Abstract
Diagnosis of Toxoplasma gondii (T.gondii) infection is of great medical importance especially for pregnant women and immunosuppressed patients. Numerous studies have shown that the recombinant production of several toxoplasma antigens, including dense granule antigens (GRAs) has a great potential as diagnostic reagents. Previous studies reported expression of amino terminal GRA8 protein in fusion with large tags. In the present study, we produced truncated GRA8 (GRA8), excluded from the signal peptide and C-terminal transmembrane domain, with a short fusion tag in Escherichia coli (E.coli). GRA8 was purified using an optimized single-step Immobilized Metal ion Affinity Chromatography (IMAC). The purity and yield of GRA8 was highest at pH = 9.25. At this pH, 13.6 mg of GRA8 was obtained with the purity of 97.97%. Immunogenicity of the protein was evaluated in Western blot analysis showing the serum sample from a rabbit immunized with GRA8 recognized a single antigen of T.gondii tachyzoite at the expected molecular weight of native GRA8. To diagnosis acute toxoplasma infection in pregnant women, an indirect immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) was developed using GRA8 resulting in a test specificity and sensitivity of 97.1% and 60.6%, respectively. These results demonstrated that immunogenic GRA8 can be produced in fusion with a short tag and purified near to homogeneity using an optimized IMAC. GRA8-IgM-ELISA was useful for detection of acute toxoplasma infection.
Keywords: Enzyme-linked immunosorbent assay, Gene expression, GRA8 protein, Immunoglobulin M, Toxoplasma gondii
Introduction
Toxoplasmosis is caused by the protozoan parasite Toxoplasma gondii in a large variety of domestic and wild mammals and birds including human being (1). The infection generally is asymptomatic in immunocompetent individuals. However, in immunocompromised patients, such as those with HIV/ AIDS, infection can cause life-threatening encephalitis and can be fatal if not recognized and treated soon (2). Furthermore, since the parasite can cross the placenta, the primary maternal infection with T.gondii can be transmitted to the fetus and may lead to severe congenital defects such as hydrocephaly, mental retardation, chorioretinitis, visual impairment or even death in utero (2–5). In live-stock, abortion of ewes causes considerable economic losses (6).
Accurate diagnosis of primary toxoplasma infection and subsequent chemotherapy reduce the risk of congenital infection (2, 3). Detection of toxoplasma infection and distinction between acute and chronic phases of the infection are mainly based on serological tests enabling the detection of anti-toxoplasma immunoglobulin G (IgG) and IgM antibodies in blood (3).
The conventional serological tests are primarily based on whole T.gondii antigens which might have inconstant quality and external antigens from mouse peritoneal cavity or cell culture. The use of these antigen preparations makes standardization of the assays difficult and also results in insufficient specificities for differentiation between acute and chronic infection (3, 7, 8). The recombinant toxoplasma antigens, which can be easily produced in large amounts and with constant quality, are the good candidates for replacement of the crude antigens. Development of serological tests using recombinant toxoplasma antigens which are representative of acute or chronic infection is a present trend in discrimination between the acute and chronic infections (7–10).
The dense granule proteins (the GRA anti-gens) of T.gondii are secreted in abundance and constitute an important fraction of the antigens which circulate in the blood stream during the first hours following infection (11). These proteins are highly immunodominant and induce strong antibody response (11). Numerous studies have shown the diagnostic potential of several GRA antigens including GRA2, GRA4, GRA6, GRA7 and GRA8 for serodiagnosis of toxoplasma infection and discriminating acute from chronic infections (12–20).
Previous studies introduced GRA8 as a marker of acute infection and showed that IgG and IgM ELISA with recombinant GRA8 was able to differentiate acute from chronic infection (15, 18–20). The sequence of GRA8 is composed of 267 amino acids from which 64 are hydrophobic proline (24% overall). GRA8 contains an amino terminal signal peptide, three degenerate proline-rich repeats in the central region and a transmembrane domain near the carboxyl terminus (21). The hydrophobic transmembrane domain greatly reduces the bacterial expression level (22) and is usually excluded from the recombinant GRA8 (15, 16, 19, 20, 23). In the previous studies, GRA8 was expressed either in fusion with large fusion tags such as glutathione S-transferase (GST) (19, 23) and the CMP–2-keto-3-deoxy octulo-sonic acid synthetase (CKS) (15) or in fusion with a relatively small tag encoded by the pUET-1 plasmid (16).
Immobilized Metal Affinity Chromatography (IMAC) is one of the most popular techniques for the purification of recombinant proteins, which use the metal binding property of certain amino acids, in particular histidine (24–27). IMAC is usually applied as the initial capture step in purification scheme (26, 28). Many studies, however, showed proteins with high purity, e.g. >95%, can be obtained through proper optimization of single-step IMAC conditions (29, 30).
In the present study, we sought to produce the truncated GRA8, with a very small fusion tag and optimize the expression and purification of the protein. The pH optimized IMAC was applied for highly efficient purification of GRA8 and the purified protein was used in IgM Enzyme-Linked Immunosorbent Assay (ELISA) for distinguishing acute from chronic infection.
Materials and Methods
Parasite, bacterial strains and plasmid
T.gondii RH strain was used for the extraction of genomic DNA. Tachyzoites were injected into the peritoneal cavity of Swiss mice. Three days later, they were harvested from peritoneal fluid, washed with phosphate-buffered saline (PBS) and stored at -70 °C.
The E.coli DH5α strain (Invitrogen) was used for cloning and E.coli BL21 (DE3), BL21 (DE3) plysS and Rosetta (DE3) strains (Promega) were used for expression of the recombinant antigen. The pET-28b(+) (Novagen) plasmid was utilized for the construction of the expression system.
Serum samples
A total number of 68 serum samples from pregnant women, each sample corresponding to a woman (provided from three medical diagnostic laboratories in Tehran, Iran), were divided into 2 groups according to the results of the standard toxoplasma-specific IgG, IgM, and IgG avidity ELISA (Euroimmun, Lubeck, Germany). Group I (acute-phase sera) consisted of 33 serum samples collected from pregnant women with clinical signs of suspected acute toxoplasmosis. They were IgG and IgM positive and have low avidity of toxoplasma-specific IgG antibodies. Group II (chronic sera) were from 35 women with the positive IgG and negative IgM response.
Construction of the recombinant expression plasmid
Genomic DNA was extracted from the tachyzoites of T.gondii RH strain using the DNA extraction kit (Bioneer) and used as the template for the PCR amplification. A truncated fragment of gra8, encoding amino acid 23 to 169, was PCR amplified using a pair of specific primers (Forward primer: 5′-GCG GAT CCC GCC ATG AAC GGT CCT TTG AGT TAT-3′ and Reverse primer: 5′-GCC TCG AGA GCT GTC GTG GTT GTC GTA ACG TAG G-3′) designed based on the published sequence of gra8 on GenBank database (AF150729). Forward and reverse primers were designed to contain the BamHI and XhoI restriction sequences, respectively. PCR was performed as follows: 94 °C for 5 min, followed by 35 cycles at 94 °C for 1 min, 57°C (+ 0.1 °C increment per cycle) for 1 min, and 72 °C for 50 sec. The final extension was 7 min at 72 °C.
The PCR product was digested with BamHI and XhoI and inserted into the pET-28b(+) expression vector digested with the same enzymes. The resulting recombinant plasmid, containing the truncated sequence of gra8, was named pET-28b(+)-GRA8. The presence of the gra8 fragment in the recombinant plasmid was investigated by the EcoRV restriction enzyme digestion and DNA sequencing determination.
Production of GRA8
To obtain the recombinant GRA8, E.coli BL21(DE3), BL21(DE3)plysS and Rosetta (DE3) strains harboring the pET-28b(+)-GRA8 plasmid was cultivated in Luria Bertani (LB), 2XTY and Terrific broth (TB) media supplemented with 25 μg/ml kanamycin and 35 μg/ml chloramphenicol, where appropriate, at 37°C with shaking at 200 rpm. Expression of GRA8 was induced at OD600 of 0.6 by addition of various concentration of Isopropyl-β-D-Thiogalactopyranoside (IPTG) ranged from 0.1-1 mM. Following incubation at 30°C for an additional 5 hr, the induced bacteria were harvested by centrifugation at 6000 × g for 15 min. The expression of GRA8 was analyzed by 12% SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE).
To assess the solubility of GRA8, the pellet of 1 ml of induced bacteria was incubated in 100 μl of the lysis buffer (500 mM NaCl, 50 mM Tris-Cl, pH = 9.25, 1 mg/ml lysozyme) at 4°C for 30 min. The cells were lyzed by gentle vortexing and centrifuged at 16000 × g for 15 min at 4°C to separate the insoluble proteins and cell debris from the soluble ones. The pellet and supernatant were analyzed by SDS-PAGE.
Purification of GRA8
The purification of GRA8 was performed by IMAC using HisTrap FF columns (5 ml) (GE Healthcare) and the Bio-Rad FPLC instrument. The induced cells from 200 ml culture were collected by centrifugation and the resulting pellet was resuspended in 15 ml of binding buffer (500 mM NaCl, 50 mM Tris-Cl, 5 mM imidazole). The purification process performed at four pH values of binding buffer: 7.5, 8.1, 8.75 and 9.25. The cells suspension was sonicated for 6 min bursts at 200-300 W using an MSE ultrasonic disintegrator (Fisons). The cell lysate was subsequently centrifuged at 12000 × g at 4°C for 20 min. The supernatant was passed through a 0.2 μm (pore size) filter. The cleared lysate was applied to the column previously equilibrated with the binding buffer at a flow rate of 1 ml/min. The column was then washed step-wised with 10 column volumes of binding buffer until the absorbance at 280 nm of the effluent was minimal. Afterwards, the column was washed step-wised with 25 ml, 20 ml and 15 ml of the binding buffer containing 35, 60 and 90 mM of imidazole at 4 ml/min. Absorbed materiel was eluted at 1 ml/min with binding buffer containing 500 mM imidazole. The purified samples were analyzed on SDS-PAGE stained with Coomassie Blue G-250 as well as silver nitrate.
Rabbit immunization with GRA8
One rabbit was immunized i.m with 100 μg of the purified GRA8 emulsified in Freund's complete adjuvant. Injection was repeated twice with two weeks intervals using Freund's incomplete adjuvant. The serum sample was collected two weeks after the last immunization. The serum obtained from another rabbit injected with the adjuvant alone was taken as the control accordingly.
Western blotting analyses
To assess the immunogenicity of GRA8, Western blotting was performed using sera of the immunized and non-immunized rabbits. About 5×106 tachyzoites of the RH strain were loaded onto SDS-PAGE. The antigens then transferred onto the nitrocellulose membrane, blocked with 2% Bovine Serum Albumin (BSA) in PBS containing 0.1% Tween 20 (PBS-T) and probed with rabbit sera diluted 1:500 in blocking buffer. The membrane was washed three times with PBS-T and incubated with Horseradish Peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (Ray Bio-tech) diluted 1:500 in blocking buffer. Finally, the protein band was revealed by the peroxidase activity on 3, 3′-diaminobenzidine tetrahydrocholoride (DAB) substrate (Sigma) as the chromogenic substrate.
Furthermore, to assess the immunoreactivity of GRA8, Western blotting was performed using pooled sera from ten pregnant women with acute toxoplasma infection and ten women with chronic infection. The purified GRA8 antigen was analyzed by SDS-PAGE and transferred onto the nitrocellulose membrane. The membrane was blocked, as explained above, and probed with acute and chronic pooled sera diluted 1:100 in blocking buffer. Bound antibodies were detected using HRP-conjugated goat anti-human IgG and IgM antibodies (Sigma) diluted 1:2000 and 1:1000 in blocking buffer, respectively.
Enzyme-linked immunosorbent assay
Maxisorp microtiter ELISA plates (Maxisorp, Nunc, Roskilde, Denmark) were coated overnight at 4°C with 4 µg/ml of GRA8 in 50 mM carbonate buffer (pH = 9.6). The plates were then washed three times with PBS-T. To avoid the nonspecific binding, the plates were blocked with PBS-T containing 1% bovine serum albumin (BSA) at 37°C for 1 hr. One hundred µl of the sample sera diluted 1:100 in blocking buffer were added to the plates. The plates were then incubated at 37°C for 1 hr and washed three times with PBS-T. One hundred µl of HRP conjugated goat anti-human IgM antibody (Sigma)-diluted 1:10000 in blocking buffer-was added to each well. The plates were incubated at 37°C for 30 min and washed 3 times. One hundred µl of tetra-methylene benzidine dihydrochloride (Kem-En-Tec) was then added as the substrate. After 15 min of incubation at room temperature, the reaction was stopped by addition of 100 µl of 1 M HCL. The optical density was measured at 450 nm by a BioTek ELISA reader (BioTek Instruments, Highland Park, VT) with a reference wave length of 630 nm. Each serum sample was screened in duplicate wells.
The result for each serum sample was determined by taking the mean value of the absorbency readings for the duplicate wells. A sample was considered positive if the final value was equal to or higher than the defined cutoff.
Results
Expression of GRA8
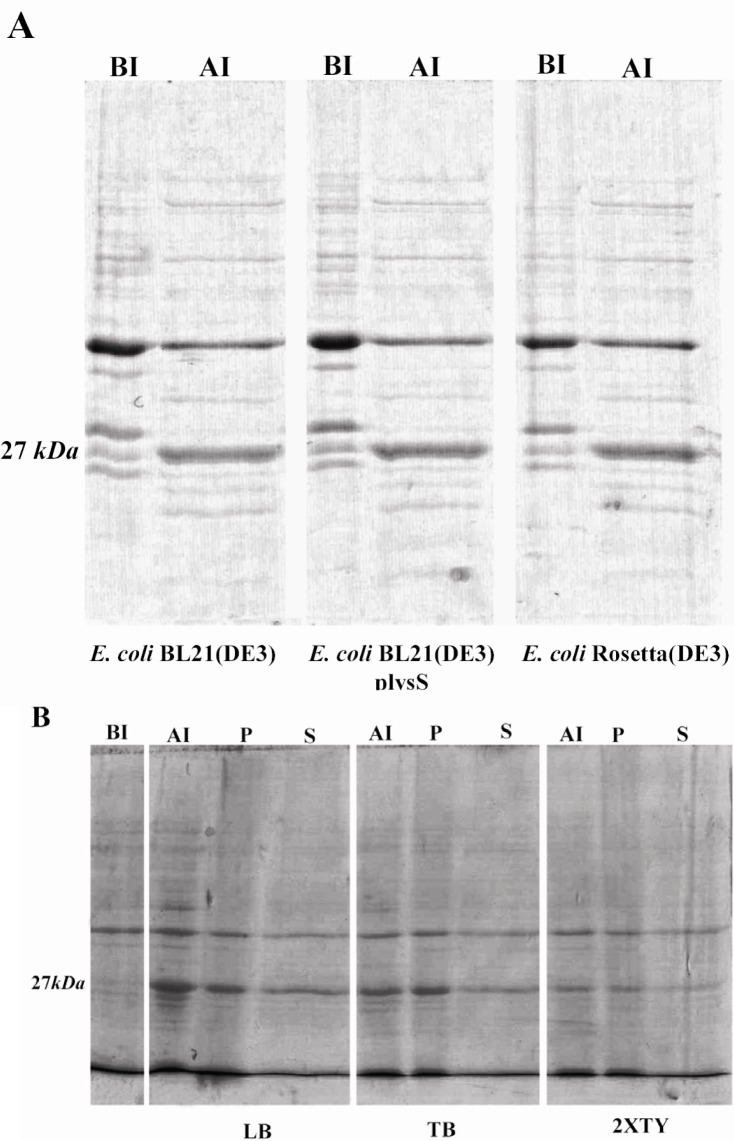
The gra8 gene fragment corresponding to amino acids 23 to 169 was PCR amplified yielding a single specific product of 458 bp. The PCR product was cloned into the pET-28b(+) vector. The presence of gra8 gene fragment in the recombinant plasmid was suggested by restriction digestion using EcoRV enzyme. Sequence analysis of the correct clone revealed 100% homology with the published sequence of gra8 (data not shown). In the pET-28b(+)-GRA8 construct, the sequences encoding the amino terminal signal peptide (amino acids 1 to 23) and the C-terminal transmembrane domain were excluded. Moreover, a short sequence encoding 35 aa including a T7 tag and a cluster of hexa-histidine residues were fused into the N-terminus of the gene. A second hexa-histidine tag was placed in C-terminal fusion of the target gene to further facilitate the subsequent purification of the recombinant protein. SDS-PAGE analysis of induced bacteria showed expression of a protein band of 27 kDa, which was absent in uninduced bacteria (Figure 1). The discrepancy observed between the calculated molecular weight of GRA8 (20.2 kDa with 189 amino acids) and the apparent band on SDS-PAGE is a common feature of GRA proteins (31). High proline content of GRA8 (53 of 189 residues) also likely contributes to the aberrant electrophoretic migration (21).
Figure 1.
SDS-PAGE analysis of expression of TGRA8 in E.coli. A) Expression level of TGRA8 in three different E.coli strains. pET-28b(+)-TGRA8 construct was transformed into the E.coli BL21(DE3), BL21(DE3) plysS and Rosetta(DE3) cells and expression of TGRA8 was induced by addition of 0.1 mM IPTG. SDS-PAGE analysis showed the expression of an approximately 27 kDa protein in induced bacteria (AI) which was absent in uninduced bacteria (BI). The expression level of the recombinant protein seemed to be equal in the three bacterial strains. B) Expression level and solubility of TGRA8 in three different culture media. Recombinant Rosetta(DE3) bacteria were cultured in LB, TB and 2XTY culture media and expression of TGRA8 was induced. SDS-PAGE analysis showed that the highest expression level was achieved in cells cultured in LB medium. The soluble and insoluble fractions of induced bacteria were analyzed on SDS-PAGE. Most of the recombinant protein was in insoluble form. However, considerable amount of the protein cultured in LB was soluble. BI) Total proteins of E.coli cells harbouring pET-28b(+)-TGRA8 before induction. AI) Total proteins after induction with 0.1 mM IPTG.
To optimize the bacterial production of GRA8, the expression was carried out in three E.coli expression hosts: BL21(DE3), BL21 (DE3)plysS and Rosetta(DE3) (Figure 1A) and three culture media: LB, 2XTY and TB (Figure 1B). The expression level of GRA8 seemed to be equal in the three bacterial hosts, however, the highest expression level of GRA8 was achieved in E.coli cells cultured in LB medium (Figure 1). To evaluate the solubility of GRA8, the recombinant Rosetta (DE3) cultivated in different medium were lyzed and fractionated into the soluble and insoluble proteins. Figure 1B showed that most of GRA8 in induced bacteria is in insoluble form, however, densitometry analysis using ImageJ software showed soluble expression of GRA8 was enhanced by cultivating in LB medium, as compared to the two other culture media (data not shown).
Optimization of immobilized metal affinity chromatography (IMAC) for purification of GRA8
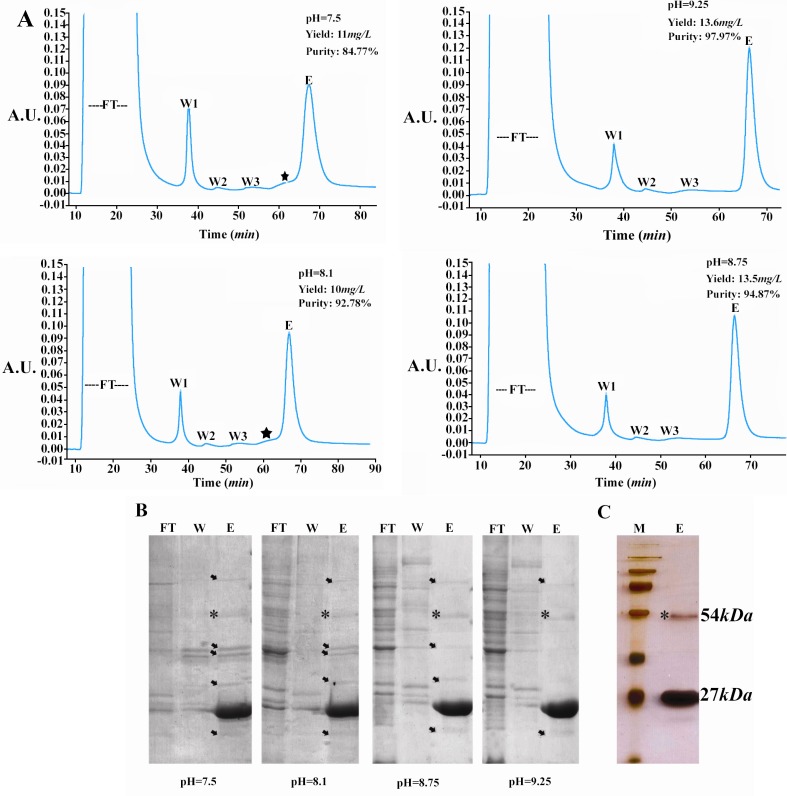
The soluble fraction of G RA8 was purified using IMAC. In the preliminary experiments, we observed marked variations in the purity and yield of GRA8 at different pH values of binding buffer. Therefore, IMAC was performed at four pH value of 7.5, 8.1, 8.75 and 9.25 as explained in the Materials and Methods. SDS-PAGE analysis of protein samples showed that the purity of GRA8 was improved when pH of binding buffer was increased from 7.5 to 9.25, and the highest purity of 97.97% was achieved at pH value of 9.25 (Figures 2A and 2B). Similarly, yield of GRA8 was increased from 10 mg/l to 13.6 mg/ ml when pH reached to 9.25 (Figure 2A).
Figure 2.
Purification of TGRA8. A) Chromatograms of the immobilized metal affinity chromatography (IMAC) purification of TGRA8. The purification was performed by using the HisTrap FF column (5 ml) and buffers made at four pH values of 7.5, 8.1, 8.75 and 9.25. Initially, the column was equilibrated with the binding buffer (5 mM imidazole, 50 mM Tris–HCl, 500 mM NaCl). The soluble E.coli lysate was passed though the column and the unbound proteins were collected (Flow Through). The column was then washed step-wised with the binding buffer containing 35 (Wash 1), 60 (Wash 2) and 90 (Wash 3) mM imidazole. Absorbed material was eluted with the binding buffer containing 500 mM imidazole (Elution). The purity and yield of TGRA8 was improved when pH was increased from 7.5 to 9.25. The star mark possibly represents shows the peak of impurities. FT) flow through, W1: wash 1, W2: wash 2, W3: wash 3 and E: Elution. B) SDS-PAGE analysis of TGRA8 purified by IMAC at four different pH. Protein samples of unbound proteins (FT), wash 1 (W) and elution (E) fractions were analyzed. The purity of TGRA8 was considerably improved with increasing the pH from 7.5 to 9.25. The arrows represent bacterial proteins co-purified with TGRA8. The 54 kDa protein band, marked with a star sign, might be the dimmer of TGRA8 as it reacts with Toxoplasma-specific antibody on 12 % SDS-PAGE stained with Coomassie Blue. C) Silver nitrate staining of the purified TGRA8. At pH 9.25 TGRA8 was purified near to homogeneity. On silver stained gel only 2 specific bands of 27 and 54 kDa could be observed. M: Protein molecular weight marker
The improved purity and yield of GRA8 at higher pH values was also evident from the corresponding chromatograms (Figure 2A). The peak of some impurities seen at pH = 7.5 and 8.1 was disappeared or weakened at highe r pH. In addition, the area under the peak of GRA8, representing the yield of protein, was apparently higher at pH = 8.75 and 9.25, compared to the lower pH values. Moreover, the bacterial impurities co-purified with GRA8 were also decreased from pH = 7.5 to pH = 9.25 (Figure 2B). The same results were obtained when Ni-NTA agarose resin was used for batch purification of GRA8 (data not shown).
The silver nitrate staining of the GRA8 purified at pH = 9.25 showed that protein was purified near to homogeneity (Figure 2C). The higher band revealed by silver nitrate staining, about 54 kDa, might be attributed to the dimmerization of GRA8. In fact, oligo-merisation of GRA proteins has been suggested in the previous studies (32–34).
Antigenicity and immunogenicity evaluation of GRA8
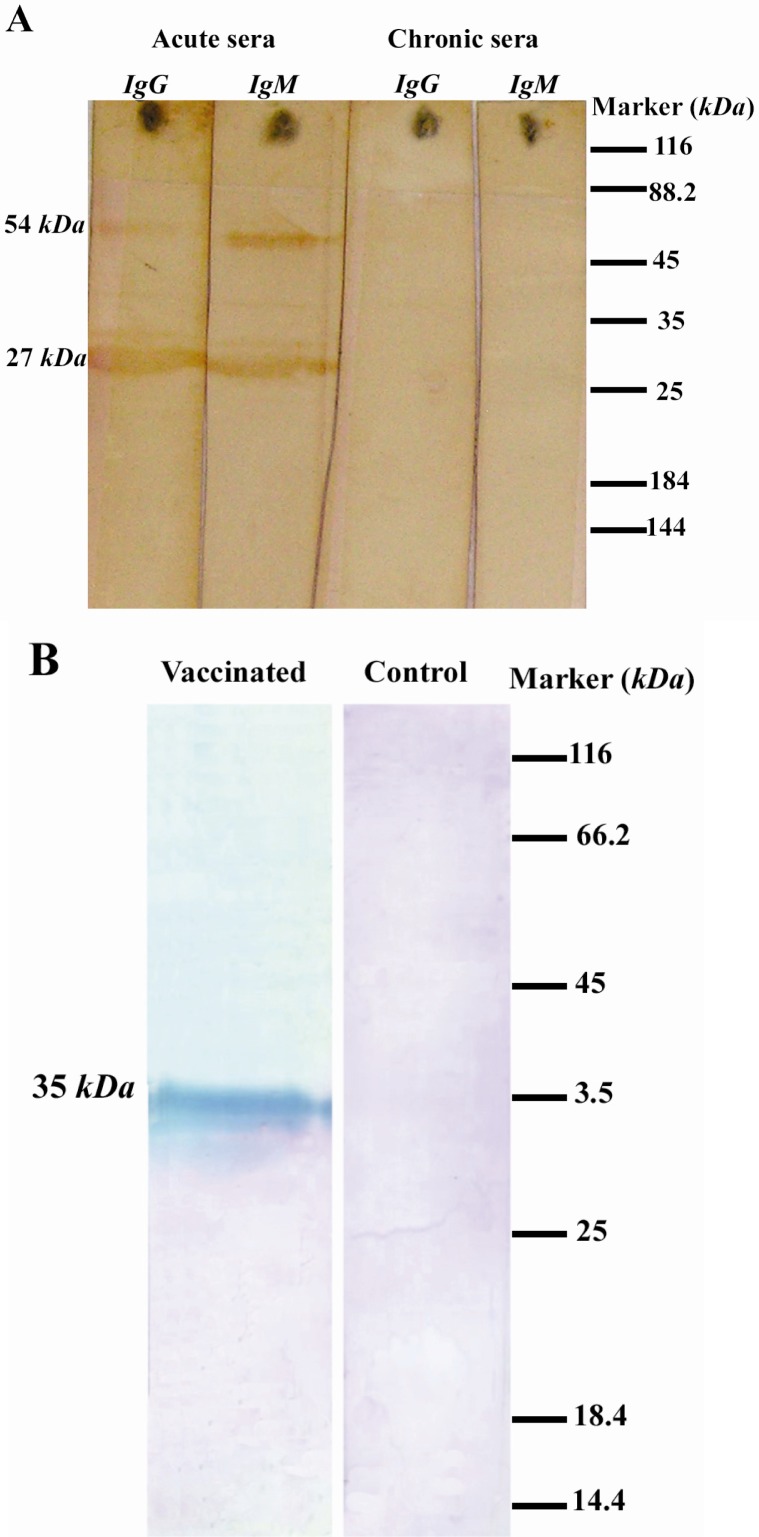
Antigenicity of GRA8 was investigated in Western blotting using pooled human sera representing the acute and chronic infection. The purified antigen strongly reacted with IgG and IgM antibodies in acute sera. No specific band, however, was observed on the blot probed with chronic sera (Figure 3A). The results demonstrated the antigenicity of GRA8 and its superior reactivity towards acute sera in comparison to chronic ones.
Figure 3.
Western blotting analyses of purified TGRA8. A) Reactivity of purified TGRA8 with human sera. Purified TGRA8 was analyzed on the SDS-PAGE, transferred onto the nitrocellulose membrane and probed with the acute and chronic pooled sera from pregnant women. The purified antigen strongly reacted with IgG and IgM antibodies in acute sera, while no specific band can be observed on the blot probed with chronic sera,. The protein band of about 54 kDa was probably attributed to the dimmer of TGRA8. B) Evaluation of immunogenicity of TGRA8. One Rabbit was injected intramuscularly with 100 μg of TGRA8 three times with two weeks intervals. Two weeks after the last injection rabbit serum sample was obtained. Tachyzoites of T.gondii RH strain (5×106 tachyzoites) were analyzed by SDS-PAGE, transferred onto nitrocellulose membrane and probed with the serum samples from the TGRA8-immunized and adjuvant-injected rabbits. The serum from the TGRA8-immunized rabbit recognized a single protein band of 35 kDa, the expected size of the native GRA8 antigen, while the serum from the adjuvant-injected rabbit failed to react with any specific protein of RH tachyzoites
Moreover, the immunoreactivity of GRA8 was confirmed by Western blotting using the serum sample from a GRA8-immunized rabbit. On the blot of RH tachyzoite probed with anti-GRA8 serum, a single protein band of 35 kDa, the same size of the native GRA8, was detected. In contrast, there was no specific band on the blot of tachyzoites probed with the serum from a rabbit injected with the adjuvant alone (Figure 3B).
Detection of acute toxoplasma infection by GRA8-IgM-ELISA
A total number of 68 sera from pregnant women with acute and chronic infection were included in this study. The sera were examined individually by GRA8-IgM-ELISA. A cut-off value (0.31) was determined on the basis of the mean value plus two standard deviations of the OD values of chronic sera. One (2.8%) of 35 chronic serum samples scored above the cut-off (No. 19, OD: 0.36), resulting in a test specificity of 97.1% (Table 1). Twenty of 33 acute serum samples had OD values equal to or higher than the cut-off, leading to a sensitivity of 60.6% for the assay (Table 1). These results indicated that IgM ELISA using the purified GRA8 was useful in discriminating acute from chronic infection.
Table 1.
Results of the TGRA8-IgM-ELISA and standard serologic tests on sera from Group I (acute-phase sera) pregnant women
| Serum No. | IgM-ELISA Euroimmun (index)a | IgG-ELISA Euroimmun (index)b | IgM-ELISA GRA8 (OD450 value)c |
|---|---|---|---|
| 1 | 2.78 | 5.05 | 0.40 |
| 2 | 6.55 | 1.43 | 0.77 |
| 3 | 2.14 | 5.34 | 0.17 |
| 4 | 2.17 | 2.24 | 0.48 |
| 5 | 8.20 | 2.20 | 0.31 |
| 6 | 1.64 | 6.25 | 0.22 |
| 7 | 4.90 | 2.88 | 0.21 |
| 8 | 5.93 | 3.22 | 0.59 |
| 9 | 8.74 | 4.85 | 0.64 |
| 10 | 5.14 | 2.49 | 0.56 |
| 11 | 4.86 | 2.63 | 0.58 |
| 12 | 2.10 | 1.27 | 0.31 |
| 13 | 6.98 | 1.46 | 0.25 |
| 14 | 1.67 | 1.58 | 0.23 |
| 15 | 1.28 | 1.58 | 0.25 |
| 16 | 7.62 | 3.32 | 0.22 |
| 17 | 4.52 | 4.13 | 0.98 |
| 18 | 6.51 | 2.87 | 1.81 |
| 19 | 3.48 | 2.28 | 1.03 |
| 20 | 5.03 | 6.04 | 0.99 |
| 21 | 3.79 | 1.48 | 1.28 |
| 22 | 2.76 | 5.42 | 0.15 |
| 23 | 4.60 | 7.01 | 0.12 |
| 24 | 6.54 | 1.30 | 1.45 |
| 25 | 3.73 | 3.85 | 0.62 |
| 26 | 2.68 | 3.50 | 0.23 |
| 27 | 2.59 | 3.52 | 0.55 |
| 28 | 9.52 | 7.32 | 0.31 |
| 29 | 1.28 | 2.52 | 0.71 |
| 30 | 1.68 | 3.81 | 0.19 |
| 31 | 1.28 | 2.21 | 0.40 |
| 32 | 1.41 | 2.47 | 0.24 |
| 33 | 1.62 | 1.27 | 0.25 |
Results printed in boldface show positive ones according to the corresponding cut-off
Positive results were defined as indexes above 1.1
Positive results were defined as indexes above 1.1
Positive values were defined as readings equal to or above 0.31.
Discussion
The truncated GRA8 antigen was expressed in fusion with a small tag and expression of the protein was analyzed in different expression hosts and culture media. The single-step purification of GRA8 was performed by IMAC at the optimized pH, and led to the highly pure protein. Western blot analyses showed proper antigenicity and immunogenicity of the protein. IgM ELISA using GRA8 was shown to be useful to distinguish between the acute and chronic toxplasma infection.
GRA8, amino acids 23 to 169 was produced in fusion with a small tag to avoid possible nonspecific reactivities of long fusion partners in ELISA tests (35). The full-length GRA8 was difficult to express (22) and exclusion of the amino terminal signal peptide and the C-terminal transmembrane domain enhanced the expressivity and solubility of the hydrophobic protein. The major drawback of using small fusion tags is low expression of the recombinant protein (35). In attempt to increase expression of GRA8, a variety of related parameters were analyzed; among them the media culture was the most influential.
The soluble fraction of GRA8 was purified using single-step IMAC. The pI of histidine residue is 6 and it is usually recommended to perform the purification at pH = 7 to 8 to make sure that the histidine residues are negatively-charged and allow biding to Ni2 + ions. Nevertheless, in our setting for IMAC purification we observed that the optimum purity and yield of GRA8 was achieved at 9.25. The greater purity and yield in pH = 8.75 and 9.25, compared to those of pH = 7.5 and 8.1, were probably due to the more specific binding of GRA8 to the Ni2 + resin. It is usually suggested by the manufacturers to avoid pH higher than 8 as additional protein-metal-coordinating groups, presumably surface amines, might contribute to chromatographic retention in IMAC at alkaline pH values and result in nonspecific binding to the resin. In contrast, we observed the highest purity of GRA8 in IMAC performed at pH = 9.25. Several studies reported the influence of pH in the performance of IMAC and found that the optimum pH might be higher than 7 to 8 (36).
The strong reactivity of the purified GRA8 toward sera from T.gondii acute-phase sera, as compared to chronic sera, is in agreement with previous studies. Besides, GRA8 was capable of induction of specific immune response in a rabbit verifying the presence of the immunodominant epitopes of the native protein in GRA8.
The performance of IgM ELISA using GRA8 for detection of acute toxoplasma infection was comparable with the previously published studies. Aubert et al used GRA8, amino acid 1-135 produced in fusion with CKS (CMP-2-keto-3-deoxy octulosonic acid synthetase), in IgM ELISA and reported a sensitivity of 46.1% (41 of 89 sera) for detection of acute infection in individuals with acute infection (20). A double-sandwich IgM ELISA using the same part of GRA8 produced in fusion with GST was developed and showed a sensitivity of 90% (18 of 20 sera) and specificity of 100% for discriminating recent from past Toxoplasma infection (19). Pfrepper et al from Mikrogen Company applied GRA8, amino acid 22-165, in IgM ELISA and showed sensitivity of 64.7% (44 of 68 sera) and specificity of 100% for detection of acute infection (18). In the present study, IgM ELISA using GRA8 showed a sensitivity and specificity of 60.6% and 97.1% for detection of acute infection.
The differences between the sensitivities reported in different studies might be attributed to the methods of antibodies detection (direct ELISA and double sandwich ELISA (19), the variations in cloning strategy of the selected epitopes and using the different local infection serum samples (3). Recently, GRA8 has been used for the development of a commercial immunoassay kit for the detection of specific toxoplasma IgG antibodies (37).
Conclusion
Taking together, soluble GRA8 was produced with a very small fusion tag and purified near to homogeneity using an optimized single-step IMAC. Our simplified extraction and purification protocols resulted in reduced losses and increased yield and purity of the recombinant protein. An IgM ELISA using GRA8 was useful in differentiation between chronic and acute toxoplasmosis.
Acknowledgement
This work was supported by the research grants No. 461 from Pasteur Institute of Iran and No. 89000846 from and Iranian National Science Foundation.
References
- 1.Blader IJ, Saeij JP. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and viru-lence. APMIS. 2009;117(5-6):458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 3.Kotresha D, Noordin R. Recombinant proteins in the diagnosis of toxoplasmosis. APMIS. 2010;118(8):529–542. doi: 10.1111/j.1600-0463.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 4.Sukthana Y. Toxoplasmosis: beyond animals to humans. Trends Parasitol. 2006;22:137–142. doi: 10.1016/j.pt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Wallon M, Kodjikian L, Binquet C, Garweg J, Fleury J, Quantin C, et al. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics. 2004;113(6):1567–1572. doi: 10.1542/peds.113.6.1567. [DOI] [PubMed] [Google Scholar]
- 6.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12-13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatkowska J, Hiszczynska-Sawicka E, Kur J, Holec L, Dlugonska H. Toxoplasma gondii: an evaluation of diagnostic value of recombinant antigens in a murine model. Exp Parasitol. 2006;114(3):220–227. doi: 10.1016/j.exppara.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Beghetto E, Spadoni A, Bruno L, Buffolano W, Gargano N. Chimeric antigens of Toxoplasma gondii: toward standardization of toxoplasmosis serodiagnosis using recombinant products. J Clin Microbiol. 2006;44(6):2133–2140. doi: 10.1128/JCM.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002;185(Suppl 1):S73–S82. doi: 10.1086/338827. [DOI] [PubMed] [Google Scholar]
- 10.Remington JS, Thulliez P, Montoya JG. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42(3):941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes HP, Van Knapen F. Characterization of a secretory antigen from Toxoplasma gondii and its role in circulating antigen production. Int J Parasitol. 1982;12(5):433–437. doi: 10.1016/0020-7519(82)90073-x. [DOI] [PubMed] [Google Scholar]
- 12.Golkar M, Rafati S, Abdel-Latif MS, Brenier-Pinchart MP, Fricker-Hidalgo H, Sima BK, et al. The dense granule protein GRA2, a new marker for the serodiagnosis of acute Toxoplasma infection: comparison of sera collected in both France and Iran from pregnant women. Diagn Microbiol Infect Dis. 2007;58(4):419–426. doi: 10.1016/j.diagmicrobio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Altcheh J, Diaz NS, Pepe CM, Martin V, Nigro M, Freilija H, et al. Kinetic analysis of the humoral immune response against 3 Toxoplasma gondii-recombinant proteins in infants with suspected congenital toxoplasmosis. Diagn Microbiol Infect Dis. 2006;56(2):161–165. doi: 10.1016/j.diagmicrobio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Nigro M, Gutierrez A, Hoffer AM, Clemente M, Kaufer F, Carral L, et al. Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn Microbiol Infect Dis. 2003;47(4):609–613. doi: 10.1016/s0732-8893(03)00156-1. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Maine G, Suzuki Y, Araujo FG, Galvan G, Remington JS, et al. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J Clin Microbiol. 2000;38(1):179–184. doi: 10.1128/jcm.38.1.179-184.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiszczyjska-Sawicka E, Kur J, Pietkiewicz H, Holec L, Gasior A, Myjak P. Efficient production of the Toxoplasma gondii GRA6, p35 and SAG2 recombinant antigens and their applications in the serodiagnosis of toxoplasmosis. Acta Parasitol. 2005;50(3):249–254. [Google Scholar]
- 17.Jacobs D, Vercammen M, Saman E. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin Diagn Lab Immunol. 1999;6(1):24–29. doi: 10.1128/cdli.6.1.24-29.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfrepper KI, Enders G, Gohl M, Krczal D, Hlobil H, Wassenberg D, et al. Seroreactivity to an avidity for recombinant antigens in toxoplasmosis. Clin Diagn Lab Immunol. 2005;12(8):977–982. doi: 10.1128/CDLI.12.8.977-982.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki Y, Ramirez R, Press C, Li S, Parmley S, Thulliez P, et al. Detection of immunoglobulin M antibodies to P35 antigen of Toxoplasma gondii for serodiagnosis of recently acquired infection in pregnant women. J Clin Microbiol. 2000;38(11):3967–3970. doi: 10.1128/jcm.38.11.3967-3970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubert D, Maine GT, Villena I, Hunt JC, Howard L, Sheu M, et al. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol. 2000;38(3):1144–1150. doi: 10.1128/jcm.38.3.1144-1150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey KL, Donahue CG, Ward GE. Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol. 2000;105(1):25–37. doi: 10.1016/s0166-6851(99)00160-7. [DOI] [PubMed] [Google Scholar]
- 22.Babaie J, Zare M, Sadeghiani Gh, Lorgard-Dezfuli M, Aghighi Z, Golkar M. Bacterial production of dense granule antigen GRA8 of Toxoplasma gondii. Iran Biomed J. 2009;13(3):145–151. [PubMed] [Google Scholar]
- 23.Li S, Galvan G, Araujo FG, Suzuki Y, Remington JS, Parmley S. Serodiagnosis of recently acquired Toxoplasma gondii infection using an enzyme-linked immunosorbent assay with a combination of recombinant antigens. Clin Diagn Lab Immunol. 2000;7(5):781–787. doi: 10.1128/cdli.7.5.781-787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porekar VG, Menart V. Perspective of immobilized metal affinity chromatography. J Biochem Biophys Met. 2001;49(1-3):335–360. doi: 10.1016/s0165-022x(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 25.Paunovic I, Schulin R, Nowack B. Evaluation of immobilized metal ion affinity chromatography for the fractionation of natural Cu complexing ligands. J Chromatogr. 2005;1100(2):176–184. doi: 10.1016/j.chroma.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 26.Sulkowski E. Purification of proteins by IMAC. Trends Biotechnol. 1985;3(1):1–7. [Google Scholar]
- 27.Jiang W, Hearn MTW. Protein interaction with immobilized metal ion affinity ligands under high ionic strength conditions. Anal Biochem. 1996;242(1):45–54. doi: 10.1006/abio.1996.0426. [DOI] [PubMed] [Google Scholar]
- 28.Chaga GS. Twenty-five years of immobilized metal ion affinity chromatography: past, present and future. J Biochem Biophys Methods. 2001;49(1-3):313–334. doi: 10.1016/s0165-022x(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 29.Gaberc-Porekar V, Menart V. Perspectives of immobilized-metal affinity chromatography. J Bio-chem Biophys Methods. 2001;49:335–360. doi: 10.1016/s0165-022x(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 30.Charlton A, Zachariou M. Immobilized metal ion affinity chromatography of histidine-tagged fusion proteins. Methods Mol Biol. 2008;421:137–150. doi: 10.1007/978-1-59745-582-4_10. [DOI] [PubMed] [Google Scholar]
- 31.Mercier C, Adjogble KD, Daubener W, Delauw MF. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all Apicomplexa parasites? Int J Parasitol. 2005;35(8):829–849. doi: 10.1016/j.ijpara.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Golkar M, Shokrgozar MA, Rafati S, Sadaie MR, Assmar M. Construction, expression and preliminary immunological evaluation of a DNA plasmid encoding the GRA2 protein of Toxoplasma gondii. Iran Biomed J. 2005;9(1):1–8. [Google Scholar]
- 33.Mercier C, Lecordier L, Darcy F, Deslee D, Murray A, Tourvieille B, et al. Molecular characterization of a dense granule antigen (Gra2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol. 1993;58(1):71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- 34.Parmley SF, Sgarlato GD, Remington JS. Genomic and corrected cDNA sequence of the P28 gene from Toxoplasma gondii. Mol Biochem Parasitol. 1993;57:163–168. doi: 10.1016/0166-6851(93)90253-t. [DOI] [PubMed] [Google Scholar]
- 35.Esposito D, Chatterjee DK. Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol. 2006;17(4):353–358. doi: 10.1016/j.copbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Tan YP, Ling TC, Tan WS, Yusoff Kh, Tey BT. Recovery of histidine-tagged nucleocapsid protein of Newcastle disease virus using immobilised metal affinity chromatography. Process Biochem. 2006;41(4):874–881. [Google Scholar]
- 37.Sickinger E, Andrieu FG, Jonas G, Schultess J, Stieler M, Smith D, et al. Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG Avidity assays. Diagn Microbiol Infect Dis. 2008;62(3):235–244. doi: 10.1016/j.diagmicrobio.2008.07.005. [DOI] [PubMed] [Google Scholar]