Abstract
Objective
Acoustic radiation force impulse (ARFI) is a new software-based technique that evaluates liver stiffness during B-mode ultrasonography. The purpose of this study was to evaluate the accuracy of ARFI in distinguishing patients with chronic autoimmune liver disease from healthy subjects.
Material and methods
We enrolled 9 adult patients (8 women, 1 man; age 48.1 ± 12.8 years) with chronic autoimmune disease (primary biliary cirrhosis (PBC, n = 3), autoimmune hepatitis (AIH, n = 2), primary sclerosing cholangitis (PSC, n = 1) and overlap syndromes, (n = 3) who underwent a liver biopsy and 11 healthy volunteers (age 34.7 ± 10.4 years; 7 women, 4 men). Liver stiffness was evaluated and expressed as the shear wave velocity (SWV) in m/sec. We used a US scanner Siemens-Acuson S2000, evaluating the right liver lobe and the left liver lobe.
Results
The SWV was significantly higher in cases (right lobe: 1.51 ± 0.44; left lobe: 1.57 ± 0.40) than in controls (right lobe: 1.08 ± 0.10; left lobe: 1.12 ± 0.13) (right lobe: P = 0.002; left lobe: P = 0.013). We found no significant correlation between right and left lobe SWVs in cases (P = 0.779) or controls (P = 0.385). The SWV cut-off that best distinguished cases from controls was 1.25 m/sec (accuracy: AUC=0.885; sensitivity: 70.6%; specificity: 95.5%).
Conclusions
ARFI elastography is a noninvasive ultrasonographic technique that can differentiate healthy subjects from patients with fibrotic stages of chronic liver disease.
Keywords: ARFI imaging, Primary biliary cirrhosis, Autoimmune hepatitis, Primary sclerosing cholangitis
Sommario
Introduzione
L'elastografia ARFI è una tecnologia integrata all'ultrasonografia convenzionale B-mode in grado di determinare la rigidità tissutale. L'obiettivo di questo studio è stato valutare se l'elastografia ARFI sia in grado di differenziare pazienti affetti da patologie epatiche croniche autoimmuni e soggetti normali.
Materiali e metodi
Sono stati arruolati 9 pazienti affetti da epatopatia cronica autoimmune sottoposti a biopsia epatica (età 48.1 ± 12.8 anni; femmine/maschi: 8/1; 3 PBC, 2 AIH, 1 PSC e 3 overlap) e 11 volontari sani (età 34.7 ± 10.4 anni; F/M: 7/4). La rigidità epatica è stata valutata mediante l'elastografia ARFI ed espressa come “shear wave velocity” (SWV) in m/sec. È stato utilizzato un ecografo Siemens-Acuson S2000 valutando entrambi i lobi epatici. Le misurazioni sono state eseguite a respiro bloccato, ad una profondità compresa tra 2 e 5 cm.
Risultati
La SWV è risultata significativamente più elevata nei casi (Dx: 1.51 ± 0.44; Sn: 1.57 ± 0.40) rispetto ai controlli (Dx: 1.08 ± 0.10; Sn: 1.12 ± 0.13) (Dx: P = 0.002; Sn: P = 0.013). Sia nei pazienti con epatopatia cronica (P = 0.779) che nei controlli (P = 0.385) non è stata rilevata una differenza statisticamente significativa della SWV fra i due lobi. Il valore ottimale di SWV per discriminare i casi dai controlli è risultato 1.25 m/sec (accuratezza: AUC = 0.885; sensibilità: 70.6%; specificità: 95.5%).
Conclusioni
L'elastografia ARFI è una metodica ultrasonografica utile nel differenziare soggetti sani da pazienti affetti da epatopatia cronica in evoluzione fibrotica.
Introduction
The prognosis and management of chronic liver diseases depends largely on the extent and progression of hepatic fibrosis, which involves replacement of the hepatic parenchyma by extracellular matrix [1]. Liver biopsy is considered the gold standard method for assessing hepatic fibrosis because it is a direct method [2]. The main limitation of this procedure is its invasiveness, which in rare cases can lead to both minor and major complications [2–4]. Abdominal pain is associated with the procedure in roughly 25% of all cases, and procedure-related complications that require hospitalization are reported in 1–3% [5–7].
Other limitations include the risk of sampling errors [8–11] and operator-dependent variability in interpreting the results [12], which can result in under – as well as overestimation of the degree of liver fibrosis. In addition, the procedure is expensive, and it cannot be repeated frequently to monitor the evolution of the disease. There are also situations in which liver biopsy are contraindicated, including patients with coagulation disorders, those who uncooperative, and those who refuse to undergo biopsy.
Because of the limitations of liver biopsy and the dynamic nature and prognostic/therapeutic relevance of hepatic fibrogenesis in patients with chronic liver disease, several simple noninvasive methods have been proposed over the last decade to obtain simple, low-cost estimates of the extent of this process, which are also accurate, repeatable, and reproducible. These methods have been widely evaluated in patients with viral liver disease caused by hepatitis virus B or C and in those with nonalcoholic fatty liver disease (NAFLD), but there is much less information on their use in rarer forms of chronic autoimmune liver disease, such as primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and the AIH-PBC overlap syndrome.
The new methods used to evaluate liver fibrosis include biologic tools based on the use of direct and indirect biohumoral markers and instrumental methods. Among biologic tools, those that have been most widely tested are the Fibrotest and the APRI score (AST to platelet ratio index). Instrumental approaches include impulse-based elastography (FibroScan) and ultrasound-based elastography (acoustic radiation force impulse elastography – ARFI – and real-time elastography).
The FibroScan has been proposed for assessment of liver stiffness as an indirect index of hepatic fibrosis. It is based on the use of mechanical waves generated by vibrations. The diagnostic performance of this method in patients with HCV-related liver disease has been evaluated in three important multicenter studies [13–15], and it has also been tested in patients with NAFLD [16]. Data on its use in patients with autoimmune liver disease are not as clear or concordant [17,18].
A more recently developed approach, ultrasound-based elastography, is a technique used with conventional B-mode sonography to evaluate the elastic properties of tissues. It has been employed mainly for the differentiation of malignant and benign thyroid, breast, and prostate lesions and for characterization of atherosclerotic plaques [19–23].
Recent studies have evaluated its use in the study of hepatic fibrosis in patients with chronic liver disease (viral in most cases), and the results have shown good concordance with histological data [24,25]. Takahashi et al., for example, found that liver stiffness measured with ARFI correlates with Metavir scores of liver fibrosis. Again, however, there are fewer data on the less common autoimmune liver diseases.
The main objective of this study was to determine patients with chronic autoimmune liver disease can be distinguished from normal subjects with comparative analysis of ARFI elastographic findings in the right and left lobes of the liver.
Materials and methods
The study population included male and female patients with chronic autoimmune liver disease who underwent liver biopsy in the Dept. of Digestive Tract Diseases and Internal Medicine of Saint Orsola-Malpighi Policlinic in Bologna, Italy. The criteria for enrollment were: a) age ≥ 18 years; b) diagnosis of one of the following diseases: PBC, AIH, PSC, or an overlap syndrome; c) indications for liver biopsy; d) patient consent to participation in the study. The diagnosis of PBC was based on the criteria of Lindor et al. [26]. AIH was diagnosed on the basis of the simplified criteria described by the International AIH Group [27]. The diagnosis of PSC was based on the criteria of Lindor et al. [28], and the criteria of Boberg et al. were used to diagnose overlap syndromes [29]. Exclusion criteria were: a) multifactorial liver disease (other than an overlap syndrome); b) ascitic decompensation; c) previous liver transplantation (OLT); d) pregnancy; e) liver tumors; f) alcohol abuse (>20 g/day); g) absence of consent to participation in the study.
The criteria for enrollment of the healthy controls were: negative history for chronic disease, in particular for current or past liver disease; alcohol intake of less than 20 g/day; physical examination negative for liver disease; body mass index (BMI) within normal limits (18.5–24.9); laboratory values, including serum transaminase levels, within normal limits.
Patients and healthy controls
We enrolled 9 consecutive patients with chronic autoimmune hepatitis and 11 healthy controls. The characteristics of the patient group were as follows: mean age 48.1 ± 12.8 years; female:male ratio 8:1. Of the 9 patients enrolled, 3 had PBC, 2 had AIH, 1 had PSC, and 3 had AIH-PBC overlap syndrome. The characteristics of the healthy control group were: mean age 34.7 ± 10.4 years and female:male ratio: 7:4 (P = 0.020 and P = 0.319 versus the patient group, respectively) (Table 1).
Table 1.
Population characteristics.
| Patients (n = 9) | Healthy controls (n = 11) | |
|---|---|---|
| Age | 48 ± 13 years | 35 ± 10 years |
| F/M ratio | 8/1 | 7/4 |
| Autoimmune liver disease | ||
| PBC | 3 | – |
| AIH | 2 | – |
| PSC | 1 | – |
| Overlap syndrome | 3 | – |
| Metavir fibrosis stage | ||
| F0 | 0 | – |
| F1 | 4 | – |
| F2 | 4 | – |
| F3 | 1 | – |
| F4 | 0 | – |
| ARFI Elastography findings | ||
| Right lobe SWV (mean ± SD) | 1.51 ± 0.44 | 1.08 ± 0.10 |
| Left lobe SWV (mean ± SD) | 1.57 ± 0.40 | 1.12 ± 0.13 |
F, female; M, male; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; PSC, primary sclerosing cholangitis; SWV, shear wave velocity.
Liver histology and quantification of fibrosis
Liver biopsies in all participants were ordered for the purpose of disease staging. All biopsy procedures were carried out percutaneously, under ultrasound guidance, by the same operator using a 17G needle. The histological specimens were examined by pathologists who had no knowledge of the elastographic findings. Alterations were classified using the Ishak score (patients with AIH, PSC, or overlap syndrome) or the Scheuer score (patients with PBC).
Semiquantitative assessment of the liver fibrosis was made using the Metavir score with appropriate conversion tables. The fibrosis was classified as follows: F0 – no fibrosis; F1 – portal fibrosis without septa; F2 – portal fibrosis with rare septa, F3 – numerous septa without cirrhosis, F4 – cirrhosis. Liver biopsy was not performed on healthy controls: these subjects were considered a priori to be free of liver fibrosis.
ARFI elastography and measurement of liver stiffness
All patients and healthy controls enrolled in the study underwent ARFI elastography. This new technology, which is available in recent Siemens-Acuson S2000 scanners (Siemens Healthcare, Erlangen, Germany), provides quantitative measurements of hepatic elasticity during the course of a conventional sonographic examination of the abdomen. It involves the delivery of short (100 s), high-frequency ultrasound pulses with the ultrasound transducer. The mechanical force of these pulses causes minimal tissue displacement, which produces a new mechanical wave that propagates transversely to the impulse delivered. The velocity of transverse propagation inside a sample volume of 1 cm2 (shear wave velocity, SWV) is assessed by the scanner and reported on the screen in meters per second. The SWV is proportional to hepatic stiffness: the higher the SWV, the stiffer the liver.
Using a 4C1 transducer, we examined both lobes of each liver. Measurements were made in segments V and VI (right lobe) with an intercostal approach (Figs. 1 and 2). Those in segments III and IV (left lobe) were obtained via a subcostal epigastric approach (Fig. 3). In both cases, care was taken to minimize the pressure exerted with the transducer. Patients were examined in the supine position with the right arm maximally abducted. They were instructed to hold their breath briefly during each measurement. The measurements themselves (5 in the right lobe, 2 in the left lobe) were made at depths ranging from 2 to 5 cm in areas where there were no blood vessels or ligaments. In one patient with PBC, we were unable to measure the SWV in the left lobe of the liver due to the presence of gas-filled intestinal loops.
Figure 1.
ARFI elastography of the right lobe (V segment) (intercostal approach).
Figure 2.
ARFI elastography of the right lobe (VI segment) (intercostal approach).
Figure 3.
ARFI elastography of the right lobe (IV segment) (epigastric approach).
Ethical standards
The study was conducted in accordance with the ethical standards of the Helsinki Declaration (Helsinki, Finland, June 1964), and the protocol had been preapproved by the local ethics commission. All patients and healthy controls provided written informed consent to take part in the study.
Statistical analyses
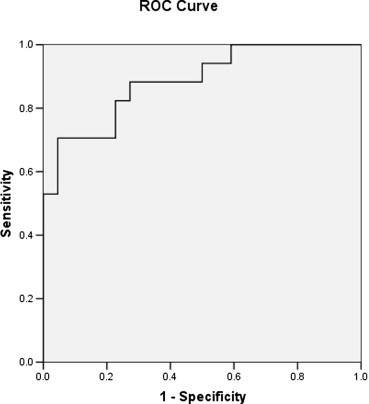
We calculated the mean of all SWV measurements made in each lobe of the liver. Differences involving continuous variables, which were expressed as means with standard deviations, were analyzed with the Mann–Whitney and Wilcoxon tests and the Spearman correlation coefficient. The Fisher exact test was used to analyze dichotomous variables. ROC curves were used to evaluate the accuracy of the SWV in distinguishing patients from healthy controls. The area under the ROC curve (AUC) was calculated, together with the standard error (SE), considering the right and left lobe measurements together. A maximum likelihood method [30] was used to identify the SWV value that best discriminated between patients and healthy controls, and the sensitivity and specificity of this cut-off value were also determined.
Results
On the basis of the histological findings, the fibrosis was classified as follows: F0: 0 patients; F1: 4 patients; F2: 4 patients; F3: 1 patient; F4: 0 patients. The SWV in the patient group (right lobe: 1.51 ± 0.44; left lobe: 1.57 ± 0.40) was significantly higher than that of controls (right lobe: 1.08 ± 0.10 [P = 0.002]; left lobe: 1.12 ± 0.13 [P = 0.013]). The SWVs of the right and left lobes were not significantly different from one another in the patient group (P = 0.779) or in the healthy controls (P = 0.374), and there was no significant correlation between the values recorded for the two lobes in either group (patients: P = 0.779; healthy controls: P = 0.385). The SWV distinguished patients from controls with good accuracy (AUC = 0.885 ± 0.054, mean ± SE). The value that best discriminated between the two groups (calculated on the basis of findings in both lobes) was 1.25 m/sec (sensitivity 70.6% [12/17], specificity 95.5% [21/22]).
Discussion
In recent years various methods have been proposed for the evaluation of liver fibrosis with the aim of reducing the risk (already quite low) associated with liver biopsy, including ultrasound-based elastography. Our preliminary study demonstrates that ARFI elastography can reliably differentiate healthy subjects from patients with liver fibrosis related to chronic liver disease. The SWV values in the patient group were significantly higher than those of the healthy controls. Therefore, ARFI elastography distinguished individuals with fibrosis (F1 or higher in the Metavir classification) from subjects who were healthy and for this reason presumably fibrosis-free (F0 in the Metavir system). Analysis of the overall SWVs for each liver (i.e., the mean of all measurements made in both lobes) showed that the highest discriminatory accuracy was achieved with a cut-off of 1.25 m/sec (accuracy: AUC = 0.885; sensitivity: 70.6%; specificity: 95.5%) (Fig. 4).
Figure 4.
ROC curve.
Our SWV data are similar to those observed in comparative studies of patients with viral hepatitis and healthy controls. In the study published by Takahashi et al in 2009 [24], where measurements were confined exclusively to the right lobe of the liver, the mean (SD) SWV for the controls (1.08 ± 0.13) was identical to that of the controls in our study (1.08 ± 0.10). Similar results were also reported by Piscaglia et al. in 2011 [31]: the mean ± SD SWVs were 1.13 ± 0.61 for the healthy controls and 1.12 ± 0.13 in the patients with chronic viral hepatitis who were fibrosis-free (Metavir stage F0). The cut-off we used to identify patients with fibrosis classified as F1 or higher (1.25 m/sec) is consistent with the findings of Piscaglia et al. [31], who found mean SWVs of 1.27 (0.77–1.62) and 1.28 (0.45–2.15) for patients with F1 and F2 fibrosis, respectively.
Unlike Piscaglia et al. [31], we found no significant difference between the left and right lobe SWVs in our series. The discrepancy is probably related to the fact that none of the patients we examined had cirrhosis, differently from the study of Piscaglia et al. The marked structural and anatomic disruption caused by this condition would more than justify the interlobe differences in SWV they observed [31].
One of the most important advantages of ARFI elastography is that it can be incorporated into the standard B-mode examination, increasing the duration of the examination by only 2–3 min. These data can be combined with the eco-color-Doppler evaluations of the portal veins system for the staging of the hepatic disease.
The main limitation of our study is the small number of subjects examined, which does not allow adequate distinction between the various types of autoimmune liver disease (which differ markedly from one another in terms of clinical manifestations, pathogenetic mechanisms, prognosis, development of hepatic fibrosis, and therapy).
In our opinion, the correlation between ARFI elastographic data and hepatic fibrosis varies depending on the type of liver disease being examined. Similar observations have been made relative to FibroScan. The diagnostic performance of the latter method is good in patients with PBC or PSC [17,32], and the same might be true of ARFI elastography. In patients with AIH or overlap syndrome, the measurements might be affected by the hypertransaminasemia (often quite severe) that accompanies the frequent flare-ups that characterize these diseases. Similar interference has been reported with FibroScan in patients of this type. The inflammatory edema that develops within the nonexpandible hepatic capsule during these flare-ups might cause parenchymal stiffness resembling that related to fibrosis. In these patients, one might observe increases in the SWV similar to the increased FibroScan liver stiffness values observed in some studies during relapse [18].
In conclusion, ARFI elastography is a new, noninvasive complement to B-mode ultrasonography, which can be useful in distinguishing healthy patients from those with fibrosis due to chronic liver disease. However, validation studies in larger, more homogenous populations are needed to evaluate the correlation between the results of this examination and the stages of fibrosis in subgroups of autoimmune liver disease.
Conflict of interest
The authors have no conflict of interest to disclose.
Appendix A. Supplementary data
References
- 1.Friedman S.L. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38(S1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Bravo A.A., Sheth S.G., Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 3.Gunneson T.J., Menon K.V., Wiesner R.H., Daniels J.A., Hay J.E., Charlton M.R. Ultrasound-assisted percutaneous liver biopsy performed by a physician assistant. Am J Gastroenterol. 2002;97:1472–1475. doi: 10.1111/j.1572-0241.2002.05789.x. [DOI] [PubMed] [Google Scholar]
- 4.McGill D.B., Rakela J., Zinsmeister A.R., Ott B.J. A 21 year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396–1400. doi: 10.1016/0016-5085(90)91167-5. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G., Boyer J.L. Outpatient liver biopsy: how safe is it? Ann Intern Med. 1993;118:150–153. doi: 10.7326/0003-4819-118-2-199301150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Janes C.H., Lindor K.D. Outcome of patient hospitalized for complications after outpatient liver biopsy. Ann Intern Med. 1993;118:96–98. doi: 10.7326/0003-4819-118-2-199301150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Castéra L., Nègre I., Samii k, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529–1530. doi: 10.1002/hep.510300624. [DOI] [PubMed] [Google Scholar]
- 8.Regev A., Berho M., Jeffers L.J., Milikowski C., Molina E.G., Pyrsopoulos N.T. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 9.Maharaj B., Maharaj R.J., Leary W.P., Cooppan R.M., Naran A.D., Pirie D. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 10.Poniachik J., Bernstein D.E., Reddy K.R., Jeffers L.J., Coelho-Little M.E., Civantos F. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 11.Guido M., Rugge M. Liver biopsy sampling in chronic viral hepatitis. Semin Liver Dis. 2004;24:89–97. doi: 10.1055/s-2004-823103. [DOI] [PubMed] [Google Scholar]
- 12.Bedossa P., Poynard T., Metavir Cooperative Group Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 13.Ziol M., Handra-Luca A., Kettaneh A., Christidis C., Mal F., Kazemi F. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 14.Castera, Vergniol J., Foucher J., Le Bail B., Chanteloup E., Haaser M. Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Arena U., Vizzutti F., Abraldes J.G., Corti G., Stasi C., Moscarella S. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57(9):1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 16.Wong V.W., Vergniol J., Wong G.L., Foucher J., Chan H.L., Le Bail B. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 17.Corpechot C., El Naggar A., Poujol-Robert A., Ziol M., Wendum D., Chazouillères O. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 18.Romanque P., Stickel F., Dufour J.F. Disproportionally high result of transient elastography in patients with autoimmune hepatitis. Liver Int. 2008;28:1177–1178. doi: 10.1111/j.1478-3231.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 19.Nightingale K.R., Palmeri M.L., Nightingale R.W., Trahey G.E. On the feasibility of remote palpation using acoustic radiation force. J Acoust Soc Am. 2001;110:625–634. doi: 10.1121/1.1378344. [DOI] [PubMed] [Google Scholar]
- 20.Palmeri M.L., Frinkley K.D., Zhai L., Gottfried M., Bentley R.C., Ludwig K. Acoustic radiation force impulse (ARFI) imaging of the gastrointestinal tract. Ultrason Imaging. 2005;27:75–88. doi: 10.1177/016173460502700202. [DOI] [PubMed] [Google Scholar]
- 21.Dahl J.J., Pinton G.F., Palmeri M.L., Agrawal V., Nightingale K.R., Trahey G.E. A parallel tracking method for acoustic radiation force impulse imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:301–312. doi: 10.1109/tuffc.2007.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahl J.J., Dumont D.M., Allen J.D., Miller E.M., Trahey G.E. Acoustic radiation force impulse imaging for noninvasive characterization of carotid artery atherosclerotic plaques: a feasibility study. Ultrasound Med Biol. 2009;35:707–716. doi: 10.1016/j.ultrasmedbio.2008.11.001. [Epub 2009 Feb 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rago T., Vitti P. Potential value of elastosonography in the diagnosis of malignancy in thyroid nodules. Q J Nucl Med Mol Imaging. 2009;53:455–464. [PubMed] [Google Scholar]
- 24.Takahashi H., Ono N., Eguchi Y., Eguchi T., Kitajima Y., Kawaguchi Y. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30(4):538–545. doi: 10.1111/j.1478-3231.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 25.Koizumi Y., Hirooka M., Kisaka Y., Konishi I., Abe M., Murakami H. Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography-establishment of the method for measurement. Radiology. 2011;258(2):610–617. doi: 10.1148/radiol.10100319. [DOI] [PubMed] [Google Scholar]
- 26.Lindor K.D., Gershwin M.E., Poupon R., Kaplan M., Bergasa N.V., Heathcote J., American Association for Study of Liver Diseases Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 27.Hennes E.M., Zeniya M., Czaja A.J., Parés A., Dalekos G.N., Krawitt E.L. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 28.Lindor K.D., Kowdley K.V., Luketic V.A.C., Harrison M.E., McCashland T., Befeler A.S. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50(3):808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boberg K.M., Chapman R.W., Hirschfield G.M., Lohse A.W., Manns M.P., Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374–385. doi: 10.1016/j.jhep.2010.09.002. [Epub 2010 Sep 18] [DOI] [PubMed] [Google Scholar]
- 30.Pezzilli R., Billi P., Miniero R., Fiocchi M., Cappelletti O., Morselli-Labate A.M. Serum interleukin-6, interleukin-8, and beta 2-microglobulin in early assessment of severity of acute pancreatitis. Comparison with serum C-reactive protein. Dig Dis Sci. 1995;40(11):2341–2348. doi: 10.1007/BF02063235. [DOI] [PubMed] [Google Scholar]
- 31.Piscaglia F., Salvatore V., Di Donato R., D'Onofrio M., Gualandi S., Gallotti A. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32(2):167–175. doi: 10.1055/s-0029-1245948. [Epub 2011 Feb 14] [DOI] [PubMed] [Google Scholar]
- 32.Floreani A., Cazzagon N., Martines D., Cavalletto L., Baldo V., Chemello L. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Dig Liver Dis. 2011;43(11):887–892. doi: 10.1016/j.dld.2011.06.011. [Epub 2011 Jul 23] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


