Abstract
Has color Doppler a role in the evaluation of mammary lesions? A schematic review of color Doppler diagnostic criteria, indicating a benign or malignant lesion, are described.
Malignant tumors have a tendency to show increased vascularization, but avascular tumors may be found as well as hypervascular benign tumors.
Number of vessels, blood-flow velocity, and intra-tumoral vessel resistance assessed by color Doppler ultrasonography don’t permit clear differentiation between malignant and benign tumors, but intra-tumoral blood-flow analysis well correlates with aggressiveness and histological grade of the mass, so a preoperative assessment using Color Doppler may give preliminary prognostic informations useful for therapeutic planning.
Color Doppler ultrasound may be valuable also in assessing the efficacy of neoadjuvant chemotherapy and in particular of antiangiogenesis treatments.
Color Doppler ultrasound has many other useful applications in the evaluation of diseases of the female and male breast, that are reviewed in the paper.
Color Doppler however is not conceived as a stand-alone examination, but associated with B-mode ultrasound that is quite effective as a diagnostic tool, often not requiring Doppler informations.
Keywords: Color Doppler, Breast, Mammary lesions
Sommario
Il color Doppler ha un ruolo nella valutazione delle lesioni mammarie? Viene presentata una rassegna schematica dei criteri diagnostici color Doppler, che indicano la benignità o la malignità di una lesione.
I tumori maligni hanno la tendenza a mostrare vascolarizzazione aumentata, ma si possono trovare tumori maligni avascolari, così come tumori benigni ipervascolarizzati.
Numero di vasi, velocità del flusso, indici di resistenza non consentono una sicura differenziazione tra tumori maligni e benigni, ma l’analisi del flusso intratumorale si correla bene con l’aggressività ed il grado istologico della neoplasia, quindi una valutazione preoperatoria, utilizzando il Color Doppler, può fornire informazioni prognostiche preliminari utili per la pianificazione terapeutica.
Può essere utile anche per valutare l’efficacia della chemioterapia neoadiuvante e, in particolare di trattamenti antiangiogenesi.
Il color Doppler ha molte altre applicazioni nella valutazione delle patologie della mammella femminile e maschile, che vengono esaminati nel presente articolo.
Il color Doppler, tuttavia, non è concepito come un esame autonomo, ma associata all’ecografia B-mode, che è molto efficace come strumento diagnostico delle lesioni mammarie e che, spesso, non richiede informazioni Doppler.
Introduction
Clinical, ultrasonographic and mammographic examinations are excellent morphological tools for the assessment of breast masses, but they cannot evaluate the vascularization of the lesions, that may give informations regarding their nature and, in the malignant subset, the aggressiveness, the response to therapy, the prognosis and the probability to metastatize.
An attempt was made in the past to evaluate the vascularization of mammary lesions, initially with angiography, and subsequently by using the Doppler. The angiographic results, however, were modest and the studies were interrupted; then, with the advent of color Doppler in the 90’s, there was a new impetus in studying the vascularization of mammary lesions.
In the first studies it was emphasized as signals were more numerous and stronger in breast tumors [1,2].
Now we know that this is not true in all instances [3,4].
Malignant tumors had a tendency to show increased vascularization, and new particular feeding vessels are stimulated to form to enable the cancers growth, but this occurs mainly in high-grade high-cellular tumors, while low-grade cancers may have no demonstrable vascularization at color Doppler.
Overlapping is not uncommon with some benign tumors that are hypervascular.
Number of vessels, blood-flow velocity, and intra-tumoral vessel resistance did not permit clear differentiation between malignant and benign tumors [4].
Color Doppler has other useful applications, such as: the capacity of distinguishing complicated cysts from some solid quite anechoic malignant lesions (medullary carcinomas, metastases to the breast); distinguishing intracystic or intraductal debris or apocrine metaplasia from solid growths like papillary lesions and intraductal carcinomas; demonstrating inflammatory hyperemia in abscesses and mastitis; assessing whether lymphadenopathy is caused by inflammation or metastatic breast carcinoma or lymphoma.
Color Doppler however is not conceived as a stand-alone examination, but associated with B-mode ultrasound that is quite effective as a diagnostic tool, often not requiring Doppler informations.
Here below are presented examples where Color Doppler is valuable.
Fibroadenomas
Fibroadenomas that have high stromal cellularity or containing adenosis (complex fibroadenomas) often show internal vascularization at Color Doppler, differently from the fibrosclerotic or hyalinized ones that may show small peripheral vessels but not internal flow.
Fibroadenomas have usually a harmonic vascularization with 3 vessel types: feeding vessels, which are prominent vessels leading from the surrounding breast tissue into the fibroadenoma; capsular vessels, which are located within the tissue capsule; and segmental vessels, which are located within the fibrous septa of the fibroadenoma (Fig. 1) [5].
Figure 1.
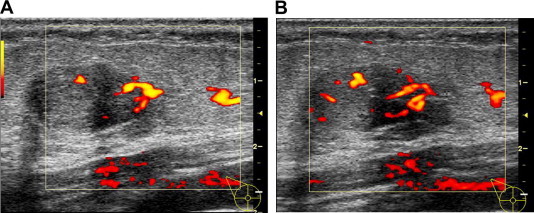
Fibroadenoma. There are 3 vessel types: feeding vessels, which are prominent vessels leading from the surrounding breast tissue into the fibroadenoma (A); capsular vessels, which are located within the tissue capsule; and segmental vessels, which are located within the fibrous septa of the fibroadenoma (B).
Cysts
Cysts can be divided in: simple, complicated and complex. Complicated cysts need to be distinguished from quite anechoic malignant lesions such as medullary carcinomas, metastases to the breast or intra-mammary metastatic lymph-nodes: color Doppler can exclude complicated cysts if vascularization is present.
In complex cysts the demonstration of one or more fibrovascular stalks (Fig. 2) in the solid components may indicate benign intracystic papilloma or intracystic papillary carcinoma (the definitive diagnosis requires histological analysis).
Figure 2.
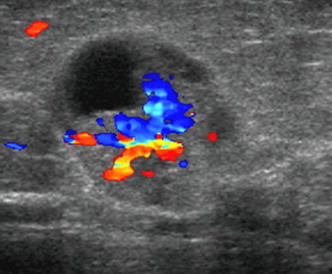
Complex cyst. US shows intracystic mural nodule with fibrovascular stalk in the solid components (histological analysis: intracystic papilloma).
Focal fibrocystic disease
Fibrocystic disease or mammary dysplasia is a group of histologically benign entities with some proliferative changes that have a potential for development of breast carcinoma.
Focal fibrocystic disease may present as a mass or focus lesion sometimes mimicking a malignant breast lesion [6,7].
In other instances US may find a complex mass with solid and cystic components (Fig. 3A) showing rich vascularization at Color Doppler (Fig. 3B) : in these cases vacuum-assisted biopsy can demonstrate the presence of atypical hyperplasia or sometimes noninvasive carcinoma.
Figure 3.
Focal fibrocystic disease. US shows a complex mass (A) with rich vascularization (B).
Giant fibroadenomas of the breast
Juvenile or giant fibroadenoma is a rare pathology usually presenting in adolescent and young girls (10–18 years old) that is characterized by massive and rapid enlargement of an encapsulated mass [8]. The etiology is believed to be an end-organ hypersensitivity to normal levels of gonadal hormones.
Treatment is usually surgical and ranges from simple excision to subcutaneous mastectomy with reconstruction.
US scan reveals a hypoechoic mass (Fig. 4A) with rich vascularization at Color Doppler (Fig. 4B).
Figure 4.
Giant fibroadenoma of the breast in girls. US scan reveals a hypoechoic mass (A) with rich vascularization (B).
Diagnosis was made with core needle biopsy.
Phyllodes tumors
Phyllodes tumors are rare fibro-epithelial neoplasms; they may be classified as benign, borderline or malignant according to histological assessment of mitotic index, stromal hypercellularity and pleomorphism, and well circumscribed or invasive borders [6–8].
They most frequently affect white Latin women in the perimenopausal age, but sometimes teenagers are affected too.
The incidence of metastases is strictly correlated to the degree of histologic aggressiveness.
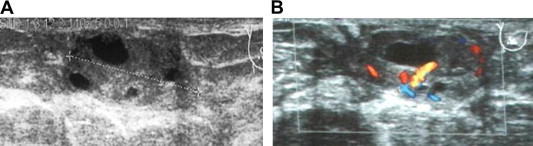
Phyllodes tumor in B-mode ultrasound is appreciated as a solid, omogeneous lesion [6,7] (Fig. 5A). Color Doppler flow image shows high vascularity in the mass [9] (Fig. 5B, C).
Figure 5.
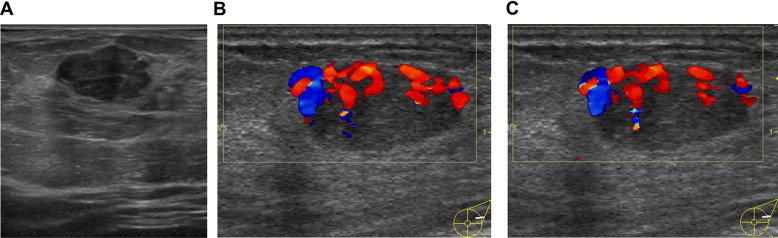
Phillodes tumors. Phillodes tumors in B-mode appares as a solid, omogeneous lesion (A). Color Doppler flow image shows high vascularity in the mass (B, C).
Inflammatory disease
Ultrasonography using Color Doppler can depict vascularization in thick-walled cysts, allowing the distinction between old fibrotic cysts with low level internal echoes and abscesses or infected galactoceles, where wall hyperemia is associated with fluid-debris (pus) levels.
Color Doppler ultrasonography permits to assess the features and evolution (Fig. 6) and post-treatment changes of the abscesses; it is useful to guide drainage especially of multilobed abscesses or a biopsy when abscessual collections have disappeared after therapy and doubts remain about an abscessual neoplasm versus non-puerperal abscess [10] (Fig. 7).
Figure 6.

Non-puerperal abscesses. US image shows a hypoechoic lesion, with irregularly shaped, acoustic enhancement, within which no vessels were observed at color Doppler but US increased vascularity around the mass (A, B).
Figure 7.
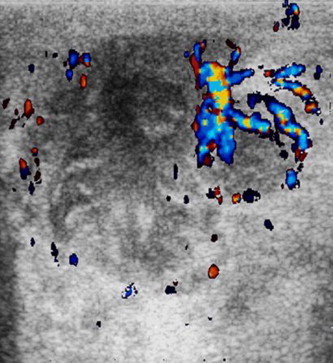
Abscessual breast cancer. US image shows a hypoechoic lesion, with irregularly shaped, acoustic enhancement, within which no vessels were observed at color Doppler but US increased vascularity around the mass.
In acute mastitis Color Doppler shows hyperemia in superficial cellulitis as well as around infected galactoceles or along infected ducts deeply in the breast.
Cancers
Previous studies using color-coded duplex sonography in the diagnosis of breast tumors revealed that the evaluation criteria of the number of vessels, blood-flow velocity, and intra-tumoral vessel resistance did not permit clear differentiation between malignant and benign tumors.
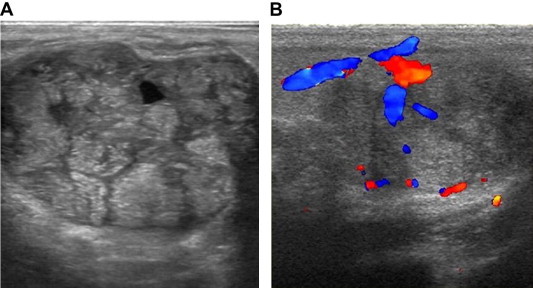
Malignant tumors had a tendency to show increased vascularization (Fig. 8), but this is not the rule and it is usually true for high-grade high-cellular cancers, while low-grade carcinomas that show prominent desmoplastic reaction behave differently.
Figure 8.
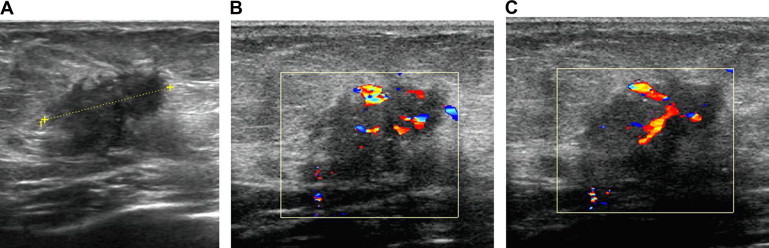
Carcinomas. US image shows a hypoechoic lesion, with irregularly shaped (A); color Doppler shows vessels with irregular course, sinusoids, and arteriovenous shunts (B, C).
The vessel architecture of malignant tumors is known from pathologic studies: tumoral vessels are characterized by caliber fluctuations, irregular course, formation of sinusoids, and arteriovenous shunts [4,5] (Fig. 8 B, C).
Papillary lesions and particularly carcinomas are hypervascularized, but caution and a very slight pressure is needed to appreciate the vascular stalks.
The evaluation of tumor response to neoadjuvant chemotherapy by means of physical examination, ultrasonography, and mammography has limitations on the basis of its morphological approach and the impossibility of distinguishing residual caner from fibrosis. Color Doppler imaging may have the potential to measure tumor response [11–13] (Fig. 9), because vascular changes often precede volumetric shrinkage of the tumor.
Figure 9.
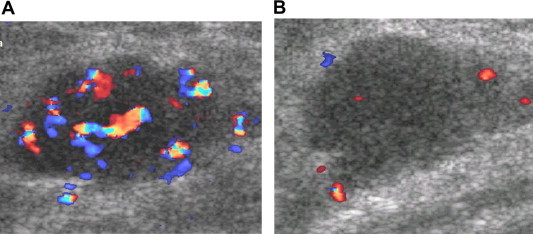
Evaluation of tumor response to neoadjuvant chemotherapy. Color Doppler imaging pre- (A) and post-chemotherapy (B) shows decreased vascularity of the tumor as positive response to chemotherapy.
Color Doppler however is unable to indicate whether a complete response has occurred.
Finally, Doppler is very useful for ultrasound-guided breast biopsy because permits to avoid the sampling of the most vascularized part of the lesion.
Axillary lymph-nodes
The diagnostic value of ultrasonography and color Doppler combined with fine needle aspiration (FNA) cytology for the detection of non-palpable axillary lymph node metastases in breast cancer patients has a high sensitivity and specificity when there are suspicious features.
Color Doppler can help in distinguishing inflammatory lymph-nodes, which show increase in arborization of the hilar vessel, from non-enlarged or aspecific metastatic lymph-nodes, whose cortical sinusoids contain tumoral cells that stimulate the formation of characteristic transcapsular vessels.
Lymphomas may develop multiple feeding vessels that however usually enter through the hilum of the lymph-nodes.
Mondor’s disease
Mondor’s disease or thrombophlebitis of subcutaneous veins of chest region is an uncommon condition that may be rarely associated with breast cancer.
Gray-scale sonography shows a non-compressible, tortuous or beaded hypoechoic structure with hyperechoic layers in the subcutaneous fat, without a demonstrable flow signal at Color Doppler.
Conclusion
Color Doppler imaging may help in differentiating between malignant and benign solid breast masses, but it doesn’t show high predictive values, so its role is only complementary to the high-sensitive B-mode evaluation raising or confirming the doubts upon indeterminate or suspicious lesions.
Magnetic Resonance Imaging is significantly better when considering vascular items both for preoperative assessment and during chemotherapic treatment of breast cancer, as well as for post-treatment changes.
However, intra-tumoral blood-flow analysis by color Doppler ultrasonography correlates well with histological grade and aggressiveness of the cancers, and can be used as first step assessment of the efficacy of neoadjuvant and antiangiogenesis treatments.
Color Doppler ultrasound is a valuable or sometimes problem-solving tool in a lot of breast conditions and its usefulness also in the male breast is out of doubt [14,15].
Conflict of interest
The authors have no conflict of interest to disclose.
Appendix.
References
- 1.Cosgrove D.O., Bamber J.C., Davey J.B., McKinna J.A., Sinnett H.D. Color Doppler signals from breast tumors. work in progress. Radiology. 1990 Jul;176(1):175–180. doi: 10.1148/radiology.176.1.2191364. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove D.O., Kedar R.P., Bamber J.C., al-Murrani B., Davey J.B., Fisher C. Breast diseases: color Doppler US in differential diagnosis. Radiology. 1993 Oct;189(1):99–104. doi: 10.1148/radiology.189.1.8372225. [DOI] [PubMed] [Google Scholar]
- 3.Draghi F., Coopmans de Yoldi G.F. Color Doppler ultrasonography of the breast. image results. Radiol Med. 1995;89:158–163. [PubMed] [Google Scholar]
- 4.Svensson W.E., Pandian A.J., Hashimoto H. The use of breast ultrasound color Doppler vascular pattern morphology improves diagnostic sensitivity with minimal change in specificity. Ultraschall Med. 2010 Oct;31(5):466–474. doi: 10.1055/s-0028-1109478. Epub 2010 Jan 21. [DOI] [PubMed] [Google Scholar]
- 5.Strano S., Gombos E.C., Friedland O., Mozes M. Color Doppler imaging of fibroadenomas of the breast with histopathologic correlation. J Clin Ultrasound. 2004 Sep;32(7):317–322. doi: 10.1002/jcu.20041. [DOI] [PubMed] [Google Scholar]
- 6.Chen J.H., Nalcioglu O., Su M.Y. Fibrocystic change of the breast presenting as a focal lesion mimicking breast cancer in MR imaging. J Magn Reson Imaging. 2008 Dec;28(6):1499–1505. doi: 10.1002/jmri.21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masciadri N., Ferranti C. Benign breast lesions: ultrasound. J Ultras. 2011;14(2):55–65. doi: 10.1016/j.jus.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan P.H., Thike A.A., Tan W.J., Thu M.M., Busmanis I., Li H. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012 Jan;65(1):69–76. doi: 10.1136/jclinpath-2011-200368. Epub 2011 Nov 2. [DOI] [PubMed] [Google Scholar]
- 9.Draghi F., Sabolla L., Campani R., Meloni G. Diagnostic imaging of phyllodes tumors: preliminary observations. Radiol Med. 1996;91:585–589. [PubMed] [Google Scholar]
- 10.Draghi F., Calliada F., Ferrozzi G. Cancer mammarie inflammatoire et abcès non puerperaux: diagnostic et suivi par mammographie, ecographie et Doppler couleur. JEMU. 1999;20(1):30–40. [Google Scholar]
- 11.Miyatake K., Kubota K., Ogawa Y., Hamada N., Murata Y., Nishioka A. Non-surgical care for locally advanced breast cancer: radiologically assessed therapeutic outcome of a new enzyme-targeting radiosensitization treatment, Kochi oxydol-radiation therapy for unresectable carcinomas, type II (KORTUC II) with systemic chemotherapy. Oncol Rep. 2010 Nov;24(5):1161–1168. doi: 10.3892/or_00000968. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A., Srivastava V., Singh S., Shukla R.C. Color Doppler ultrasonography for treatment response prediction and evaluation in breast cancer. Future Oncol. 2010 Aug;6(8):1265–1278. doi: 10.2217/fon.10.93. [DOI] [PubMed] [Google Scholar]
- 13.Roubidoux M.A., LeCarpentier G.L., Fowlkes J.B., Bartz B., Pai D., Gordon S.P. Sonographic evaluation of early-stage breast cancers that undergo neoadjuvant chemotherapy. J Ultrasound Med. 2005 Jul;24(7):885–895. doi: 10.7863/jum.2005.24.7.885. [DOI] [PubMed] [Google Scholar]
- 14.Draghi F., Ferrozzi G., Calliada F. B-mode, color and power Doppler ultrasonography of the male breast. Breast Dis. 1998:309–312. [Google Scholar]
- 15.Draghi F., Tarantino C.C., Madonia L., Ferrozzi G. Ultrasonography of the male breast. J Ultras. 2011;14(3):122–129. doi: 10.1016/j.jus.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


