Abstract
Purpose
The efficacy of platelet-rich plasma (PRP) in the treatment and healing of chronic tendinopathy through stimulation of cell proliferation and total collagen production has been demonstrated by both in vitro and in vivo studies. The aim of this study is to evaluate the effectiveness of ultrasound (US)-guided autologous PRP injections in patellar and Achilles tendinopathy.
Materials and methods
Autologous PRP was injected under US-guidance into the Achilles and patellar tendons (30 Achilles tendons, 28 patellar tendons) in 48 prospectively selected patients (30 males, 18 females, mean age 38 ± 16 years, range 20–61 years). All patients were previously evaluated according to the Victoria Institute of Sport Assessment (VISA) scale, which assessed pain and activity level, and they all underwent US of the tendon before treatment and at follow-up after 20 days and 6 months. Statistical analysis was performed with Chi-square and Wilcoxon tests.
Results
20 days after PRP injection the patients presented a non-significant improvement of clinical symptoms. At the 6-month follow-up VISA score increased from a mean value of 57–75.5 (p < .01). US evaluation revealed a reduction of hypoechoic areas in 26 tendons (p < .01) associated with a widespread improvement of fibrillar echotexture of the tendon and reduced hypervascularity at power Doppler.
Conclusion
PRP injection in patellar and Achilles tendinopathy results in a significant and lasting improvement of clinical symptoms and leads to recovery of the tendon matrix potentially helping to prevent degenerative lesions. US-guidance allows PRP injection into the tendon with great accuracy.
Keywords: Platelet-rich plasma, Achilles tendon, Patellar tendon, Jumper's knee, Achilles tendinopathy
Sommario
Scopo
Il plasma ricco di piastrine (PRP), provocando un iperafflusso di citochine nel sito di iniezione si è dimostrato efficace, sia in vitro che in studi clinici in vivo, come promotore della rigenerazione tissutale.
Lo scopo di questo studio è quello di valutare l’efficacia dell’iniezione ecoguidata di PRP autologo nel trattamento delle tendinopatie croniche del rotuleo e dell’achilleo.
Materiali e metodi
Il PRP autologo è stato iniettato sotto guida ecografica in tendini rotulei e achillei (30 tendini di Achille, 28 tendini rotulei) di 48 pazienti selezionati in modo prospettico (30 maschi, 18 femmine, età media 38 ± 16 anni, range 20–61 anni). Tutti i pazienti erano stati precedentemente valutati mediante questionario Victoria Institute of Sport di valutazione (VISA) in cui si analizza il punteggio del dolore e il livello di attività.
È stata poi eseguita una valutazione ecografica dei tendini mirata a valutarne l’ecostruttura e vascolarizzazione. La valutazione statistica dei dati ottenuti è stata eseguita mediante i test del Chi-quadrato e di Wilcoxon.
Risultati
Venti giorni dopo l’iniezione di PRP è stato rilevato un miglioramento dei sintomi clinici non significativo. Il punteggio VISA è migliorato da una base di 57 a 75,5 a 6 mesi (p < .01). La valutazione ecografica ha rilevato una riduzione delle aree ipoecogene tendinee in 26 tendini a 6 mesi (p < 0,01) con un generale miglioramento dell’ecostruttura fibrillare tendinea e riduzione dell’ipervascolarizzazione al power Doppler.
Conclusione
La terapia infiltrativa con PRP per il trattamento della tendinopatia rotulea e achillea si è rivelata efficace, determinando un miglioramento significativo e duraturo dei sintomi clinici e permettendo un recupero della fisiologica struttura tendinea. La guida ecografica permette inoltre di iniettare il PRP all'interno del tendine con elevata precisione.
Introduction
Chronic musculoskeletal diseases are the most common cause of severe long-term pain and physical disability [1]. Patellar tendinopathy (a degeneration of the tendon enthesis caused by a repetitive and/or chronic overloading that exceeds the adaptive capacity of the tendon) and Achilles tendinopathy (a degenerative tendinous disorder that commonly involves the hypovascular area located from 2 to 6 cm above the calcaneal insertion) are commonly considered the result of multiple micro-tears that do not heal because of poor tendon vascularity [2,3]. Histological features in the early stages of the disease are focal areas of asymptomatic intratendineous damage without signs of inflammation [4,5]. Histological appearance of degenerative damage begins and progresses long before onset of symptoms. This delay causes late therapeutic approaches to be less successful [6,7]. Patellar tendinopathy, in which degeneration is located at the proximal enthesis, is also known as jumper's knee. This disease mostly affects young athletes and is associated with running, jumping, soccer, and volleyball [5]. Prevalence has been estimated to approx. 40%–50% among professional volleyball players and 35%–40% among basketball players. Some intrinsic factors (e.g. gender, age and overweight) and extrinsic factors (e.g. functional overload and training errors) play an important role in the development of chronic tendinopathy [5–9].
Achilles tendinopathy is a common condition in the adult population. In 59% of patients it is related to sports activities, and 53% of them are runners [10,11]. At present, patients affected by jumper's knee and Achilles tendinopathy are primarily treated with rest, ice packs, physical therapy, non-steroidal anti-inflammatory drugs (NSAIDs) and steroid injections, although there is no evidence of the effectiveness of these therapies [10–13].
New theories of tendon regeneration have been focused on the role of platelets and the development of different therapeutic strategies in the treatment of chronic tendinopathy [14]. Platelets are involved in the mechanism of thrombus formation and participate in the healing process by removing necrotic tissues and stimulating regeneration and healing of the tissues [15,16]. Platelet-rich plasma (PRP) with a high concentration of platelets has been used in cardiac, maxillofacial, plastic, neuro, and orthopedic surgery [17–19]. More recently, PRP injections have been used to treat muscle, tendon, and cartilage injuries [20,21].
In this preliminary study, we evaluated the effectiveness of ultrasound (US)-guided intratendineous administration of PRP to treat jumper's knee and Achilles tendinopathy in a population of young athletes.
Materials and methods
Patients
Institutional Review Board approval and patients' informed consent were obtained.
A total of 28 patellar tendons in 24 patients (14 men, 10 women) and 30 Achilles tendons in 24 patients (16 men, 8 women) were prospectively evaluated. Patients affected by patellar tendinopathy (group 1) were aged between 21 and 56 years (mean age 37.4 years), while the age of patients affected by Achilles tendinopathy (group 2) ranged from 20 to 61 years (mean age 38.6 years). All patients practiced sports at the competitive or amateur level.
The inclusion criteria for the study were the presence of patellar or Achilles tendinopathy, pain at palpation and during physical activity for at least three months and US or magnetic resonance imaging (MRI) evidence of tendon degeneration.
All patients had previously undergone other treatments (NSAIDs, laser therapy, US, shock waves, local administration of corticosteroids) with unsatisfactory outcome.
Exclusion criteria were systemic disease (diabetes, rheumatoid arthritis, coagulopathy, severe cardiovascular disease, infection, immunosuppression), antiplatelet therapy in progress, intake of NSAIDs less than 5 days before the procedure, hemoglobin <11 g/dl and platelets <150.000/mmc.
Pre-treatment clinical and US evaluation
Pre-treatment clinical evaluation was performed using the Victoria Institute of Sport Assessment questionnaire for knee (VISA-P) and for the Achilles tendon (VISA-A) [22,23] adapted to the Italian language [24,25]. The VISA-P and VISA-A questionnaires consist of 8 questions, of which 6 deal with pain experienced during a range of everyday activities and 2 questions deal with the ability to perform sports activities. Separate scales are used [22–25] and high scores indicate a low level of pain or impairment. The maximum VISA-P and VISA-A score for an asymptomatic subject who regularly exercises is 100 points and the theoretical minimum is 0 points.
US examination was performed using US equipment (MyLab 70 XvG, Esaote, Italy) and a high-resolution linear transducer (13-6 MHz). For each patient the following parameters were recorded: patellar or Achilles tendon thickness, presence/absence of hypoechoic areas and vascularity on power Doppler according to a semiquantitative scale (0 = absent; 1 = few spots; 2 = moderate; 3 = intense).
Percutaneous US-guided PRP injection
In all patients, after thorough disinfection of the skin and subcutaneous administration of local anesthesia (4 ml of 2% mepivacaine), US-guided scarification of the degenerated region of the tendon was performed using a 21G needle in order to produce a small amount of bleeding.
Afterward, using the same needle, 6 ml of autologous PRP prepared from a previously collected sample (110 ml of venous blood collected from the patient a few hours before the procedure was centrifuged and activated in the laboratory) were injected into the degenerated region of the tendon under US-guidance Fig. 1.
Figure 1.
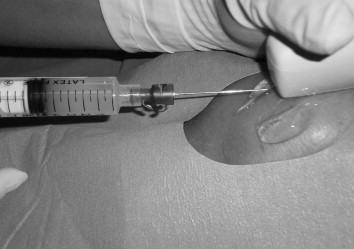
US-guided injection of platelet-rich plasma (PRP) in a patellar tendon of a 24-year-old football player.
In both patellar and Achilles tendons, 2 treatments were performed at a mean distance of 3 ± 0.52 weeks (mean ± standard deviation). Fig. 2 shows images of a pre-and post-treatment patellar tendon.
Figure 2.
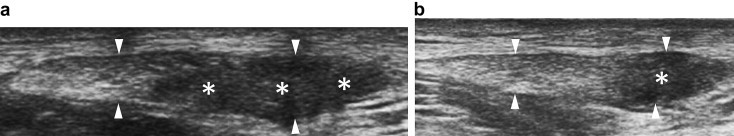
A 26-year-old rugby player: axial scan of a patellar tendon: (a) The tendon is thickened (arrowheads) with a large focal hypoechoic area (asterisks); (b) Six months after US-guided platelet-rich plasma (PRP) injection, tendon thickness (arrows) is slightly decreased compared to baseline, but the hypoechoic area is markedly reduced. VISA-P score changed from 51 to 77.
Patients were asked to minimize physical activity for 48 h after treatment and to avoid maximum efforts during the following two weeks. After that, physiokinesitherapy was recommended associated with a gradual return to sports activities. Patients were also told to avoid drug therapies with particular reference to corticosteroids and NSAIDs.
Follow-up
Pain occurring between the first and second PRP administration was assessed using a visual analogue scale (VAS). In all patients, clinical evaluation was performed using VISA-P or VISA-A questionnaires. US evaluation included patellar or Achilles tendon thickness, presence/absence of hypoechoic areas and vascularity on power Doppler 20 days and 6 months after the second PRP administration. At the 6-month follow-up, patients were also asked about their overall satisfaction with the treatment using a semiquantitative scale (poor, good, excellent).
Statistical analysis
VISA-P and VISA-A scores, patellar and Achilles tendon thickness at baseline and at different time points (20 days and 6 months) were compared using the Wilcoxon test. Presence/absence of hypoechoic areas at US examination at different time points compared to baseline was assessed using the Chi-square test. Vascularity on power Doppler at different time points was assessed using the two-way ANOVA test with post-hoc analysis. A P-value less than 0.05 was considered as significant. The Bonferroni correction was applied when needed.
Results
No peri- and post-procedural complications were observed.
Patellar tendinopathy
Patients experienced moderate post-procedural pain in 27/28 tendons (96%) which lasted for 3 ± 2 days after PRP administration (mean VAS = 4.8); they were treated with local application of ice packs. One patient experienced intense pain over one treated tendon (1/28; 4%) from the day following the treatment (VAS = 8) and the pain lasted for about 2 weeks. Twenty-day follow-up was performed in all patients but 6-month follow-up was performed in 18 patients only (20 tendons). In all treated cases, there was a gradual return to sports activities in an average period of 6 weeks from the second PRP administration.
Regarding the VISA-P score, a minimal(non-significant) improvement compared to baseline was observed at the first follow-up (20 days) and a significant improvement at the second follow-up (6 months). The 20-day follow-up revealed a non-significant reduction in the number of tendons with focal hypoechoic areas; at the 6-month follow-up this reduction was statistically significant. Tendon thickness was not significantly reduced at the 20-day follow-up compared to baseline, but it was significantly reduced after 6 months. Intratendinous vascularity was significantly increased both at the 20-day and 6-month follow-up compared to baseline. Detailed data are reported in Table 1 and graphically represented in Fig. 3. Overall results related to all tendons are reported in Table 3.
Table 1.
Clinical and US evaluation of 28 tendons in 24 patients affected by jumper's knee who underwent US-guided PRP injection.
| Baseline | Twenty days | Six months | |
|---|---|---|---|
| Victoria Institute sport assessment - P | 56 ± 18 | 60 ± 19 | 74 ± 14 |
| P | 0.644a | 0.044a | |
| Tendon thickness; mean ± standard deviation (mm) | 17 ± 8 | 16 ± 9 | 11 ± 5 |
| P | 0.925a | 0.031a | |
| Hypoechoic areas; tendons | 28/28 | 26/28 | 15/28 |
| P | 0.874b | 0.021b | |
| Power Doppler; median (25th–75th percentile) | 2 (2–3) | 3 (2–3) | 1 (1–2) |
| P | 0.411c | 0.001c |
P values are calculated in comparison to baseline using the:
Wilcoxon test.
Chi-square test.
two-way ANOVA test.
Figure 3.
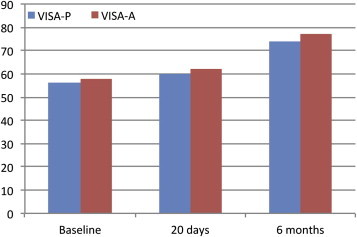
VISA evaluation of patellar and Achilles tendinopathy: Baseline, 20 days and 6 months after US-guided percutaneous injection of platelet-rich plasma (PRP).
Achilles tendinopathy
Patients experienced moderate post-procedural pain in 28/30 tendons (93%) which lasted for 4 ± 2 days after PRP administration (mean VAS = 5.1); they were treated with local application of ice packs. Two patients experienced intense pain over two of these tendons (2/30; 7%) from the day following the treatment (VAS = 8.6) and the pain lasted for about 2 weeks.
Twenty-day follow-up was performed in all patients but 6-month follow-up was performed in 19 patients only (23 tendons). As regards the VISA-A score, minimal (non-significant) improvement compared to baseline was observed at the 20-day follow-up and a significant improvement at the 6-month follow-up. The 20-day follow-up revealed a non-significant reduction in the number of patients presenting focal hypoechoic areas; this reduction was statistically significant at the 6-month follow-up. Tendon thickness was not significantly reduced at the 20-day follow-up compared to baseline; at the 6-month follow-up this reduction was statistically significant. Intratendinous vascularity was significantly increased both at the 20-day and 6-month follow-up compared to baseline. Detailed data are reported in Table 2 and graphically represented in Fig. 3.
Table 2.
Clinical and US evaluation of 30 tendons in 24 patients affected by Achilles tendinopathy who underwent US-guided PRP injection.
| Baseline | Twenty days | Six months | |
|---|---|---|---|
| Victoria Institute sport assessment - A | 58 ± 16 | 62 ± 20 | 77 ± 12 |
| P | 0.144a | 0.001a | |
| Tendon thickness; mean ± standard deviation (mm) | 15 ± 5 | 14 ± 6 | 10 ± 3 |
| P | 0.285a | 0.004a | |
| Hypoechoic areas; tendons | 30/30 | 27/30 | 17/30 |
| P | 0.075b | 0.001b | |
| Power Doppler; median (25th–75th percentile) | 2 (2–2) | 3 (2–3) | 1 (1–2) |
| P | 0.094c | 0.002c |
P values are calculated in comparison to baseline using:
Wilcoxon test.
Chi-square test.
two-way ANOVA test.
Overall results related to all tendons are reported in Table 3.
Table 3.
Overall clinical and US evaluation of 58 patellar and Achilles tendons which underwent US-guided PRP injection.
| Baseline | Twenty days | Six months | |
|---|---|---|---|
| Victoria Institute sport assessment – P and A | 57 ± 17 | 61 ± 19.5 | 75.5 ± 13 |
| P | 0.548a | 0.004a | |
| Hypoechoic areas; number | 58/58 | 52/58 | 32/58 |
| P | 0.011b | 0.001b | |
| Power Doppler; median (25th–75th percentile) | 2 (2–3) | 3 (2–3) | 1 (1–2) |
| P | 0.072c | 0.001c |
P values are calculated in comparison to baseline using:
Wilcoxon test.
Chi-square test.
two-way ANOVA test.
At the 6-month follow-up, overall satisfaction was rated by patients in whom patellar tendon was treated as positive in 20 tendons (71%; excellent = 8/28 tendons; good = 12/28 tendons) and poor in 8 tendons (29%).
At the 6-month follow-up, overall satisfaction was rated by patients in whom Achilles tendon was treated as positive in 21 tendons (70%; excellent = 10/30 tendons; good = 11/30 tendons) and poor in 9 tendons (30%).
Overall satisfaction at the 6-month follow-up was rated by patients of both groups as positive in 41 tendons (70%; excellent = 18/58 tendons; good = 23/58) and poor in 17 tendons (30%).
Discussion
Patellar tendinopathy and Achilles tendinopathy are common conditions in elite and amateur athletes as a result of overuse during sports activity. In both cases, repetitive overloading of the tendon stimulates the local release of cytokines resulting in an impaired reparative process [26–28].
Several treating options are available for these conditions [4,29]. Physiotherapy and steroid injections have proved to have no long-term efficacy [30,31]. Shock waves may be effective but are painful when performed in acute conditions [32], while surgical debridement is effective but expensive and invasive [33,34]. Recently, autologous growth factors have been proposed to treat different pathologic conditions [14] with the aim of promoting and supporting the biological reparative response of tissues to obtain complete regeneration.
The therapeutic effect of PRP has been proposed on the basis of the knowledge of the effects of platelet-derived growth factors in the stages of reparative cascade [35–38]. In vitro studies have shown that PRP may increase protein-coding gene expression of the extracellular matrix, collagen production and tenocyte proliferation [39–41]. Tendon tissue is known to heal slower than connective tissues because of a poor intrinsic vascularization [42]. No inflammatory response is observed in histological samples taken from chronic tendon injuries, but rather an abnormal response to fibroblast and vascular degeneration, known as angiofibroblastic hyperplasia [43]. Based on these data, new therapeutic strategies have been proposed. These strategies aim at stimulating inflammation rather than suppressing it, by trying to modify the poor intrinsic reparative capacity of the tendons.
Some studies of PRP administration in tendinopathy have demonstrated the validity of such a therapeutic option in cases which were unresponsive to conservative therapy alone [36,38]. However, other studies of large series have reported that PRP treatment of tendinopathy is no more effective than placebo [44,45]. Thus, this issue remains challenging and requires further studies to clearly define the effectiveness of PRP therapy.
In the present study, the 6-month follow-up showed that US-guided PRP injection combined with dry needling improved symptoms and tendon structure. The significantly improved VISA scores at the 6-month follow-up were consistent with results previously obtained by other authors [38,39,46]. Also, the fact that tendon thickness and hypoechoic areas were reduced may be a sign of tendon regeneration, as collagen fibers were more closely packed like in normal tendons. Finally, the increased power Doppler signal, both at the 20-day and 6-month follow-up, is a sign of an induced vascular response needed to improve tendon regeneration.
The absence of peri- and post-procedural complications proves the safety of PRP injection. The 3 cases of severe postprocedure pain may be due to stimulation of the body's natural inflammatory response.
As previously described by other authors [47–50], a therapy proposed for treating tendinopathy of the patellar and Achilles tendon is effective only if sports activity is suspended for a period and the therapy is followed by eccentric rehabilitation and a subsequent gradual return to sports activity. PRP treatment of jumper's knee and Achilles tendinopathy should therefore not be performed if the patient is scheduled for demanding physical activity in the near future. All patients treated in connection with the present study were told to return gradually to sports activity over an average period of 6 weeks from the second PRP administration.
The main limitation of the present study is the lack of a control group. This is particularly important, as it is not clear whether the effectiveness of the treatment is a result of PRP administration or of the concurrent performance of dry needling. However, this preliminary study was mainly focused on testing the feasibility of the PRP procedure in the authors' clinical setting. The sample is furthermore inhomogeneous as both patellar and Achilles tendons were treated.
Conclusions
The present study shows that US-guided treatment of jumper's knee and Achilles tendinopathy using PRP is feasible as it is an effective and minimally invasive treatment option. Further randomized controlled studies performed on a larger sample size are warranted to confirm these preliminary results.
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Oral communication prize winner at the 23rd SIUMB Congress in Rome 2011.
Appendix A. Supplementary data
References
- 1.Lawrence R.C., Helmick C.G., Arnett F.C., Deyo R.A., Felson D.T., Giannini E.H. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Cook J.L., Khan K.M., Harcourt P.R., Kiss Z.S., Fehrmann M.W., Griffiths L. Patellar tendon ultrasonography in asymptomatic active athletes reveals hypoechoic regions: a study of 320 tendons. Victorian Institute of Sport Tendon Study Group. Clin J Sport Med. 1998;8:73–77. doi: 10.1097/00042752-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Zantop T., Tillmann B., Petersen W. Quantitative assessment of blood vessels of the human achilles tendon: an immunohistochemical cadaver study. Arch Orthop Trauma Surg. 2003;123:501–504. doi: 10.1007/s00402-003-0491-2. [DOI] [PubMed] [Google Scholar]
- 4.Maffulli N., Sharma P., Luscombe K.L. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97:472–476. doi: 10.1258/jrsm.97.10.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lian Ø., Refsnes P.E., Engebretsen L., Bahr R. Performance characteristics of volleyball players with patellar tendinopathy. Am J Sports Med. 2003;31:408–413. doi: 10.1177/03635465030310031401. [DOI] [PubMed] [Google Scholar]
- 6.van Sterkenburg M.N., van Dijk C.N. Mid-portion achilles tendinopathy: why painful? An evidence-based philosophy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1367–1375. doi: 10.1007/s00167-011-1535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josza L.G., Kannus P. Human Kinetics Books; Champaign, IL, USA: 1997. Human tendons. anatomy, physiology, and pathology. 178–184. [Google Scholar]
- 8.Cook J.L., Khan K.M., Kiss Z.S., Purdam C.R., Griffiths L. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med. 2000;19:473–479. doi: 10.7863/jum.2000.19.7.473. [DOI] [PubMed] [Google Scholar]
- 9.Kettunen J.A., Kvist M., Alanen E., Kujala U.M. Long-term prognosis for jumper’s knee in male athletes: a prospective follow-up study. Am J Sports Med. 2002;30:689–692. doi: 10.1177/03635465020300051001. [DOI] [PubMed] [Google Scholar]
- 10.Kader D., Saxena A., Movin T., Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sconfienza L.M., Silvestri E., Cimmino M.A. Sonoelastography in the evaluation of painful achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28:373–378. [PubMed] [Google Scholar]
- 12.Paavola M., Kannus P., Paakkala T., Pasanen M., Järvinen M. Long-term prognosis of patients with achilles tendinopathy. An observational 8-year follow-up study. Am J Sports Med. 2000;28:634–642. doi: 10.1177/03635465000280050301. [DOI] [PubMed] [Google Scholar]
- 13.Peers K.H., Lysens R.J. Patellar tendinopathy in athletes: current diagnostic and therapeutic recommendations. Sport Med. 2005;35:71–87. doi: 10.2165/00007256-200535010-00006. [DOI] [PubMed] [Google Scholar]
- 14.de Vos R.J., van Veldhoven P.L., Moen M.H., Weir A., Tol J.L., Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010;95:63–77. doi: 10.1093/bmb/ldq006. [DOI] [PubMed] [Google Scholar]
- 15.Anitua E., Andia I., Ardanza B., Nurden P., Nurden A.T. Autologus platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 16.Katsura T., Tohyama H., Kondo E., Kitamura N., Yasuda K. Effects of administration of trasforming growth factor (TGF)-beta1 and anti-TGF-beta1 antibody on the mechanical properties of the stress-shielded patellar tendon. J Biomech. 2006;39:2566–2572. doi: 10.1016/j.jbiomech.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Everts P.A., Hoogbergen M.M., Weber T.A., Devilee R.J., van Monftort G., de Hingh I.H. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Curr Pharm Biotechnol. 2012;13(7):1163–1172. doi: 10.2174/138920112800624346. [DOI] [PubMed] [Google Scholar]
- 18.Sheth U., Simunovic N., Klein G., Fu F., Einhorn T.A., Schemitsch E. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Jt Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 19.Sariguney Y., Yavuzer R., Elmas C., Yenicesu I., Bolay H., Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008;24(3):159–167. doi: 10.1055/s-2008-1076752. [DOI] [PubMed] [Google Scholar]
- 20.Magra M., Maffulli N. Nonsteroidal antiinflammatory drugs in tendinopathy: friend or foe. Clin J Sport Med. 2006;16:1–3. doi: 10.1097/01.jsm.0000194764.27819.5d. [DOI] [PubMed] [Google Scholar]
- 21.Sànchez M., Azofra J., Anitua E., Andía I., Padilla S., Santisteban J. Plasma rich in growth factors to treat an articular cartilage avulsion: a case report. Med Sci Sports Exerc. 2003;35:1648–1652. doi: 10.1249/01.MSS.0000089344.44434.50. [DOI] [PubMed] [Google Scholar]
- 22.Visentini P.J., Khan K.M., Cook J.L., Kiss Z.S., Harcourt P.R., Wark J.D. The VISA score: an index of severity of symptoms in patients with jumper's knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22–28. doi: 10.1016/s1440-2440(98)80005-4. [DOI] [PubMed] [Google Scholar]
- 23.Robinson J.M., Cook J.L., Purdam C., Visentini P.J., Ross J., Maffulli N. The VISA-A questionnaire: a valid and reliable index of the clinical severity of achilles tendinopathy. Br J Sports Med. 2001;35:335–341. doi: 10.1136/bjsm.35.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maffulli N., Longo U.G., Testa V., Oliva F., Capasso G., Denaro V. VISA-P score for patellar tendinopathy in males: adaptation to Italian. Disabil Rehabil. 2008;30:1621–1624. doi: 10.1080/09638280701786070. [DOI] [PubMed] [Google Scholar]
- 25.Maffulli N., Longo U.G., Testa V., Oliva F., Capasso G., Denaro V. Italian translation of the VISA-A score for tendinopathy of the main body of the Achilles tendon. Disabil Rehabil. 2008;30:1635–1639. doi: 10.1080/09638280701785965. [DOI] [PubMed] [Google Scholar]
- 26.Tibesku CO, Pässler HH. Jumper's knee, a review. Sportverletz Sportschaden ;19(2):63–71. [Review. German]. [DOI] [PubMed]
- 27.Paavola M., Kannus P., Järvinen T.A., Khan K., Józsa L., Järvinen M. Achilles tendinopathy. J Bone Joint Surg Am. 2002;84-A:2062–2076. doi: 10.2106/00004623-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Gisslén K., Alfredson H. Neovascularisation and pain in jumper's knee: a prospective clinical and sonographic study in elite junior volleyball players. Br J Sports Med. 2005;39:423–428. doi: 10.1136/bjsm.2004.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan S.C., Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608–1615. doi: 10.1080/09638280701792268. [DOI] [PubMed] [Google Scholar]
- 30.Young M.A., Cook J.L., Purdam C.R., Kiss Z.S., Alfredson H. Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med. 2005;39:102–105. doi: 10.1136/bjsm.2003.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredberg U., Bolvig L., Pfeiffer-Jensen M., Clemmensen D., Jakobsen B.W., Stengaard-Pedersen K. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper's knee and achilles tendinitis: a randomized, double-blind, placebo-controlled study. Scand J Rheumatol. 2004;33:94–101. doi: 10.1080/03009740310004126. [DOI] [PubMed] [Google Scholar]
- 32.Wang C.J. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. 2003;26:220–232. [PubMed] [Google Scholar]
- 33.Orava S., Osterback L., Hurme M. Surgical treatment of patellar tendon pain in athletes. Br J Sports Med. 1986;20:167–169. doi: 10.1136/bjsm.20.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paavola M., Kannus P., Orava S., Pasanen M., Järvinen M. Surgical treatment for chronic Achilles tendinopathy: a prospective seven month follow up study. Br J Sports Med. 2002;36:178–182. doi: 10.1136/bjsm.36.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch S.E., Nixon J.C., Colvin R.B., Antoniades H.N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci USA. 1987;84:7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filardo G., Kon E., Della Villa S., Vincentelli F., Fornasari P.M., Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34(6):909–915. doi: 10.1007/s00264-009-0845-7. Epub 2009 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiegerinck J.I., Reilingh M.L., de Jonge M.C., van Dijk C.N., Kerkhoffs G.M. Injection techniques of platelet-rich plasma into and around the Achilles tendon: a cadaveric study. Am J Sports Med. 2011;39:1681–1686. doi: 10.1177/0363546511401577. [DOI] [PubMed] [Google Scholar]
- 38.Gaweda K., Tarczynska M., Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med. 2010;31:577–583. doi: 10.1055/s-0030-1255028. [DOI] [PubMed] [Google Scholar]
- 39.Schnabel L.V., Mohammed H.O., Miller B.J., McDermott W.G., Jacobson M.S., Santangelo K.S. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 40.de Mos M., van der Windt A.E., Jahr H., van Schie H.T., Weinans H., Verhaar J.A. Can platelet rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Wang J.H. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38:2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 42.Anitua E., Andia I., Sanchez M., Azofra J., del Mar Zalduendo M., de la Fuente M. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF productions by human tendon cells in culture. J Orthop Res. 2005;23:281–286. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 44.de Jonge S., de Vos R.J., Weir A., van Schie H.T., Bierma-Zeinstra S.M., Verhaar J.A. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39:1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 45.de Vos R.J., Weir A., Tol J.L., Verhaar J.A., Weinans H., van Schie H.T. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45:387–392. doi: 10.1136/bjsm.2010.076398. [DOI] [PubMed] [Google Scholar]
- 46.Kon E., Filardo G., Delcogliano M., Presti M.L., Russo A., Bondi A. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598–603. doi: 10.1016/j.injury.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Sampson S., Gerhardt M., Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1:165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visnes H., Hoksrud A., Cook J., Bahr R. No effect of eccentric training on jumper's knee in volleyball players during the competitive season: a randomized clinical trial. Clin J Sport Med. 2005;15:227–234. doi: 10.1097/01.jsm.0000168073.82121.20. [DOI] [PubMed] [Google Scholar]
- 49.Kingma JJ, de Knikker R, Wittink HM, Takken T. Eccentric overload training in patients with chronic Achilles tendinopathy: a systematic review. Br J Sports Med ;41:e3. [DOI] [PMC free article] [PubMed]
- 50.Wasielewski N.J., Kotsko D.M. Does eccentric exercise reduce pain and improve strength in physically active adults with symptomatic lower extremity tendinosis? A systematic review. J Ath Train. 2007;42:409–421. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


