Abstract
Hemangiomas are benign congenital tumors of endothelial origin that are typically seen in childhood and that undergo spontaneous involution with time. They are usually found on the face or less commonly on the trunk. Very few cases of chest-wall angiomas have been reported. This report describes chest-wall angiomas in two children (a 7-year-old boy and a 1-year-old girl) with emphasis on their sonographic features.
Keywords: Hemangioma, Ultrasonography, Chest wall
Sommario
Gli emangiomi sono lesione benigne di natura endoteliale, congenite, tipiche dei bambini e con tendenza all’involuzione spontanea nel corso degli anni. Comunemente interessano il volto, raramente il tronco e sono documentate solo rare localizzazioni a livello della parete toracica.
Riportiamo due casi di emangiomi della parete toracica, in bambini rispettivamente di sette anni e di un anno, con particolare attenzione agli aspetti ecografici.
Introduction
Hemangiomas are benign neoplastic lesions that originate from the proliferation of endothelial cells. They are typically seen in children and usually congenital. With age, they generally undergo a process of involution and sometimes disappear entirely [1]. Hemangiomas are frequently found on the face and neck; they are especially common in the periorificial areas. The chest wall is a rare location for a hemangioma. Color Doppler ultrasonography is an excellent tool for rapid, reliable diagnosis of hemangiomas. We report the sonographic findings in two hemangiomas involving the chest wall of a 7-year-old boy and a 1-year-old girl. The cases confirm the clinical characteristics and natural history of chest-wall hemangiomas, which are generally associated with typical sonographic findings that are highly diagnostic.
The patient provided informed consent to publication of this report.
Case reports
Patient 1
The patient was a 7-year-old boy referred to our staff for a chest-wall mass with discoloration of the overlying skin.
The sonographic examination revealed a complex, spongy mass with multiple vascular spaces (Figs. 1 and 2), which displayed low-resistance flow on color Doppler that was compatible with a venous malformation.
Fig. 1.
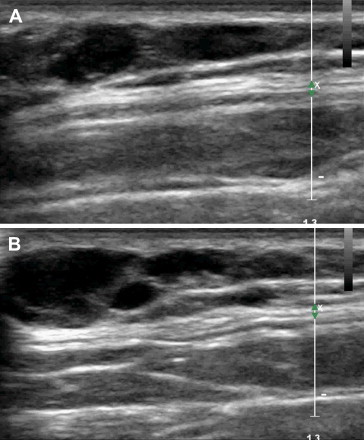
Angioma on the back of a 7-year-old boy (Patient 1). Ultrasound shows a complex mass characterized by anechoic vascular spaces (A, B).
Fig. 2.
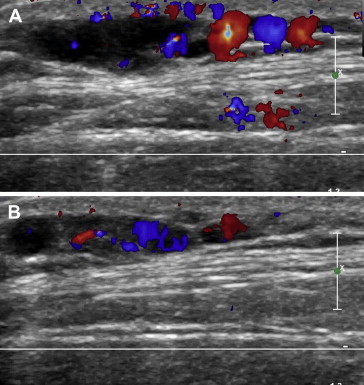
The same patient shown in Fig. 1. Color Doppler confirms that the anechoic spaces consist of vascular structures (A, B).
During the following year, sonographic follow-up revealed decreases in the volume of the lesion.
Patient 2
This patient was a 1-year-old girl with a clinically appreciable lesion in the left subclavian region that had been present since birth.
Ultrasonography revealed a complex formation, approximately 3 cm in diameters, composed of cutaneous and subcutaneous tissues (Fig. 3) and numerous blood vessels (Fig. 4A), most of which were venous (Fig. 4B).
Fig. 3.
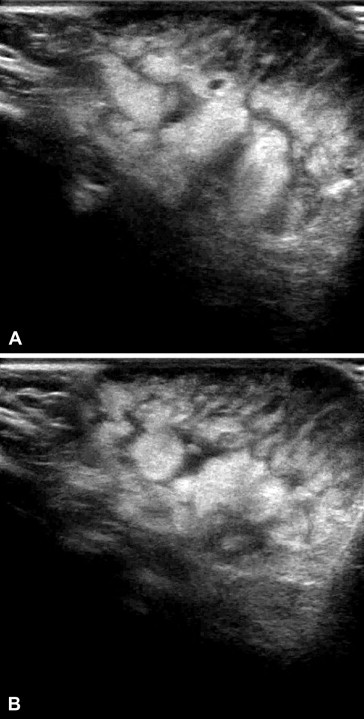
Angioma on the back of a 1-year-old girl. Ultrasonography confirms the presence of a complex mass composed of cutaneous and subcutaneous tissues (A, B).
Fig. 4.
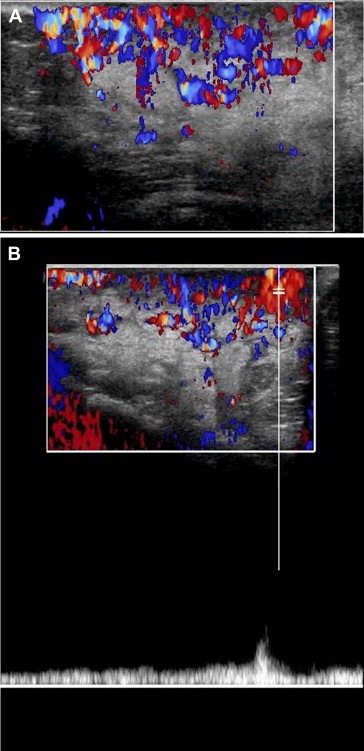
The same patient shown in Fig. 1. Color Doppler reveals the presence within the lesion of numerous vessels (A), most of which are veins (B).
The sonographic findings were indicative of a capillary angioma.
During the year after the diagnosis, sonographic follow-up revealed decreases in the volume of the lesion.
Discussion and conclusions
Hemangiomas are vascular tumors, solitary in most cases, that originate from the proliferation of epithelial cells. They are the most common benign soft-tissue tumor diagnosed in children [2].
At birth, the lesion may be quite small or even absent. Their rate of growth exceeds that of the child’s somatic growth. Later, they undergo a process of slow involution that may end with complete regression [3,4].
Many hemangiomas are small, innocuous tumors, but in some cases the tumor causes functional deficits. They may also be associated with congenital structural anomalies. Functional deficits may arise when the angioma is located near a sensory organ or an anatomic structure with important physiological functions. For example, periorbital hemangiomas, particularly those located on the upper eyelid, can cause serious visual impairments, such as astigmatism, myopia, ptosis, strabismus, and amblyopia. Hemangiomas located in the temporoauricular region can obstruct the external auditory canal, causing disturbances like mechanical hypoacusis, which can delay language development. Hemangiomas in the oral cavity can cause feeding problems; those in the nasal cavity can impair breathing to variable degrees.
It is important to recall that a capillary malformation may be part of a complex or systemic malformation syndrome. Flat angiomas in the craniofacial area innervated by the trigeminal nerve (V1) may be a manifestation of Sturge-Weber-Krabbe syndrome, which includes homolateral lesions of the eyes and leptomeninges together with neurological symptoms (neonatal seizures, motor disturbances with contralateral hemiparesis or hemiplegia, impaired intellectual development) and ocular problems (glaucoma, retinal detachment, blindness) [5,6]. Flat angiomas can also be the first neonatal manifestation of Klippel-Trenaunay syndrome. In this case the capillary malformation typically involves an entire lower limb and sometimes the homolateral hemipelvis or hemithorax and is associated with a complex venous malformation and gigantism of the involved limb. The presence on a limb of an extensive flat angioma can also be the first sign of Parkes-Weber syndrome, which is characterized by arteriovenous microfistulas and hypertrophy of bone and soft tissues that gradually becomes more severe [7].
On the basis of the predominant vascular component, hemangiomas are classified as capillary, cavernous, arteriovenous, venous, or mixed [8]. However, it is more accurate to describe them in terms of their presentation, depending on where they originate within the skin (subcutaneous forms characterized superficial development within the skin layers; tuberous forms characterized by exophytic growth that rises above the cutaneous plane; mixed forms, which include hypodermic and superficial tuberous components) or by their developmental stage (proliferative, quiescent, involutional).
There are typically three phases of development: the first is characterized by rapid postnatal growth (cellular hyperplasia), which begins a few weeks to a few months after birth and ends at 9–10 months of age, when growth peaks. This is followed by amore or less stable plateau phase in which the size remains fairly stable and the lesion appears quiescent. The third phase is characterized by gradual but progressive reabsorption (programmed cell death or apoptosis). The color of the cutaneous portion of the lesion becomes increasingly lighter and gray-white spots develop within it. The subcutaneous portion shrinks and the mass becomes softer, more compressible, with reduced flow that can be demonstrated sonographically.
This final phase generally ends before the child begins school but in rare cases it can last through the eighth or tenth year of life.
Hemangiomas can develop in any part of the body, but the majority (60%) are found on the face or neck; less common sites include the trunk (25%) and extremities (15%) [9]. In some cases, more than one site may be involved [10].
On ultrasound, they appear as complex masses, often with numerous phleboliths.
Color Doppler reveals low resistance arterial flow and/or venous flow within the mass. Color Doppler sonography can be useful for defining the depth of the mass, for identifying the afferent arterioles, and above all for monitoring the evolution of the hemangioma during its proliferative phase. At this point, the flowmetric pattern resembles that of arteriovenous microfistulas (primarily high velocity, diastolic flow) [11–13].
In the cases reported above, the clinical characteristics and natural history are typical of hemangiomas involving the chest wall, which are no different from those located in other regions of the body. These cases illustrate the sonographic features of these lesions, which are general characteristic and more than sufficient for making a correct diagnosis.
Conflict of interest statement
The authors have no conflict of interest.
Appendix. Supplementary material
References
- 1.Castellote A., Vázquez E., Vera J., Piqueras J., Lucaya J., Garcia-Peña P. Cervicothoracic lesions in infants and children. Radiographics. May 1999;19:583–600. doi: 10.1148/radiographics.19.3.g99ma08583. [DOI] [PubMed] [Google Scholar]
- 2.Dohil M.A., Baugh W.P., Eichenfield L.F. Vascular and pigmented birthmarks. Pediatr Clin North Am. 2000;47:783–812. doi: 10.1016/s0031-3955(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 3.Bruckner A.L., Frieden I.J. Hemangiomas of infancy. J Am Acad Dermatol. 2003;48:477–493. doi: 10.1067/mjd.2003.200. [DOI] [PubMed] [Google Scholar]
- 4.Requena L., Sangeguza O.P. Cutaneous vascular proliferations: part II. Hyperplasias and benign neoplasms. J Am Acad Dermatol. 1997;37:887–919. doi: 10.1016/s0190-9622(97)70065-3. [quiz 920-2. Review] [DOI] [PubMed] [Google Scholar]
- 5.Kihiczak N.I., Schwartz R.A., Józwiak S., Silver R.J., Janniger C.K. Sturge–Weber syndrome. Cutis. 2000 Mar;65(3):133–136. [PubMed] [Google Scholar]
- 6.Jay V. Sturge–Weber syndrome. Pediatr Dev Pathol. 2000 May–Jun;3(3):301–305. doi: 10.1007/s100249910040. [DOI] [PubMed] [Google Scholar]
- 7.Roebuck D.J. Klippel–Trenaunay and Parkes-Weber syndromes. AJR Am J Roentgenol. 1997 Jul;169(1):311–312. doi: 10.2214/ajr.169.1.9207562. [DOI] [PubMed] [Google Scholar]
- 8.Chiller K.G., Passaro D., Frieden I.J. Hemangiomas of infancy. Clinical characteristics, morphologic subtypes and their relationship to race, ethnicity and sex. Arch Dermatol. 2002;138:1567–1576. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 9.Finn M.C., Glowacki J., Mulliken J.B. Congenital vascular lesions: clinical application of a new classification. J Pediatr Surg. 1983;18:894–900. doi: 10.1016/s0022-3468(83)80043-8. [DOI] [PubMed] [Google Scholar]
- 10.Senthilkumar M., Thappa D.M. Vascular nevi in children. Indian J Dermatol Venereol Leprol. 2006;72:19–23. doi: 10.4103/0378-6323.19712. [DOI] [PubMed] [Google Scholar]
- 11.Enjolras O. Management of hemangiomas. Dermatol Nurs. 1997 Feb;9(1):11–17. [PubMed] [Google Scholar]
- 12.Frieden I.J., Eichenfield L.F., Esterly N.B., Geronemus R., Mallory S.B. Guidelines of care for hemangiomas of infancy. American academy of dermatology guidelines/outcomes committee. J Am Acad Dermatol. 1997 Oct;37(4):631–637. doi: 10.1016/s0190-9622(97)70183-x. [DOI] [PubMed] [Google Scholar]
- 13.Haimowitz J.E. Guidelines of care: hemangiomas of infancy. J Am Acad Dermatol. 1998 Oct;39(4 Pt 1):662. doi: 10.1016/s0190-9622(98)70028-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


