDNA was synthesized using the first strand cDNA synthesis kit (Fermentas, Germany) on total RNA. According to the protocol, total RNA was incubated for 5 min at 65°C at first because the desired genes were rich in %G + C and then 60 min incubation with MuLV reverse transcriptase at 42°C was done. The product of the first strand cDNA synthesis reaction was used directly in PCR.
Primer design and proliferation
The sequences were obtained from NCBI and primers were designed using Gene Runner software with required cut sites. These primers were constructed by Gene Fanavaran Company (Table 1).
Table 1.
List of used primers and PCR conditions
| Primer name | Sequence | Enzyme site | Fragment length | PCR conditions |
|---|---|---|---|---|
| F-COL1A2 | 5′GGTACCCAGTATGATGGAAAAGGAGTTGG3′ | KpnI | 3120 | Annealing: 60°C |
| R-COL1A2 | 5′AGATCTAGCCCTGTAGAAGTCTCCATC3′ | BglII | Extension: 6 min 10 s | |
| F-COL1A1 | 5′GAATTCCAGCTGTCTTATGGCTATG3′ | EcoRI | 3183 | Annealing: 68°C |
| R-COL1A1 | 5′AGATCTAGCCCGGTAGTAGCG3′ | BglII | Extension: 6 min 20 s | |
| F-Amp | 5′AGATCTTATATATGAGTAAACTTGGTCTGACAG3′ | BglII | 923 | Annealing: 45°C |
| R-Amp | 5′AGATCTAATAATATTGAAAAAGGAAGAGTATG3′ | BglII | Extension: 120 s | |
| F-Oligo | 5′TCGAGAAAAGAGAGGCTGAAGCTGGTACCTTT3′ | XhoI | ||
| R-Oligo | 5′CTAGAAAGGTACCAGCTTCAGCCTCTCTTTTC3′ | XbaI | ||
| F-Backbone | 5'AATCTTAAGCACGTCCGACGGCGGCCC 3' | AflII | 4120 | Annealing: 68°C |
| R-Backbone | 5'ATTAGCTAGCGGTTTAGTTCCTCACCTTGTCGTATTATACTATGC3' | NheI | Extension: 4 min 20 s | |
| F-Hygro | 5'ATTAGCTAGCATGAAAAAGCCTGAACTCA 3' | NheI | 1126 | Annealing: 56°C |
| R-Hygro | 5'ATACTTAAGCTATTCCTTTGCCCTCG 3' | AflII | Extension: 65 s |
COL1A1 and COL1A2 gene fragments were amplified by PCR from total cDNA. Ampicillin and Hygromycin resistant genes were also proliferated by PCR from pTZ57R and pCDNA3.1Hygro vectors, respectively.
Due to the long size of pPICZαA backbone, this gene fragment was amplified using Long PCR Enzyme Mix kit (Fermentas Co.). The size of this fragment was 4120 bp. At the end of the amplification, the size of the PCR product was determined to compare with 1 kb DNA marker, using 1% agarose gel electrophoresis followed by staining with ethidium bromide. Then, these PCR products were purified using DNA gel extraction kit (Fermentas) and then used for cloning.
Cloning steps
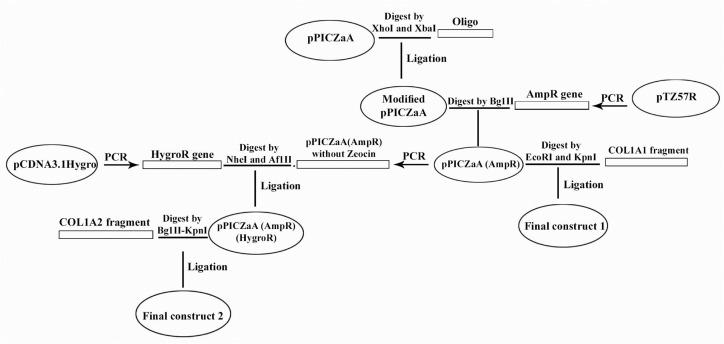
pPICZαA vector was digested with XhoI and XbaI restriction enzymes and was replaced by the previously designed oligonucleotide containing these cut sites. Then, pPICZαA vector was linearized with BglII and Amp R gene was ligated into the linearized vector. COL1A1 and COL1A2 fragments were flanked by artificial 5′_ EcoRI and 3′_ KpnI sites, and 5′_ KpnI and 3′_ BglII sites, respectively. Then, COL1A1 PCR products were digested with EcoRI and KpnI and co-ligated into EcoRI-KpnI digested pPICZαA, which led to the production of pPICZαA construct containing the COL1A1 gene.
In order to produce the second construct containing COL1A2, Hygromycin resistant gene and also pPICZαA(AmpR) which didn't include zeocin, were both flanked by artificial cut sites, and then digested with NheI and AflII. These two fragments were generated by PCR, such that the second one was extended from the last codon of the Zeocin resistant gene up to its initiation codon. Then, these PCR products were co-ligated; and ZeoR gene was replaced by Hygro R. Finally, COL1A2 was digested with BglII- KpnI and cloned into BglII-KpnI digested Amp R hygro R pPICZαA vector in order to generate the second construct.
All plasmids were transformed into the E.coli Top10F′ strain to proliferate. Bacteria were cultured in LB-Broth medium (Merck-Germany) containing 100 µg/ml ampicillin. Schematic diagram of cloning steps is shown in Figure 2.
Figure 2.
Schematic diagram of cloning steps
Confirmation
All clones and subclones were confirmed by clony PCR, digestion and sequencing. The constructs were directly sequenced by the dideoxy chain termination method (Gene Fanavaran Company) using designed primers.
Results
Design result
Final shuttle vector was designed so COL1A1 and COL1A2 genes were placed to correct the position of the share between the desired and correct translation frame in the two structures separately. In addition, the Ampicillin resistance gene was placed into BglII restriction site of pPICZαA vector and in the second structure of pPICZαA vector, Zeocin resistance gene was replaced by Hygromycin resistance gene. The size of Amp R gene was 923 bp and Hygro R gene previously proliferated by PCR was 1126 bp.
Total RNA extraction result
If total RNA is extracted properly, two bands in 28S and 18S related to ribosomal RNAs would be observed by gel electrophoresis. Electrophoresis of the product was determined with the Total RNA fully obtained (Figure 3).
Figure 3.
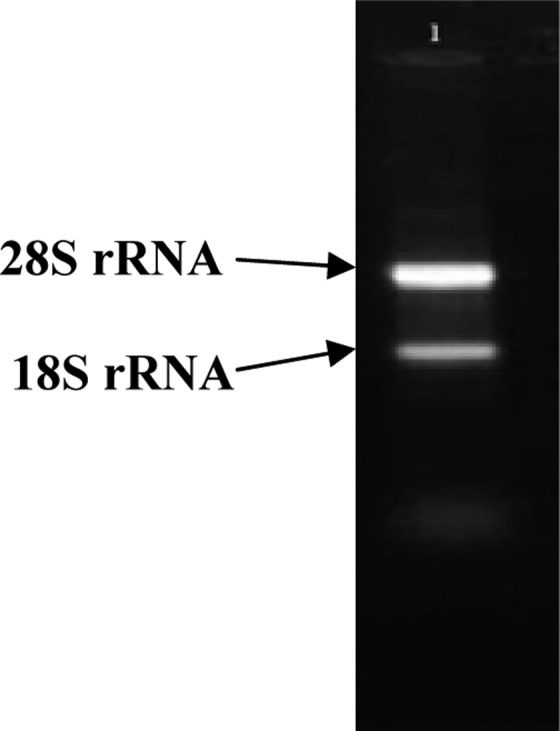
Total RNA electrophoresis. Upper band shows 28S rRNA and lower band shows 18SrRNA
cDNA synthesis and PCR results
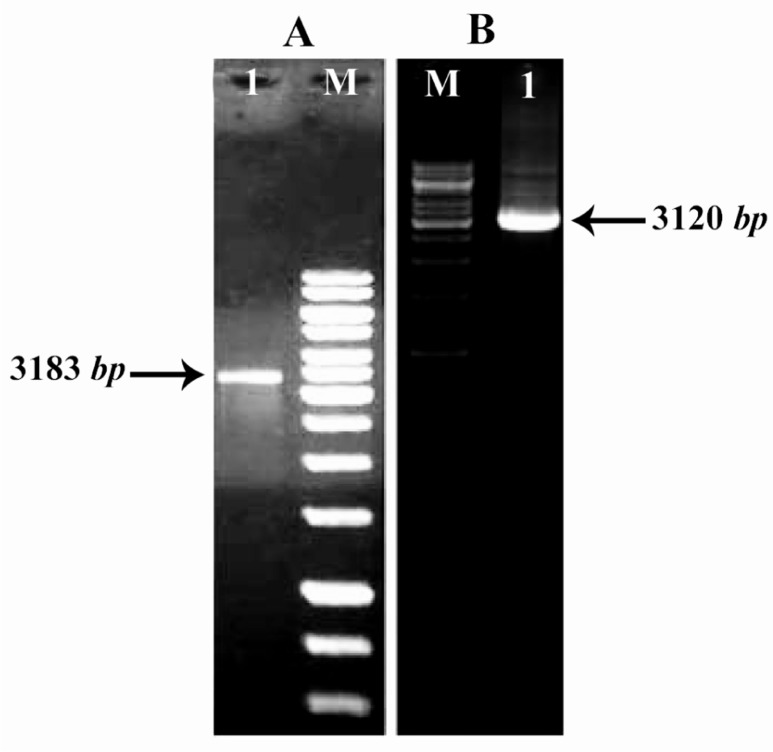
cDNA synthesis protocol properly was done, then complete cDNA was confirmed by PCR. COL1A1 and COL1A2 gene segments were amplified by PCR. The size of collagen fragments are 3183 bp (COL1A1) and 3120 bp (COL1A2) (Figure 4).
Figure 4.
Figure A) Lane M: 1 kb DNA ladder. Lane 1: PCR product of COL1A (3183 bp). Figure B) Lane M: DNA Ladder and lane 1 PCR product of COL1A2 (3120 pb)
Final shuttle vector confirmation
Final vectors were analyzed by digestion, PCR and sequencing. The relevant parts of all results indicated that all of these steps were performed correctly.
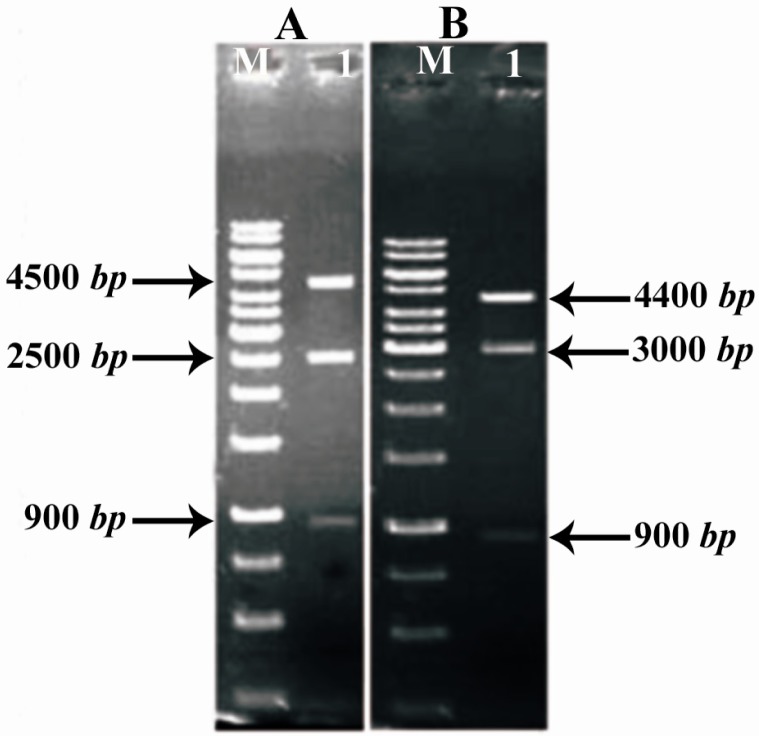
In regards to the three enzymes BglII cut sites existing in the final structure (two locations on both sides of AmpR gene and a location at the 3′ end of COL1A1 and COL1A2 genes), this enzyme was used to confirm the cloning. In cutting with this enzyme, the structure contains COL1A1; if the parts are correctly cloned, three bands of approximately 900 bp, 2500 bp and 4500 bp will be observed (Figure 5A) and in structure containing COL1A2, if the gene entering is correct, three bands of approximately 900 bp, 3000 bp and 4400 bp will be made (Figure 5B).
Figure 5.
Restriction analysis of pPICZα-COL1A1 and pPICZα-COL1A2 by BglII. A) Lane 1: Digestion of pPICZα-COL1A1. B) Lane 1: Digestion of pPICZα-COL1A2. Lane M: 1 kb DNA ladder
Results of enzymatic digestion with BglII, indicate correct entry into COL1A1 and COL1A2, so two vectors containing COL1A1 and COL1A2 were produced (Figures 6A and B). The results of sequencing were analyzed by chromas and were aligned by Mega4 and there were not any mutation, deletion or frame shift.
Figure 6.
Schematic picture of final constructs. Part A) shows final construct containing COL1A1 and part B) shows final construct containing COL1A2
Discussion
Due to the complexity of collagen biosynthesis, it is a challenge to produce it in recombinant systems. Besides, several post translational modifications are required and its expression in bacterial systems is not possible. Several systems are used to produce recombinant collagen and gelatin (2, 8). Several systems have been developed such as mammalian cells, insect cells, yeast and E.coli and transgenic animals such as mice, silkworms, tobacco and barley plants (15, 16).
In 1998, Fichard et al and Tyler et al began collagen production in cell lines, and despite their efforts, the collagen which was produced, did not have enough quality and due to the high cost of cell culture, it was not suitable for commercial production (20). The genomic fragment of collagen type I was cloned in mice by Toman in 1999 and the collagen was produced in mice milk. The resulted collagen had much lower hydroxy proline in its structure and was not desirable (21).
One year later Ruggiero cloned collagen type I gene into tobacco plants and collagen was expressed in tobacco (22), and Human collagen type I homotrimer was produced in tobacco by Merle, but in both of them, hydroxy proline amount was low (23). Human collagen type III was expressed in silkworm, but the produced collagen fragments were not hydroxylated, and collagen triple helix was not formed (24). Ritala tried to express collagen in barley cell cultures in 2008, but their efforts did not produce satisfactory results and triple helix did not formed (25).
Production in microorganism systems is the most desirable method of protein production. Thus, the efforts to produce collagen were carried out in microbial systems. In non-biological methods, production rate is much higher and the cost is lower (2, 8).
The efforts to produce human recombinant collagen in E.coli eventually succeeded to produce gelatin fragments (26). In 2000, recombinant human type I procollagen trimers was expressed in Saccharomyces cerevisiae (27). The best results were obtained when Vuorela et al expressed collagen type III in P.pastoris, in 1997 (9). Four years later collagen type I was produced as a complete triplet and with high yields in P.pastoris (12).
P.pastoris has the ability to express several foreign genes simultaneously, and this is one of the most important characteristics which is needed to produce collagen (28). Production of stable recombinant collagen triple helix structure requires prolyl 4-hydroxylase enzyme (29) and P.pastoris expressing prolyl 4-hydroxylase enzyme is engineered to produce high (g/l5/1-1) hydroxylated triple helixes of recombinant human collagen type I, II and III (8, 30). The recombinant human collagen produced in P.pastoris has a similar structure and content of hydroxy proline to compare with human wild collagen molecules (2). Therefore, this is why we have followed this system to produce collagen; thus, we have designed two yeast shuttle vectors containing the gene expression cassettes of human collagen type I.
Using optimized synthetic genes for expression in P.pastoris increased 25% in the amount of products (2). Researchers’ results showed that N-propeptide is not necessary for the collagen triple helix formation. When N-propeptide was separated from the gene, the triple helix formed and the amount of human recombinant collagen type III was increased about 25% in comparison to the presence of N-propeptide (31). These results indicate that the N-propeptides do not play a role in the accumulation of procollagen molecules within the endoplasmic reticulum lumen. Therefore, to make gene cassettes containing the coding sequences of α1 and α2 collagen chains, according to previous studies for optimizing gene, regions of the genes were amplified without N-propeptide ends using PCR.
Resistant to Zeocin antibiotic was the selective marker gene for COL1A1 construction. In order to determine entry of both final constructions into the yeast, COL1A2 construction's selection marker was changed by Hygromycin.
Conclusion
As already mentioned, by cell culture, we gained a number of human skin fibroblast cells. We extracted total RNA with sigma protocol from cells. After synthesis of cDNA, we amplified COL1A1 and COL1A2 fragments by designed primers. Two desired constructs were made by several clones and subclones. As mentioned above prolyl 4-hydroxylase is needed to produce stable collagen protein, so in this project we had to construct two additional vectors containing expression cassette of α and β genes of prolyl 4-hydroxylase. In future steps of this study, P.pastoris will be transformed by these four vectors. At the end, collagen expression will be characterized by SDS-PAGE and western.
Acknowledgement
Finally, the authors express their gratitude to Drs. Noori Inanlu, Ahangary, Movassagh and colleagues in B.C.G and Cell Bank of Pasture Institute of Iran for their cooperation during the procedure performed in the project.
References
- 1.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, et al. Engagement of collagen-binding integrins promotes matrix metallo-proteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67(5):2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 2.Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, et al. Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev. 2003;55(12):1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Koch FP, Yekta SS, Merkel C, Ziebart T, Smeets R. The impact of bisphosphonates on the osteoblast proliferation and collagen gene expression in vitro. Head Face Med. 2010;6:12. doi: 10.1186/1746-160X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akashi T, Minami J, Ishige Y, Eishi Y, Takizawa T, Koike M, et al. Basement membrane matrix modifies cytokine interactions between lung cancer cells and fibroblasts. Pathobiology. 2005;72(5):250–259. doi: 10.1159/000089419. [DOI] [PubMed] [Google Scholar]
- 5.Alvares K, Siddiqui F, Malone J, Veis A. Assembly of the type 1 procollagen molecule: selectivity of the interactions between the alpha 1(I)- and alpha 2(I)-carboxyl propeptides. Biochemistry. 1999;38(17):5401–5411. doi: 10.1021/bi9821824. [DOI] [PubMed] [Google Scholar]
- 6.Bernocco S, Finet S, Ebel C, Eichenberger D, Maz-zorana M, Farjanel J, et al. Biophysical characterization of the C-propeptide trimer from human procollagen III reveals a tri-lobed structure. J Biol Chem. 2001;276(52):48930–48936. doi: 10.1074/jbc.M108611200. [DOI] [PubMed] [Google Scholar]
- 7.Koch M, Veit G, Stricker S, Bhatt P, Kutsch S, Zhou P, et al. Expression of type XXIII collagen mRNA and protein. J Biol Chem. 2006;281(30):21546–21557. doi: 10.1074/jbc.M604131200. [DOI] [PubMed] [Google Scholar]
- 8.Baez J, Olsen D, Polarek JW. Recombinant microbial systems for the production of human collagen and gelatin. Appl Microbiol Biotechnol. 2005;69(3):245–252. doi: 10.1007/s00253-005-0180-x. [DOI] [PubMed] [Google Scholar]
- 9.Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T, Kivirikko KI. Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast pichia pastoris: formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J. 1997;16(22):6702–6712. doi: 10.1093/emboj/16.22.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutter M, Siepmann J, Hennink WE, Jiskoot W. Recombinant gelatin hydrogels for the sustained release of proteins. J Control Release. 2007;119(3):301–312. doi: 10.1016/j.jconrel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber RE, Blease K, Ambrosio A, Amburn E, Sosnowski B, Sampath TK. Bone induction by AdBMP-2/collagen implants. J Bone Joint Surg Am. 2005;87(5):1059–1068. doi: 10.2106/JBJS.D.02025. [DOI] [PubMed] [Google Scholar]
- 12.Nokelainen M, Tu H, Vuorela A, Notbohm H, Kivirikko KI, Myllyharju J. High-level production of human type I collagen in the yeast Pichia pastoris. Yeast. 2001;18(9):797–806. doi: 10.1002/yea.730. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Makareeva E, Kuznetsova NV, DeRidder AM, Sutter MB, Losert W, et al. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J Biol Chem. 2010;285:22276–22281. doi: 10.1074/jbc.M110.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31(9):2583–2591. doi: 10.1016/j.biomaterials.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Keizer-Gunnink I, Vuorela A, Myllyharju J, Pihla-janiemi T, Kivirikko KI, Veenhuis M. Accumulation of properly folded human type III procollagen molecules in specific intracellular membranous compartments in the yeast Pichia pastoris. Matrix Biol. 2000;19(1):29–36. doi: 10.1016/s0945-053x(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 16.Myllyharju J. Recombinant collagen trimers from insect cells and yeast. Methods Mol Biol. 2000;139:39–48. doi: 10.1385/1-59259-063-2:39. [DOI] [PubMed] [Google Scholar]
- 17.Eskelin K, Ritala A, Suntio T, Blumer S, Holkeri H, Wahlstrom EH, et al. Production of a recombinant full-length collagen type I alpha-1 and of a 45-kDa collagen type I alpha-1 fragment in barley seeds. Plant Biotechnol J. 2009;7(7):657–672. doi: 10.1111/j.1467-7652.2009.00432.x. [DOI] [PubMed] [Google Scholar]
- 18.Cereghino GP, Cereghino JL, Ilgen C, Cregg JM. Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris. Curr Opin Bio-technol. 2002;13(4):329–332. doi: 10.1016/s0958-1669(02)00330-0. [DOI] [PubMed] [Google Scholar]
- 19.Cregg JM, Cereghino JL, Shi J, Higgins DR. Re-combinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16(1):23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 20.Fichard A, Tillet E, Delacoux F, Garrone R, Rug-giero F. Human recombinant alpha1(V) collagen chain. Homotrimeric assembly and subsequent processing. J Biol Chem. 1997;272:30083–30087. doi: 10.1074/jbc.272.48.30083. [DOI] [PubMed] [Google Scholar]
- 21.Toman PD, Pieper F, Sakai N, Karatzas C, Platen-burg E, de Wit I, et al. Production of recombinant human type I procollagen homotrimer in the mammary gland of transgenic mice. Transgenic Res. 1999;8(6):415–427. doi: 10.1023/a:1008959924856. [DOI] [PubMed] [Google Scholar]
- 22.Ruggiero F, Exposito JY, Bournat P, Gruber V, Perret S, Comte J, et al. Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett. 2000;469(1):132–136. doi: 10.1016/s0014-5793(00)01259-x. [DOI] [PubMed] [Google Scholar]
- 23.Merle C, Perret S, Lacour T, Jonval V, Huda-verdian S, Garrone R, et al. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in trans-genic tobacco plant. FEBS Lett. 2002;515(1):114–118. doi: 10.1016/s0014-5793(02)02452-3. [DOI] [PubMed] [Google Scholar]
- 24.Tomita M, Munetsuna H, Sato T, Adachi T, Hino R, Hayashi M, et al. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol. 2003;21(1):52–56. doi: 10.1038/nbt771. [DOI] [PubMed] [Google Scholar]
- 25.Ritala A, Wahlstrom EH, Holkeri H, Hafren A, Makelainen K, Baez J, et al. Production of a recombinant industrial protein using barley cell cultures. Protein Expr Purif. 2008;59(2):274–281. doi: 10.1016/j.pep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg I, Salerno AJ, Patterson T, Williams JI. Cloning and expression of a collagen analog encoding synthetic gene in Escherichia coli. Gene. 1989;80(2):305–314. doi: 10.1016/0378-1119(89)90294-1. [DOI] [PubMed] [Google Scholar]
- 27.Toman PD, Chisholm G, McMullin H, Giere LM, Olsen DR, Kovach RJ, et al. Production of recombinant human type I procollagen trimers using a four-gene expression system in the yeast Saccharomyces cerevisiae. J Biol Chem. 2000;275(30):23303–23309. doi: 10.1074/jbc.M002284200. [DOI] [PubMed] [Google Scholar]
- 28.Hartner FS, Ruth C, Langenegger D, Johnson SN, Hyka P, Lin-Cereghino GP, et al. Promoter library designed for fine-tuned gene expression in Pichia pastoris. Nucl Acids Res. 2008;36(12):76. doi: 10.1093/nar/gkn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vuorela A, Myllyharju J, Pihlajaniemi T, Kari I. Coexpression with collagen markedly increases the half-life of the recombinant human prolyl 4-hydroxylase tetramer in the yeast Pichia pastoris. Matrix Biol. 1999;18(5):519–522. doi: 10.1016/s0945-053x(99)00040-2. [DOI] [PubMed] [Google Scholar]
- 30.Cregg JM, Tolstorukov I, Kusari A, Sunga J, Mad-den K, Chappell T. Expression in the yeast Pichia pastoris. Methods Enzymol. 2009;463:169–189. doi: 10.1016/S0076-6879(09)63013-5. [DOI] [PubMed] [Google Scholar]
- 31.Olsen DR, Leigh SD, Chang R, McMullin H, Ong W, Tai E, et al. Production of human type I collagen in yeast reveals unexpected new insights into the molecular assembly of collagen trimers. J Biol Chem. 2001;276(26):24038–24043. doi: 10.1074/jbc.M101613200. [DOI] [PubMed] [Google Scholar]