Abstract
Musa sapientum (M.sapientum) commonly known as ‘banana’ is widely used in Bangladeshi folk medicine for the treatment of various ailments including diarrhea. Hence, the present study was designed to investigate antidiarrheal, antioxidant and antibacterial potential of the methanolic extract of M.sapientum seed (MMSS). The extract was studied for antidiarrheal property using castor oil and magnesium sulfate induced diarrheal model and charcoal induced gastrointestinal motility test in mice. Total phenolic and flavonoids content, total antioxidant activity, scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, as well as nitric oxide (NO) and assessment of reducing power were used to evaluate antioxidant potential of MMSS. In addition, disc diffusion methods were used for antibacterial assay using various diarrheal induced bacterial strains. At the doses of 100 and 200 mg/kg body weight, the extract reduced the frequency and severity of diarrhea in test animals throughout the study period. At the same doses, the extracts significantly (p<0.001) delayed the intestinal transit of charcoal meal in test animals as compared to the control. In DPPH and NO scavenging method, MMSS showed good antioxidant potentiality in a dose dependent manner with the IC50 value of 12.32±0.33 µg/ml and 18.96±1.01 µg/ml, respectively with a significant (p<0.001) good reducing power. The extract also displayed strong anti-bacterial effect against when tested against Escherichia coli, Shigella dysenteriae, and Pseudomonas aeruginosa. Altogether, these results suggest that the MMSS could be used as a potential antidiarrheal agent along with its antioxidant and antibacterial potentiality.
Keywords: Antibacterial agents, Diarrhea, Free radicals, Musa sapientum
Introduction
Diarrhea is an alteration in the normal bowel movement, characterized by increased frequency of bowel sound and movement, wet stool, and abdominal pain (1). Clinically it is used to describe increased liquidity of stool, usually associated with increased stool weight and frequency (2). Regardless of the understanding causes, treatment and prevention of diarrheal diseases, an estimated 4.6 million people, with 2.5 million children, die from diarrhea every year, particularly in developing countries (3). Diarrhea, may be acute or chronic. Acute diarrhea being the most common, is usually caused by an infectious agent, even though drugs, poisons or acute inflammatory reactions can contribute a lot (4). Now a days, rotavirus is the major causative agent for infectious diarrhea, particularly in young children, however, other viral (adenovirus, enterovirus and norovirus), bacterial (Escherichia coli, Salmonella sp., Shigella sp., Camphylobacter and Vibrio cholerae) and parasitic (Cryptosporidium and Giardia) agents are important pathogens (5).
Oral Rehydration Therapy (ORT) has been identified as a key factor in the decline of child mortality rate due to diarrhea, although it does not reduce the volume or duration of diarrhea (6). Likely, antibiotics and gut motility suppressing agents bid the other treatment option, wherein reverse dehydration, shorten the length of illness and reduce the period of time when an individual is infected (7). Treatment with pharmacological agents that are pathogen specific or that suppress severe symptoms would be of benefit to patients suffering from prolonged diarrhea (8).
Despite the availability of a vast spectrum of approaches for diarrheal management, a vast majority of the people of Bangladesh have been known to treat diarrhea with a variety of medicinal plants one of which being M.sapientum (9). M.sapientum L. (family Musaceae) grows in humid lowland to upland tropical areas whose vernacular name is banana in Bengali. Agarwal et al (10) has studied the wound healing activity of plantain banana extracts. Plants parts like fruits, leaves, peels, root, and stalks from banana plants have been utilized orally or topically as a medicine for treating diarrhea and dysentery. It is also used in inflammation, pains and snakebite (11) as well as it has antilithic (12), antiulcerogenic (13), hypoglycemic (14), hypolipidemic and antioxidant actions (15). Houghton and Skari (16) have also reported the antivenom action of the stem juice from banana plant.
Literature reviews indicated that no studies combining the antidiarrheal, antioxidants as well as antibacterial of the seeds of M.sapientum have so far been undertaken. Taking this in view and as a part of our ongoing research (17, 18) on Bangladeshi medicinal plants, the present study aimed to evaluate the antidiarrheal activity of seeds extracts of M.sapientum along with their antioxidant and anti-bacterial activities.
Materials and Methods
Plant materials
The fruits of M.sapientum were collected from the local market in Mirpur, Dhaka, Bangladesh in the month of April, 2008 and identified by experts in Bangladesh National Herbarium, Mirpur, Dhaka where the Voucher specimen no: 38765 has been retained for future reference.
Preparation of plant extract
The seeds of M.sapientum were manually separated from the whole fruits, dried in hot air woven, pulverized into coarse powder using mechanical grinder, seiving through sieve #40, and stored in an air tight container. The dried powder material (500 g) was refluxed with methanol for three hr. The total filtrate was concentrated to dryness, in vacuum at 40°C to render the methanolic extract (80 g).
Chemicals
Ammonium molybdate, Folin-chiocaltu phenol reagent, sodium nitroprusside, were purchased from E. Merck (Germany). 1,1-diphenyl- 2-picryl-hydrazyl (DPPH), sodium nitroprusside, ascorbic acid, quercetin, and potassium ferric cyanide were purchased from Sigma Chemical Co. Ltd, (St. Louis, MO, USA). All other chemicals and reagents were of analytical grade.
In vivo antidiarrheal activity
Castor oil-induced diarrhea: The experiment was performed according to the method described by Shoba & Thomas (19). Briefly, mice fasted for 24 hr were randomly allocated to four groups of five animals each. The animals were all screened initially by giving 0.5 ml of castor oil. Only those showing diarrhea were selected for the final experiment. Group I received 1% carboxy-methyl cellulose (CMC) (10 ml/kg, p.o), groups III and IV received p.o the drug extract (100 and 200 mg/kg), respectively. Group II was given antidiarrheal drug loperamide (3 mg/kg, p.o) in suspension. After 60 min, each animal was given 0.5 ml of castor oil, each animal was placed in an individual cage, the floor of which was lined with blotting paper which was changed every hour, observed for 4 hr and the characteristic diarrheal droppings were recorded.
Magnesium sulfate-induced diarrhea
Diarrhea was induced by oral administration of magnesium sulfate at the dose of 2 g/kg to the animals 30 min after pre-treatment with vehicle (1% Tween 80 in water, 10 ml/kg, p.o) to the control group, loperamide (3 mg/kg) to the positive control group, and the methanol extract at the doses of 100 and 200 mg/kg to the test groups (20).
Effect on gastrointestinal motility
Animals were divided into four groups of five mice each and each animal was given p.o 1 ml of charcoal meal (5% activated charcoal suspended in 1% CMC) 60 min after an oral dose of drugs or vehicle. Group I was administered 1% CMC (10 ml/kg) and animals in groups III and IV received extract at the dose of 100 mg/kg and 200 mg/kg body weight, respectively. Group II received atropine sulfate (0.1 mg/kg), which decreased gastroin-testinal tract motility and was used as the standard drug. After 30 min, animals were killed by light ether anaesthesia and the intestine was removed without stretching and placed lengthwise on moist filter paper. The intestinal transit was calculated as a percentage of the distance travelled by the charcoal meal compared to the length of the small intestine (21).
Acute toxicity study
Animals were divided into groups of five mice each. The test was performed using increasing doses of test extract, given p.o, in a 10 ml/kg volume to different groups serving as test groups (22). Another group of mice was administered saline (10 ml/kg, p.o) as negative control. The mice were allowed food ad libitum during the 24 hr test and kept under regular observation for mortality.
In vitro antioxidant activity
Determination of total antioxidant capacity: The antioxidant activity of the extract was evaluated by the phosphomolybdenum method according to the procedure of Prieto et al (23). The assay is based on the reduction of Mo (VI)–Mo(V) by the extract and subsequent formation of a green phosphate/ Mo(V) complex at acid pH. Extract (0.3 ml) was combined with 3 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 95°C for 90 min. Then the absorbance of the solution was measured at 695 nm using a spectrophotometer (Shimadzu, UV-150-02) against blank after cooling to room temperature. Methanol (0.3 ml) was used as the blank experiment. The antioxidant activity is expressed as the number of equivalents of ascorbic acid using the following formula:
where: C-total antioxidant activity, mg/g plant extract, in Ascorbic acid; c-the concentration of ascorbic acid established from the calibration curve, mg/ml; V-the volume of extract, ml; m-the weight of pure plant extract, g.
Free radical scavenging activity measured by 1,1-diphenyl-2-picryl-hydrazyl (DPPH)
The free radical scavenging activity of extract, based on the scavenging activity of the stable 1,1-diphenyl-2- picrylhydrazyl (DPPH) free radical, was determined by the method described by Braca et al (24). Plant extract (0.1ml) was added to 3 ml of a 0.004% MeOH solution of DPPH. Absorbance at 517 nm was determined after 30 min, and the percentage inhibition activity was calculated from [(A0–A1)/A0] x 100, where A0 is the absorbance of the control, and A1 is the absorbance of the extract/ standard. IC50 value was calculated from the equation of line obtained by plotting a graph of concentration (µg/ml) versus % inhibition.
Nitric oxide radical scavenging assay
The procedure is based on the method, where sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions that can be estimated using Greiss reagent. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitrite ions. For the experiment, sodium nitroprusside (10 mM) in phosphate buffered solution (pH = 7.4) was mixed with different concentrations of extract dissolved in 10% DMSO and incubated at room temperature for 150 min. The same reaction mixture without the extract but the equivalent amount of the solvent used served as the control. After incubation, 0.5 ml of Griess reagent (1% sulfanilamide, 2% H3PO4 and 0.1% N-(1- naphthyl) ethylenediamine dihydrochloride was added. The absorbance was measured at 546 nm and the percentage inhibition activity was calculated from [(A0–A1)/A0] × 100, where A0 is the absorbance of the control, and A1 is the absorbance of the extract/ standard (25). IC50 value was calculated from the equation of line obtained by plotting a graph of concentration (µg/ml) versus % inhibition.
Reducing power activity
The reducing power of M.sapientum seed extract was determined according to the method previously described (26). Extract at different concentrations in 1 ml of 10% DMSO were mixed with 2.5 ml of phosphate buffer (0.2 M, pH = 6.6) and 2.5 ml potassium ferricyanide [K3Fe (CN) 6] (1%), and then the mixture was incubated at 50 °C for 30 min. Afterwards, 2.5 ml of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of upper layer solution was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (0.1%), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
The amount of phenolic compounds and flavonoids
The total phenolic content of extract was determined using Folin-Ciocalteu reagent (27). M.sapientum seed extracts (100 µl) were mixed with the Folin-Ciocalteu reagent (500 µl) and 20% sodium carbonate (1.5 ml). The mixture was shaken thoroughly and made up to 10 ml with distilled water. The mixture was allowed to stand for 2 hr. Then the absorbance at 765 nm was determined with a Shimadzu UV-160A spectrophotometer (Kyoto, Japan). These data were used to estimate the phenolic contents using a standard curve obtained from various concentration of gallic acid.
The flavonoids content was determined by aluminium chloride colorimetric method (28). Quercetin was used to make the calibration curve. The different concentration of extract (0.5 ml) were separately mixed with 95% ethanol (1.5 ml), 10% aluminum chloride (0.1 ml), 1 M potassium acetate (0.1 ml) and distilled water (2.8 ml). After incubation at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm. The amount of 10% aluminum chloride was substituted by the same amount of distilled water in blank. All the determinations were carried out in duplicates. These data were used to estimate the flavonoid contents using a standard curve obtained from various concentration of quercetin.
Antimicrobial activity
Sterile 6.0 mm diameter blank discs (BBL, Cocksville, USA) were impregnated with test substances at the dose of 500 µg/disc. This disc, along with standard discs (Ciprofloxacin, Oxoid Ltd, UK) and control discs were placed in petri dishes containing a suitable agar medium seeded with the test organisms using sterile transfer loop and kept at 4°C to facilitate maximum diffusion. The plates then kept in an incubator (37°C) to allow the growth of the bacteria. The antibacterial activities of the test agents were determined by measuring the diameter of the zone of inhibition in terms of millimeter. Antimicrobial activity was tested against Staphylococcus aureus, Escherichia coli, Pseudomonus aeruginosa, Salmonella typhi, Shigella boydii, Shigella flexneri and Shigella dysenteriae were obtained from International Centre for Diarrheal Disease Research, Bangladesh (ICDDR, B) (29).
Statistical analysis
All values were expressed as the mean ± standard error of the mean (SEM) of three replicate experiments and were analyzed using the GraphPad program (GraphPad, San Diego, CA, USA). The analysis was performed using student's t-test. P<0.001 was considered to be statistically significant.
Results
Effect on castor oil-induced diarrhea
In the castor oil induced diarrheal mice, the methanolic extract of the seeds of M.sapientum, at the dose of 100 and 200 mg/kg, significantly (p<0.001) lessened the total number of faeces as well as delayed the onset of diarrhea in a dose dependent manner (Table 1).
Table 1.
Effect of M.sapientum seed extract on castor oil-induced diarrhea in mice
| Dose (mg/kg) | Onset of diarrhea (min) | Animals with diarrhea | No. of faeces in 4 hr | % Inhibition of defecation | |
|---|---|---|---|---|---|
| Group I | Vehicle | 24.45±2.19 | 5/5 | 22.7±0.98 | |
| Group II | 10 | 180±0.13** | 1/5 | 2.8±0.28** | 87.38 |
| Group III | 100 | 50.67±2.73** | 3/5 | 11.6±1.05** | 47.75 |
| Group IV | 200 | 61.23±3.03** | 2/5 | 7.8±0.79** | 64.86 |
Values are mean±SEM, (n = 5); p<0.001, student's t-test compared to control. Group I animals received vehicle (1% Tween 80 in water), Group II received Loperamide 10 mg/kg body weight, Group III and Group IV were treated with 100 and 200 mg/kg body weight (p.o.) of the MMSS
Effect on magnesium sulfate-induced diarrhea
M.sapientum seed extract exhibited significant antidiarrheal activity against magnesium sulfate-induced diarrhea (Table 2). The extract at both dose levels significantly (p<0.001) reduced the extent of diarrhea and also notably delayed the onset of diarrhea in a dose dependent manner.
Table 2.
Effect of M.sapientum seed extract on magnesium sulfate-induced diarrhea in mice
| Dose (mg/kg) | Onset of diarrhea (min) | Animals with diarrhea | No. of faeces in 4 hr | % Inhibition of defecation | |
|---|---|---|---|---|---|
| Group I | Vehicle | 41.23 ± 1.11 | 5/5 | 19.0 ± 0.61 | |
| Group II | 10 | 188 ± 0.10** | 0/5 | 3.2 ± 0.18** | 83.15 |
| Group III | 100 | 56.69 ± 2.03 | 2/5 | 13.2 ± 1.25 | 30.53 |
| Group IV | 200 | 78.22 ± 2.19** | 1/5 | 9.4 ± 0.99** | 50.53 |
Values are mean±SEM, (n = 5); p<0.001, student's t-test compared to control. Group I animals received vehicle (1% Tween 80 in water), Group II received Loperamide 10 mg/kg body weight, Group III and Group IV were treated with 100 and 200 mg/kg body weight (p.o.) of the MMSS
Effect on gastrointestinal motility
With the gastrointestinal transit experiment, the methanolic extract, at the dose of 100 and 200 mg/kg, retarded (p<0.001) the intestinal transit of charcoal meal in mice when compared to the control (Table 3).
Table 3.
Effect of M.sapientum seed extract on charcoal meal stimulated gastrointestinal transit in mice
| Dose (p.o) | Mean intestinal length (cm) | Mean distance traveled by charcoal (cm) | % GI transit | |
|---|---|---|---|---|
| Group I | 0.4 ml/mouse | 68.4±0.98 | 50.4±1.18 | 73.86±3.57 |
| Group II | 0.1 mg/kg | 63.2±1.61 | 20±1.04** | 31.62±1.32** |
| Group III | 100 mg/kg | 63.8±2.41 | 35±0.91** | 55.05±1.39** |
| Group IV | 200 mg/kg | 66.2±2.60 | 24.8±1.61** | 37.82±3.04** |
Values are presented as mean±SEM, (n = 5); p<0.001, student's t test compared to control. Group I animals received vehicle (1% Tween 80 in water), Group II received atropine sulfate 0.1 mg/kg body weight, Group III and Group IV were treated with 100 and 200 mg/kg body weight (p.o.) of the MMSS
Acute toxicity studies
Methanolic seed extract of M.sapientum (500-5000 mg/kg, body weight) given p.o did not cause any death in the different dose groups. The LD50 value for oral administration of the plant extract was found to be greater than 5000 mg/kg.
Total phenolic and flavonoid contents
The total phenols and flavonoids content was found to be 15.94±0.12 mg/g plant extract (in GAE) and 29.98±0.32 mg/g plant extract (in quercetin equivalent), respectively, in crude extract of M.sapientum seeds (Table 4).
Table 4.
Yield, total amount of plant phenolic compounds, flavonoids and total antioxidant capacity of methanolic extract of M.sapientum seeds
| Sample | Yield (%) | Total phenols mg/g plant extract (in GAE)a | Total flavonoids mg/g plant extract (in QA)b | Total antioxidant capacity mg/g extract (in ASC)c |
|---|---|---|---|---|
| MMSS | 30.0% | 15.94±0.12 | 29.98±0.32 | 197.24±0.69 |
Gallic acid equivalents (GAE), (mg/g of each extract) for the total phenolic content
Quercetin equivalents (QA), (mg/g of each extract) for the total flavonoid content
Ascorbic acid equivalents (ASC), (mg/g of each extract) for the total antioxidant capacity
The GAE, QA and ASC values are expressed as means±SEM of triplicate experiments
Total antioxidant capacity
Percentage yield of methanol extract of M. sapientum seeds and its total antioxidant capacity are given in Table 4. Total antioxidant capacity of the extract is expressed as the number of equivalents of ascorbic acid and was found to be 197.24±0.69 mg/g equivalent of ascorbic acid.
DPPH radical scavenging activity
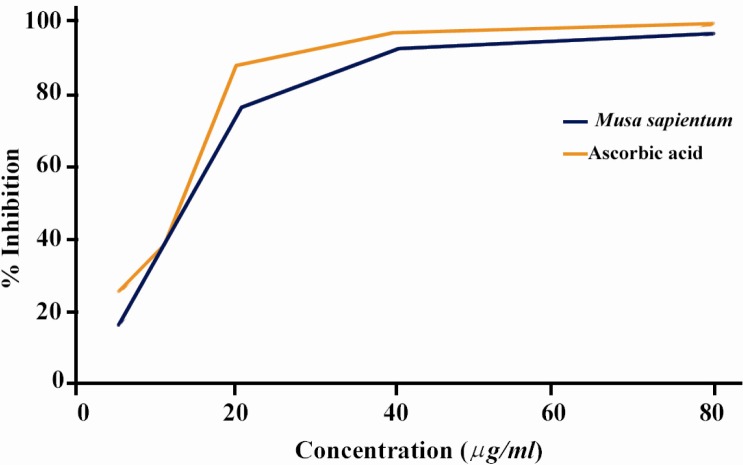
The percentage (%) scavenging of DPPH radical was found to be concentration dependent i.e. concentration of the extract between 5-80 µg/ml greatly increasing the inhibition activity (Figure 1). Crude extract of M.sapientum seed (IC50 value 12.32±0.33 µg/ml) showed similar activity than the standard ascorbic acid (IC50 value 12.30±0.15 µg/ml).
Figure 1.
Free radical scavenging activity of different concentrations of crude extract of M.sapientum seed and ascorbic acid by DPPH radicals
Nitric oxide (NO) scavenging activity
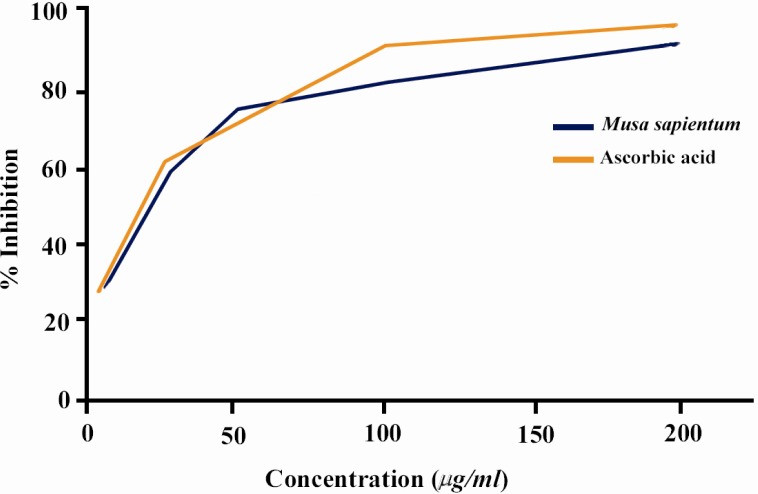
The percentage inhibition of nitric oxide production was illustrated in Figure 2. It is observed that scavenging of nitric oxide by the extract is also concentration dependent and statistically significant (p<0.001). The IC50 value of the extract of M.sapientum seed was 18.96±1.01 µg/ml, while ascorbic acid showed the value of 8.22±0.22 g/ml.
Figure 2.
Percentage inhibition of nitric oxide radical by different concentrations of crude extract of M.sapientum seed and ascorbic acid
Reducing power ability
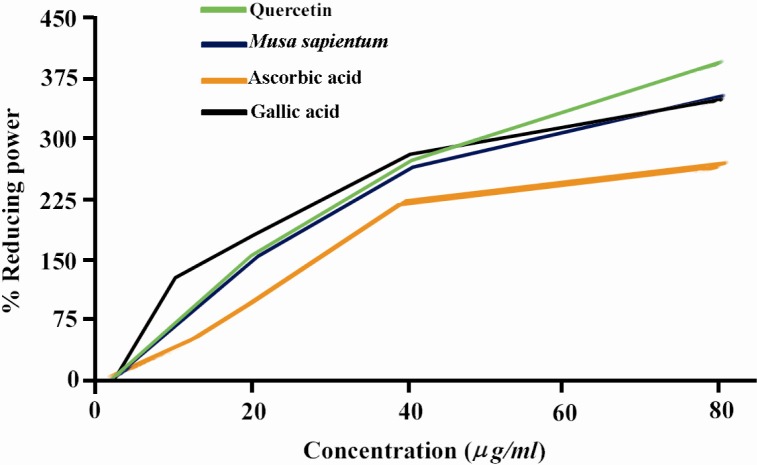
For the measurement of the reductive ability, we investigated the Fe3 + to Fe2 + transformation in the presence of crude extract of M.sapientum. Like the antioxidant activity, the reducing power of M.sapientum seed extract increased with increasing concentration of the sample and effect was statistically significant (p<0.001). Figure 3 shows the reductive capabilities of the M.sapientum compared with quercetin, gallic acid and ascorbic acid.
Figure 3.
Reducing power of MeOH extract of M.sapientum and quercetin, ascorbic acid and gallic acid by spectrophotometric detection of Fe3 + to Fe2 + transformation
Antibacterial activity
Table 5 expressed the antibacterial activity (zone of inhibitions) of the seed extract of the M. sapientum. The extract showed significant activity against the entire tested bacterial flora except Shigella flexneri and Shigella boydii. The highest zone of inhibition was found against Escherichia coli (zone of inhibition 18.59±0.22 mm), followed by Shigella dysenteriae (zone of inhibition 16.92±0.62 mm) and the moderate activity was shown against Pseudomonas aeruginosa (zone of inhibition 12.21±0.14 mm). The weakest activity was shown against Staphylococcus aureus.
Table 5.
Antibacterial activity of methanolic extract of M. sapientium seeds
| Bacterial strain | Diameter of zone of inhibition (mm) | |
|---|---|---|
|
| ||
| Ciprofloxacin | MMSS | |
| Staphylococcus aureus | 28.03±0.12 | 8.89±0.14 |
| Pseudomonas aeruginosa | 29.13±0.21 | 12.21±0.14 |
| Salmonella typhi | 25.41±0.11 | 10.39±0.12 |
| Shigella flexneri | 27.34±0.12 | NA |
| Shigella dysenteriae | 28.01±0.11 | 16.92±0.62 |
| Shigella boydii | 29.39±0.14 | NA |
| Escherichia coli | 30.23±0.18 | 18.59±0.22 |
Assay was performed in triplicate and results are the mean of three values±Standard Deviation. NA- Zone of inhibition<5 mm consider as no activity
Discussion
Several mechanisms have been previously proposed to explain the diarrheal effect of castor oil including inhibition of intestinal Na+, K+-ATPase activity to reduce normal fluid absorption (30), activation of adenylate cyclase or mucosal cAMP mediated active secretion (31), stimulation of prostaglandin formation (32), platelet activating factor and recently nitric oxide has been claimed to contribute to the diarrheal effect of castor oil (33). However, it is well evident that castor oil produces diarrhea due to its most active component recinoleic acid which causes irritation and inflammation of the intestinal mucosa, leading to release of prostaglandins, which results in stimulation of secretion (34). Since the methanol extract of the seeds of M. sapientum successfully inhibited the castor oil-induced diarrhea, the extract might have exerted its antidiarrheal action via antisecretory mechanism which was also evident from the reduction of total number of wet faeces (not shown separately) in the test groups in the experiment. Again, flavonoids present in the plant extract (13) are reported to inhibit release of autacoids and prostaglandins, thereby inhibit motility and secretion induced by castor oil (35).
On the other hand, magnesium sulfate has been reported to induce diarrhea by increasing the volume of intestinal content through prevention of reabsorption of water. It has also been reported that it promotes the liberation of cholecystokinin from the duodenal mucosa, which increases the secretion and motility of small intestine and thereby prevents the reabsorption of sodium chloride and water (36). The methanol extract was found to improve the diarrheal condition in this model. The extract may increase the absorption of water and electrolyte from the gastrointestinal tract, since it delayed the gastrointestinal transit in mice as compared to the control. The delay in the gastrointestinal transit prompted by the extract might have contributed, at least to some extent, to their antidiarrheal activity by allowing a greater time for absorption.
To determine the efficacy of natural antioxidants either as pure compounds or as plant extract, a great number of in vitro methods have been developed in which antioxidant compounds act by several mechanisms. The phosphomolybdenum method was based on the reduction of Mo(VI) to Mo(V) by the compounds having antioxidant property and is successfully used to quantify vitamin E in seeds (23).
DPPH is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule (37) and is usually used as a substrate to evaluate the antioxidant activity of a compound (28). Based on the data obtained from this study, DPPH radical scavenging activity of M.sapientum seed extract (IC50 12.32±0.16 µg/ml) was similar than the standard (IC50 12.30±0.11 µg/ml). It was revealed that M.sapientum seed extract did show the proton donating ability and could serve as free radical inhibitor or scavenger. In fact, the radical scavenging capability of phenolic compounds are due to their hydrogen donating ability/number of hydroxyl groups present, which in turn is closely related both to the chemical structure and spatial conformation, that can modify the reactivity of the molecules (38).
A direct correlation between antioxidant capacity and reducing power of certain plant extracts has been reported. The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (39). Moreover, it has been reported that the phenol and polyphenolic compound (flavonoids)-constituents of the plant-possess antioxidant properties mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers. In addition, they have a metal chelation potential (40).
In the present study this possibility is supported by the estimation of total polyphenols and flavonoids (41), which was found to be present in high concentration in the Musa sp. extracts. Our study also supported the previous study (42) on producing the antioxidant effect of the hydroalcoholic extract of M. sapientum against various in vitro model.
Flavonoids and sugars obtained from selected traditional medicinal plants in Bangladesh were reported by Rahman and Wilcock having antidiarrheal properties (43). Longanga Otshudi et al screened a number of medicinal plants and showed that antidiarrheal activity of those plants were due to tannins alkaloids, saponins, flavonoids, sterols, triterpenes and reducing sugars contained in them (44). The flavonoids presence of these types of compounds, such as kaemferol, myricetin, apigenin, and leucocyanidin in M.sapientum is likely to contribute to its grastrointestinal effects (41). Moreover, nitric acid mechanism has also been shown to be involved in castor oil induced diarrhea (33). In our study plant extract showed remarkable NO scavenging capacity may partially contribute the antidiarrheal activity.
The MMSS was shown to exhibit good antibacterial activity when tested against Escherichia coli, Shigella dysenteriae, and Pseudomonas aeruginosa and also supported to the previous study (45). Phytoconstituents such as saponin, phenolic compounds, flavonoids and glycosides have been reported to inhibit bacterial growth and to be protective to plants against bacterial and fungal infections (46). In the present study this possibility is supported by the estimation of total polyphenols and flavonoids (41), which was found to be present in high concentration and was found to be 15.94±0.12 mg equivalent of gallic acid/ g plant extract and 29.98 mg equivalent of quercetin/g plant extract, respectively. Moreover, Mokbel et al,(47) isolated various antibacterial compound viz. ß-sitosterol, malic acid, succinic acid, palmatic acid, 12-hydro-xystrearic acid, glycoside, the d-malic and 12-hydroxystrearic acid. So the antibacterial activity showed by the extract may be due to the presence of those compounds. Also some plants show antidiarrheal properties by their antimicrobial activities (48).
Conclusion
The MMSS showed antidiarrheal as well as antioxidant activities in a number of models of diarrheic condition in the test animals and in vitro antioxidant assay method, respectively. The obtained results thus give the experimental basis to understand the use of M.sapientum in traditional medicine, as an antidiar-rheal agent. However, further bioassay guided phytochemical and pharmacological studies are required to identify the active principles and exact mechanism(s) of action.
Acknowledgement
We express our sincere thanks and gratitude to Professor Dr. Kabir Hossain Talukder, Dean, Faculty of Biological Science, Atish Dipankar University of Science & Technology, Banani, Dhaka, Bangladesh for providing laboratory facilities and necessary reagents support while doing the study.
References
- 1.Guerrant RL, Van Gilder T, Steiner TS, Theilman MN, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infec Dis. 2001;32(3):331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 2.Suleiman MM, Dzenda T, Sani CA. Antidiarrhoeal activity of the methanol stem-bark extract of Annona senegalensis Pers. (Annonaceae) J Ethnopharmacol. 2008;116(1):125–130. doi: 10.1016/j.jep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Kosek M, Bern C, Guerrant RL. The global burden of diarrheal disease, as estimated from studies published 1992 between and 2000. Bull World Health Organ. 2003;81(3):197–204. [PMC free article] [PubMed] [Google Scholar]
- 4.Thapar N, Sanderson IR. Diarrhea in children: an interface between developing and developed countries. Lancet. 2004;363(9409):641–653. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- 5.Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhea. Cochrane Database Syst Rev. 2004;(2):CD003048. doi: 10.1002/14651858.CD003048.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Subbotina MD, Timchenko VN, Vorobyov MM, Konunova YS, Aleksandrovih YS, Shushunov S. Effect of oral administration of tormentil root extract (Potentilla tormentilla) on rotavirus diarrhea in children: a randomized, double blind, controlled trial. Pediatr Infect Dis J. 2003;22(8):706–711. doi: 10.1097/01.inf.0000078355.29647.d0. [DOI] [PubMed] [Google Scholar]
- 7.Palombo EA. Phytochemicals from traditional medicinal plants used in the treatment of diarrhea: Modes of action and effects on intestinal function. Phytother Res. 2006;20(9):717–724. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Matsuda M, Ohashi K, Taniguchi K, Nakagomi O, Abe Y, et al. Analysis of antirotavirus activity of extract from stevia rebaudiana. Antiviral Res. 2001;49(1):15–24. doi: 10.1016/s0166-3542(00)00134-0. [DOI] [PubMed] [Google Scholar]
- 9.Rahmatullah M, Mollik MAH, Paul AK, Jahan R, Khatun A, Seraj S, et al. A comparative analysis of medicinal plants used to treat gastrointestinal disorders in two sub-districts of greater Khulna division, Bangladesh. Adv Nat Appl Sci. 2010;4(1):22–28. [Google Scholar]
- 10.Agarwal PK, Singh A, Gaurav K, Goel S, Khanna HD, Goel RK. Evaluation of wound healing activity of extracts of plantain Banana (Musa sapientum Var. Paradisiaca) in rats. Indian J Exp Bio. 2009;47:32–40. [PubMed] [Google Scholar]
- 11.Coe FG, Anderson GJ. Ethnobotany of the Sumu (Ulwa) of southeastern Nicaragua and comparisons with Miskitu plant lore. Econ Bot. 1999;53(4):363–386. [Google Scholar]
- 12.Prasad KV, Bharathi K, Srinivasan KK. Evaluation of Musa (Paradisiaca Linn. cultivar) “Puttubale” stem juice for antilithiatic activity in albino rats. Indian J Physiol Pharmacol. 1993;37(4):337–341. [PubMed] [Google Scholar]
- 13.Lewis DA, Fields WN, Shaw GP. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. var. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. J Ethnopharmacol. 1999;65(3):283–288. doi: 10.1016/s0378-8741(99)00005-7. [DOI] [PubMed] [Google Scholar]
- 14.Ojewole JA, Adewunmi CO. Hypoglycemic effect of methanolic extract of Musa paradisiaca (Musaceae) green fruits in normal and diabetic mice. Methods Find Exp Clin Pharmacol. 2003;25(6):453–456. doi: 10.1358/mf.2003.25.6.769651. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan K, Vijayalakshmi NR. Alterations in lipids & lipid peroxidation in rats fed with flavonoid rich fraction of banana (Musa paradisiaca) from high background radiation area. Indian J Med Res. 2005;122:540–546. [PubMed] [Google Scholar]
- 16.Houghton PJ, Skari K. The effect of Indian plants used against snakebite on blood clotting. J Pharm Pharmacol. 1992;44:1054–1060. [Google Scholar]
- 17.Alam MB, Hossain MS, Ekramul Haque M. Anti-oxidant and anti-inflammatory activities of the leaf extract of Brassica nigra. Int J Pharm Sci Res. 2011;2(2):303–310. [Google Scholar]
- 18.Jha MK, Alam MB, Hossain MS, Islam A. In vitro antioxidant and cytotoxicity potential of Costus speciosus (Koen.) smith rhizome. Int J Pharm Sci Res. 2010;1(10):138–144. [Google Scholar]
- 19.Shoba FG, Thomas M. Study of antidiarrheal activity of four medicinal plants in castor oil induced diarrhea. J Ethnopharmacol. 2001;76:73–76. doi: 10.1016/s0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 20.Doherty SN. Inhibition of arachidonic acid release, mechanism by which glucocorticoids inhibit endotoxin-induced diarrhea. Br J Pharmacol. 1981;73:549–554. doi: 10.1111/j.1476-5381.1981.tb10454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdullahi Al, Agho MO, Amos S, Gamaniel KS, Wambebe C. Antidiarrheal activity of the aqueous extract of Terminalia avicemmoides roots. Phytoth Res. 2001;15:431–434. doi: 10.1002/ptr.860. [DOI] [PubMed] [Google Scholar]
- 22.Sanmugapriya E, Venkataraman S. Toxicological investigations on Strychnos potatorum seeds in experimental models. J Health Sci. 2006;52(4):339–343. [Google Scholar]
- 23.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 24.Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 25.Sreejayan N. Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49(1):105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 26.Oyaizu M. Studies on products of the browning reaction antioxidative activities of browning reaction products prepared from glucosamine. Japanese J Nutr. 1986;44(6):307–315. [Google Scholar]
- 27.Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50(6):1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- 28.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–182. [Google Scholar]
- 29.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Path. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 30.Nell G, Rummel W. Action mechanism of secretagogue drugs. In: Csaky TZ, editor. Pharmacology of Intestinal Permeation. Berlin: Springer-Verlag; 1984. pp. 464–474. [Google Scholar]
- 31.Capasso F, Mascolo N, Izzo AA, Gaginella TS. Dissociation of castor oil induced diarrhea and intestinal mucosal injury in rat: effect of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1994;113(4):1127–1130. doi: 10.1111/j.1476-5381.1994.tb17113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galvez A, Zarzuelo ME, Crespo MD, Lorente M, Ocete A, Jimenez J. Antidiarrhoeic activity of Euphorbia hirta extract and isolation of active flavonoid constituents. Planta Med. 1993;59(4):333–336. doi: 10.1055/s-2006-959694. [DOI] [PubMed] [Google Scholar]
- 33.Mascolo N, Izzo AA, Gaginella TS, Capasso F. Relationship between nitric oxide and platelet activating factor in castor oil induced mucosal injury in the rat duodenum. Naunyn Schmiedebergs Arch Pharmacol. 1996;353(6):680–684. doi: 10.1007/BF00167187. [DOI] [PubMed] [Google Scholar]
- 34.Gaginella TS, Stewart JJ, Olsen WA, Bass P. Action of recinoleic acid and structurally related fatty acid on the gastrointestinal tract. II. Effect on water and electrolyte absorption in vitro. J Pharmacol Exp Ther. 1975;195(2):355–356. [PubMed] [Google Scholar]
- 35.Hasan R, Hossain M, Akter R, Jamila M, Mazumder MEH, Islam I, et al. Antioxidant, antidiarrhoeal and cytotoxic properties of Punica granatum Linn. Latin Am J Pharm. 2009;28(5):783–788. [Google Scholar]
- 36.Zavala MA, Perez S, Perez C, Vargas R, Perez RM. Antidiarrhoeal activity of Waltheria americana, Commelina coelestis and Alternanthera repens. J Ethnopharmacol. 1998;61(1):41–47. doi: 10.1016/s0378-8741(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama T. Suppression of hydroperoxide-induced cytotoxicity by polyphenols. Cancer Res. 1994;54(7 Suppl):1991s–1993s. [PubMed] [Google Scholar]
- 38.Gorelik S, Ligumsky M, Kohen R, Kanner J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid per-oxidation products. FASEB J. 2008;22(1):41–46. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- 39.Duh PD, Tu YY, Yen GC. Antioxidant activity of the aqueous extract of harng Jyur (Chrysanthemum morifolium Ramat) Lebensm Wiss Technol. 1999;32:269–277. [Google Scholar]
- 40.El-Seedi HR, Nishiyama S. Chemistry of bio-flavonoids. Indian J Pharm Educ. 2002;36:191–194. [Google Scholar]
- 41.Kongkachuichai R, Charoensiri R, Sungpuag P. Carotenoid, flavonoid profiles and dietary fiber contents of fruits commonly consumed in Thailand. Int J Food Sci Nutr. 2010;61(5):536–548. doi: 10.3109/09637481003677308. [DOI] [PubMed] [Google Scholar]
- 42.Lim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007;103(3):1003–1008. [Google Scholar]
- 43.Rahman MA, Wilcock CC. A report on flavonoid investigation in some Bangladesh asclepiads. Bangladesh J Botany. 1991;20(2):175–178. [Google Scholar]
- 44.Longanga OA, Vercruysse A, Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhea in Lomela area, Democratic Republic of Congo (DRC) J Ethnopharmacol. 2000;71(3):411–423. doi: 10.1016/s0378-8741(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 45.Fagbemi JF, Ugoji E, Adenipekun T, Adelowotan O. Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), lemon grass (Cymbopogon citratus S.) and turmeric (Curcuma longa L.) on pathogens. Afr J Biotech. 2009;8(7):1176–1182. [Google Scholar]
- 46.Alam MB, Hossain MS, Ekramul-Haque M. Evaluation of antimicrobial and toxicity of different fractions of Dillenia indica Linn. Bark extract. J Global Pharma Tech. 2010;2(11):37–42. [Google Scholar]
- 47.Mokbel MS, Hashinaga F. Antibacterial and anti-oxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am J Biochem Biotech. 2005;1(3):126–132. [Google Scholar]
- 48.Ilyas M, Haruna AK, Ilyas N. Plant constituents with antidiarrhoeal properties. Bull Sci Assoc Nigeria. 1995;10:5–12. [Google Scholar]