Abstract
Congenital muscular dystrophy (CMD) comprises a heterogeneous group of disorders present at birth with muscle weakness, hypotonia and contractures. Congenital muscular dystrophy (CMD) comprises a heterogeneous group of disorders with muscle weakness, hypotonia and contractures present at birth. A particular subset of classic CMD is characterized by a complete absence of merosin. Merosin-deficient congenital muscular dystrophy (MDCMD) is a rare genetic disease involving the central and peripheral nervous system in the childhood. High signal intensities are often observed throughout the centrum semiovale, periventricular, and sub-cortical white matters on T2-weighted images in MRI brain in children with MDCMD. Apparent diffusion coefficient (ADC) map may reveal increased signal intensity and apparent diffusion coefficient values in the periventricular and deep white matters. These white matter findings, observed in late infancy, decrease in severity with age. The pathogenesis of these changes remains uncertain at present. In this article, we outline the specific MR imaging findings seen in a patient with documented MDCMD and also suggest the causes.
Keywords: Merosin, Congenital muscular dystrophy, Magnetic resonance imaging, Laminin
CASE REPORT
A 3-year old girl, born full term and enjoyed uneventful perinatal period, presented with proximal muscle weakness. She was noted to walk with waddling gait. Gross motor developmental delay was documented as she started walking at 19 months of age. She had normal intelligence and the other parts of physical examination were unremarkable.
Blood tests showed raised creatine kinase. Neural conduction studies showed normal nerve conduction velocity (NCV), with no neuropathy or evidence of peripheral demyelination. However, her electromyography (EMG) demonstrated myopathic pattern. Muscle biopsy was performed and confirmed myopathy with merosin deficient muscular dystrophy.
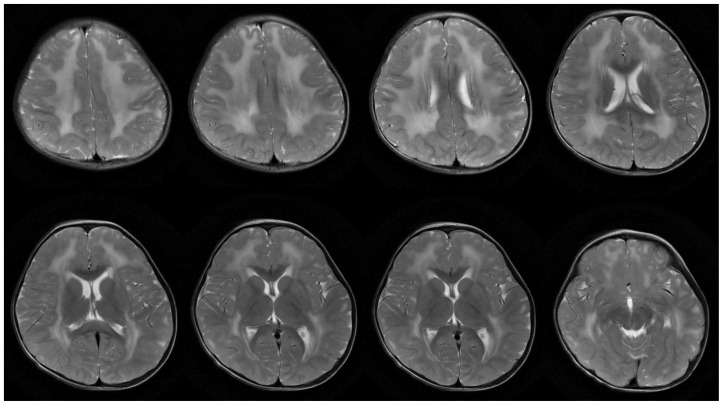
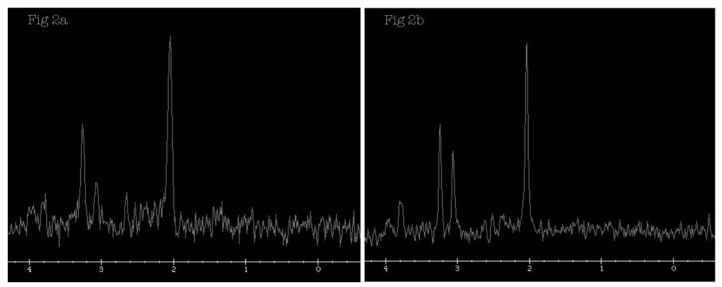
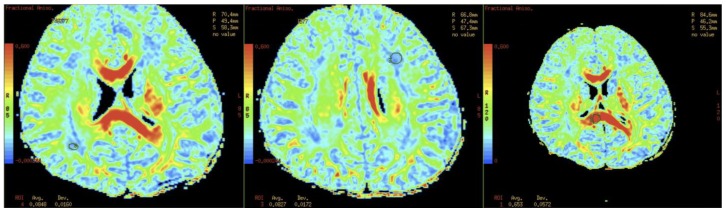
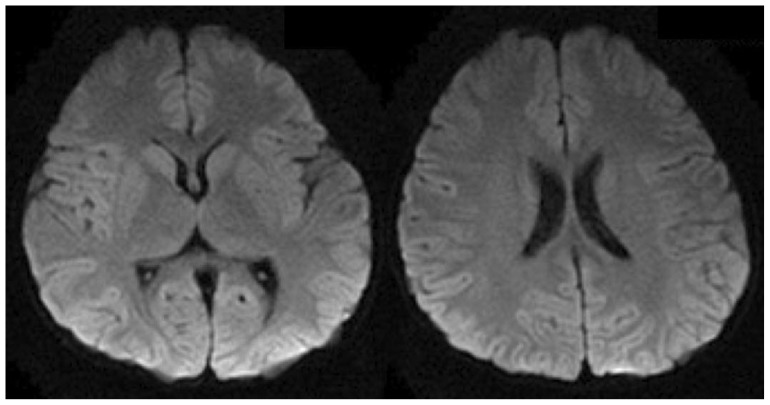
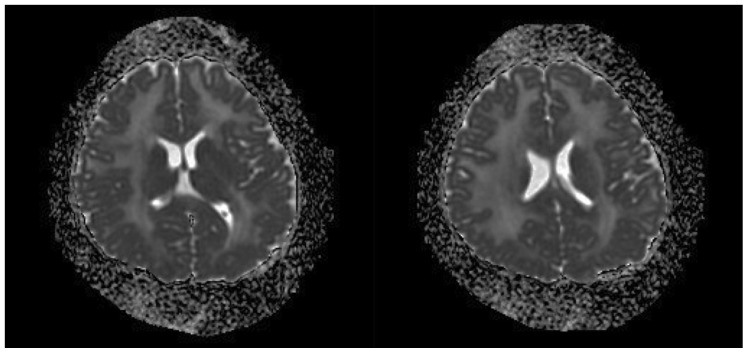
MRI brain of our patient showed diffuse and symmetrical abnormal T2 prolongation in cerebral white matter, without the involvement of corpus callosum; internal capsule; brain stem and cerebellum or other structural abnormalities (Fig. 1). MR spectroscopy (MRS) was performed at TE 288.0 (Fig. 2). White matter metabolites, including N-acetyl aspartate (NAA), choline (Cho) and creatine (Cr) were reduced with increased Cho/Cr ratio. No abnormal lactate peak was seen. Diffusion Tensor Imaging (DTI) also showed reduced Fractional Anisotrophy (FA) in affected white matter (FA<0.18) (Fig. 3). There was no evidence of restricted diffusion in Diffusion Weighted Imaging (DWI) scan (Fig. 4). Apparent Diffusion Coefficient (ADC) mapping revealed abnormally high free-water concentrations in periventricular white matter, especially at bilateral occipital regions (Fig. 5).
Figure 1.
A 3-year-old girl suffered from merosin-deficient congenital muscular dystrophy (MDCMD). Magnetic Resonance Imaging (MRI) brain shows diffuse and symmetrical increase in T2W signal in white matter of cerebral hemispheres. Signal characteristic of corpus callosum, internal capsule, basal ganglia and thalami are normal. Brain stem and cerebellum are structurally normal (not shown). Ventricles are not dilated. The major abnormality is abnormal T2 prolongation in cerebral white matter, without involvement of corpus callosum, internal capsule, brain stem and cerebellum or other structural abnormalities. (Protocol: Magnet strength 1.5 Tesla, TR 4500, TE 1086/Ef, without contrast, 5 mm slice thickness)
Figure 2.
A 3-year-old girl suffered from merosin-deficient congenital muscular dystrophy (MDCMD). Magnetic Resonance Spectroscopy (MRS) is performed at TE 288.0 at left occipital white matter (Fig. 2a). White matter metabolites, including NAA, choline (Cho) and creatine (Cr), are reduced (White matter (WM): NAA = 36, Cho = 28, Cr = 8; Basal ganglia: NAA = 46, Cho = 25, Cr = 19), with increased Cho/Cr ratio. MRS at left basal ganglia is also performed (Fig. 2b) and included here for comparison. (Protocol: Magnet strength 1.5, TR 1800, TE 288.0)
Figure 3.
A 3-year-old girl suffered from merosin-deficient congenital muscular dystrophy (MDCMD). Diffusion Tensor Imaging (DTI) also shows reduced Fractional Anisotrophy (FA) in affected white matter (FA<0.18). FA at corpus callosum is normal. (Protocol: Magnet strength 1.5, TR 15000, TE 86.4/FE, without contrast)
Figure 4.
A 3-year-old girl suffered from merosin-deficient congenital muscular dystrophy (MDCMD). Diffusion Weighted Imaging (DWI) demonstrates no evidence of restricted diffusion. (Protocol: Magnet strength 1.5, TR 10000, TE 81.6/FE, without contrast, 5 mm slice thickness)
Figure 5.
A 3-year-old girl suffered from merosin-deficient congenital muscular dystrophy (MDCMD). Apparent Diffusion Coefficient (ADC) mapping reveals abnormally high free-water concentrations in periventricular white matter, especially at bilateral occipital regions. (Protocol: Magnet strength 1.5, TR 10000, TE 81.6/FE, without contrast, 5 mm slice thickness)
The patient was referred to paediatric neurological clinic for long-term follow-up and subsequent genetic testing confirmed LAMA2 gene mutation.
DISCUSSION
Congenital muscular dystrophies (CMDs) are a heterogeneous group of disorders that are characterized by the early onset of hypotonia, muscular weakness, and dystrophic findings at muscle biopsy. The mode of inheritance is generally autosomal recessive. A dystrophic pattern is present on muscle biopsy specimens [1, 2]. The incidence and prevalence of CMD in populations are not well documented because of the limited molecular genetic confirmation of the diagnosis and the use of different diagnostic classification systems in the past. The estimated incidence is about 0.68 to 2.5 per 100,000. The disease affects both male and female equally and the disease often starts at 6–12 months of age. Unfortunately, there is no definitive treatment for this disease to date.
There are two well-recognized forms of the disease. The classic or pure form of CMD occurs in patients with normal or near-normal intelligence. A particular subset of classic CMD is characterized by a complete absence of merosin. The second form differs from the pure form in that severe mental retardation and brain anomalies are present [2]. This second group includes the Japanese variant known as Fukuyama CMD, the Walker-Warburg syndrome, and the Santavuori syndrome (muscle-eye-brain disease) [3]. Fukuyama, Walker-Warburg, and Santavuori syndromes have a variety of neuropathologic abnormalities that include abnormal cerebral and cerebellar gyral patterns, cerebellar cysts, and white matter changes on MR images [2–8]. The white matter findings, observed in late infancy, decrease in severity with age [2–8]. The pathogenesis of these changes remains uncertain at present.
Primary merosin deficiency is more frequently complete than partial and is associated with a normal intellectual development. It is caused by mutations in the gene encoding the laminin ?2 chain, LAMA2, located on chromosome 6q2 [9]. Merosin is the predominant homologue of laminin ?2 chain in the basal laminae of striated muscle and Schwann cells. Merosin binds ?-dystroglycan and in turn is linked to the subsacrolemmal cytoskeleton via the dystrophin-glycoprotein complex. Deficiency of merosin can disrupt the link between the extracellular matrix and the subsacrolemmal cytoskeleton, and thus causing muscle degeneration [10].
Clinically, patients with MDCMD have severe congenital hypotonia, an inability to achieve independent walking; severe and early joint contractures; normal or near-normal intelligence; and white matter changes at MR imaging. In the literature, CNS involvement is rarely clinically evident, but MR white matter abnormalities are always present [4,6]. Children with the merosin-positive form of CMD generally have a less severe phenotype and no MR imaging abnormalities at presentation [11].
MR imaging findings were strikingly similar in patients suffering from MDCMD. On T2-weighted images, diffuse and symmetrical increase in signal is seen in the white matter of the cerebral hemispheres and normal white matter signal in the cerebellum. MR imaging may be of limited value during the neonatal period and the first few months of life, because of diffusely un-myelinated white matter. After normal myelination in-utero, myelination of the neonatal brain is far from complete. The first myelination is seen as early as the 16th week of gestation, in the column of Burdach, but it really takes off only from 24th week. Myelination of the brain during infancy progresses in an orderly and predictable manner. It does not reach maturity until 2 years of age or so. It correlates very closely with developmental milestones. At birth only certain structures are myelinated, namely: dorsal brainstem, ventrolateral thalamus, lentiform nuclei, central corticospinal tracts, and posterior portion of internal capsule. The progression of myelination is predictable and abides by a few simple general rules, such as myelination progresses from central to peripheral; caudal to rostral; and dorsal to ventral. White matter signal changes in myelinating brain shows gradual shift from hyper- to hypo-intense relative to grey matter in T2 weighted images in children between the ages of 1 and 2. The only area to remain hyper-intense after the age of 2 years, and often for quite some time, is the peritrigonal region. Taking into consideration that the white matter changes in children suffering from MDCMD may not be apparent until 6 months of age and are progressive, longitudinal imaging assessments are useful. There is no report of associated structural abnormalities or neuronal migration anomalies, such as focal occipital agyria, white matter cysts, enlarged lateral ventricles, or hypoplasia of the cerebellar vermis and pons. The corpus callosum, internal capsule, brain stem and cerebellum are structurally normal.
In the brain, merosin has been found in the basement membrane of blood vessels. It has been postulated that in MDCMD, the lack of laminin ?2 may lead to disruption of the blood-brain barrier causing an increase in water content and resulting in abnormal white matter signal intensity. Increased T2 prolongation time on an MR image may be attributable to increased water content in the white matter owing to an abnormal blood-brain barrier rather than to decreased or abnormal myelination. The literature supports this hypothesis, and findings reporting normal brain stem auditory evoked responses in patients with MDCMD [6]. The metabolite (choline, creatine, N-acetyl aspartate) level reductions, fractional anisotropy (FA) reduction and increased apparent diffusion coefficient (ADC) in the white matter suggest increased free extracellular water concentrations in the affected white matter of patients with MDCMD.
Three well-defined syndromes, Fukuyama congenital muscular dystrophy (CMD), Walker-Warburg syndrome and muscle-eye-brain disease, which are all associated with structural brain changes, are differential diagnosis of MDCMD. However, they all have distinct features in MRI brain. In Fukuyama CMD, MRI brain shows structural changes consisting of pachygyria and polymicrogyria and abnormal signal intensity within the white matter associated with delayed myelination. Ventricular dilatation has also been frequently recorded. Cerebellar hypoplasia may be seen but is not frequent. In Walker-Warburg syndrome, MRI brain shows a type II lissencephaly with the typical micropolygyric cobblestone cortex. Cerebellar hypoplasia, mainly affecting the vermis, is also a constant feature. White matter is also severely abnormal showing dysmyelination or cystic changes. Other features, such as brain stem hypoplasia, Dandy-Walker syndrome or encephaloceles have been described, in various combinations. In muscle-eye-brain disease, MRI brain shows extensive abnormalities of neuronal migration, such as pachygyria and polymicrogyria. Brain stem and cerebellar hypoplasia are also frequent but not constantly observed. The white matter can show areas of abnormal signal but these are not very prominent and, when present, are localized in the periventricular areas. Other differential diagnosis also include congenital myotonic dystrophy, mitochondrial myopathy, Leigh syndrome/subacute necrotizing encephalopathy, X-linked myotubular myopathy and peroxisomal disorders. There is a range of associated brain abnormalities in these diseases. MR brain imaging will help in correctly diagnosing infants present with hypotonia, weakness or contractures in the newborn period.
TEACHING POINT
Magnetic resonance (MR) imaging of the brain in children with merosin-deficient congenital muscular dystrophy (MDCMD) reveals a consistent pattern of white matter abnormality and abnormally high free-water concentrations in apparent diffusion coefficient (ADC) mapping and MR spectroscopy. Imaging studies play an important role in the diagnosis of MDCMD, as subtle T2 prolongation, reduction of metabolite (choline, creatine, N-acetyl aspartate) levels and fractional anisotropy and increased ADC in the white matter may be seen in early infancy. Imaging studies also aid in the diagnosis of this form of CMD; and delineate any structural abnormalities in cases of differential diagnosis or neuronal migration anomalies, which are not commonly seen in MDCMD.
Table 1.
Summary table of Merosin-Deficient Congenital Muscular Dystrophy
| Etiology | Mutations in the gene encoding the laminin α2 chain, LAMA2, located on chromosome 6q2 |
| Incidence | 0.68 to 2.5 per 100,000 (Incidence and prevalence of CMD in populations are not well documented because of limited molecular genetic confirmation of the diagnosis and use of different diagnostic classification systems in the past.) |
| Gender ratio | N/A |
| Age predilection | Onset at first 6–12 months of life |
| Risk factors | Family history |
| Treatment | To date no treatment available |
| Prognosis | Poor |
| Findings on imaging | Magnetic Resonance Imaging (MRI) shows diffuse and symmetrical increase in signal in the white matter of the cerebral hemispheres and normal white matter signal in the cerebellum in T2W images. Apparent Diffusion Coefficient (ADC) mapping and Magnetic Resonance Spectroscopy (MRS) reveal reduced white matter metabolites and abnormally high free-water concentrations in the white matter. |
Table 2.
Differential table of Merosin-Deficient Congenital Muscular Dystrophy
| MRI (general) | DWI &ADC | DTI | MRS | |
|---|---|---|---|---|
| MDCMD | Diffuse and symmetrical increase in signal in WM of the cerebral hemispheres and normal WM signal in the cerebellum. | No restricted diffusion in DWI scan. ADC mapping revealed abnormally high free-water concentrations in periventricular WM. |
Reduced Fractional Anisotropy (FA) in affected WM. | WM metabolites, including NAA, Cho and Cr, were reduced with increased Cho/Cr ratio. No abnormal lactate peak was seen. |
| Fukuyama CMD | Polymicrogyria, pachygyria, cerebellar involvement, abnormal T2W signal in WM. Sequential MRI studies have demonstrated that the abnormal WM appearance is associated with delayed myelination rather than with dysmyelination or demyelination as the abnormal signal intensity improves with age. Frontal lobes are the last areas in which the abnormal signal persists. | |||
| Walker–Warburg syndrome | Type II lissencephaly with the typical micropolygyric cobblestone cortex. Cerebellar hypoplasia, mainly affecting the vermis, is also a constant feature. WM is also severely abnormal showing dysmyelination or cystic changes. Other features, such as brain stem hypoplasia, Dandy–Walker syndrome or encephaloceles have been described, in various combinations. |
|||
| Muscle–eye–brain disease | Pachygyria and Polymicrogyria. Brainstem and cerebellar hypoplasia, periventricular WM signal abnormalities frequently but not constantly observed. | |||
| Congenital moronic dystrophy | Ventricular dilatation in all, associated with periventricular or deep WM changes, cortical atrophy, small corpus callosum and small brain stem. | |||
| Mitochondrial myopathy | Cortical and subcortical atrophy and dysmyelination. | Elevated lactate throughout the brain but much more marked in WM. | ||
| Leigh syndrome/subacute necrotizing encephalopathy | Typical pattern with abnormal signal in the bilateral lenticular nuclei and in the caudate with subsequent cavitation. Cerebellar hypoplasia is also frequent. | High levels of lactate in the basal ganglia, even in the absence of increased serum lactate. | ||
| X-linked myotubular myopathy | Diffuse abnormal signal intensity in WM with small petechial foci consistent with hemorrhage. Widened extra-cerebral space is frequent. | |||
| Peroxisomal disorders (Refsum’s disease and Neonatal adrenoleukodystrophy) | Signal abnormalities in WM. Involvement of the brain may not be easily detectable in the neonatal period when it is difficult to differentiate between the unmyelinated white matter and signs of dysmyelination, which is also true for all the above differential diagnosis. | |||
ABBREVIATIONS
- ADC
Apparent diffusion coefficient
- Cho
Choline
- CMD
congenital muscular dystrophy
- Cr
Creatine
- DTI
Diffusion tensor imaging
- DWI
Diffusion weighted imaging
- FA
Fractional anisotrophy
- Fig
Figure
- MDCMD
Merosin-deficient congenital muscular dystrophy
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- NNA
N-acetyl aspartate
- WM
White Matter
REFERENCES
- 1.Caro PA, Scavina M, Hoffman E, et al. MR imaging findings in children with merosin-deficient congenital muscular dystrophy. AJNR Am J Neuroradiol. 1999;20(2):324–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Leyten QH, Gabreels FJ, Renier WO, et al. White matter abnormalities in congenital muscular dystrophy. J NeurolSci. 1995;129:162–169. doi: 10.1016/0022-510x(94)00264-o. [DOI] [PubMed] [Google Scholar]
- 3.Trevisan CP, Martinello F, Ferruzza E, et al. Divergence of central nervous system involvement in 2 Italian sisters with congenital muscular dystrophy: a clinical and neuroradiological follow-up. EurNeurol. 1995;35:230–235. doi: 10.1159/000117134. [DOI] [PubMed] [Google Scholar]
- 4.Trevisan CP, Carollo C, Segalla P, et al. Congenital muscular dystrophy: brain alterations in an unselected series of Western patients. J NeurolNeurosurg Psychiatry. 1991;54:330–334. doi: 10.1136/jnnp.54.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aida N, Tamagawa K, Takada K, et al. Brain MR in Fukuyama congenital muscular dystrophy. AJNR Am J Neuroradiol. 1996;17:605–613. [PMC free article] [PubMed] [Google Scholar]
- 6.Mercuri E, Muntoni F, Berardinelli A, et al. Somatosensory and visual evoked potentials in congenital muscular dystrophy: correlation with MRI changes and muscle merosin status. Neuropediatrics. 1995;26:3–7. doi: 10.1055/s-2007-979711. [DOI] [PubMed] [Google Scholar]
- 7.Reed UC, Marie SK, Vainzof M, et al. Congenital muscular dystrophy with cerebral white matter hypodensity: correlation of clinical features and merosin deficiency. Brain Dev. 1996;18:53–58. doi: 10.1016/0387-7604(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka M, Kuroki S, Mizue H. Congenital muscular dystrophy of non-Fukuyama type with characteristic CT images. Brain Dev. 1987;9:316–318. doi: 10.1016/s0387-7604(87)80052-9. [DOI] [PubMed] [Google Scholar]
- 9.Helbling-Leclerc A, Zhang X, TopalogÏlu H, et al. Mutations in the laminin a2-chain gene (LAMA2) cause merosin deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- 10.Vuolteenaho R, Nissinen M, Sainio K, et al. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994;124:381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pegoraro E, Marks H, Garcia CA, et al. Laminin alpha-2 muscular dystrophy: genotype/phenotype studies of 22 patients. Neurology. 1998;51:101–110. doi: 10.1212/wnl.51.1.101. [DOI] [PubMed] [Google Scholar]