Abstract
Askin tumor is a rare disease which had previously been reported as being thallium-201 and gallium-67 avid. Varying data regarding 18F- fluorodeoxyglucose metabolism has been described with Ewing family of soft tissue tumors. In this case, we present a patient found to have an Askin tumor of the left chest wall which demonstrated indium-111 pentetreotide and technetium-99m MIBI avidity. The lesion did not show 18F- fluorodeoxyglucose hypermetabolism in this case despite the aggressiveness of the tumor. The patient was treated with surgical excision of the tumor and chemotherapy. Subsequently, contrast enhanced CT, indium-111 pentetreotide and technetium 99m-MIBI showed that the lesion had regressed. These findings suggest that Askin tumor can demonstrate Indium-111 pentetreotide and technetium 99m-MIBI uptake and need not be hypermetabolic on 18F-fluorodeoxyglucose exam.
Keywords: Askin, Primitive neuroectodermal tumor, PNET, Ewing, FDG, MIBI, Pentetreotide, Chest wall mass, Pediatric radiology
CASE REPORT
In this case, a 17 year old girl with a past medical history of scoliosis, cerebral palsy and developmental delay presented with shortness of breath. A subsequent chest radiograph (figure 1) and contrast enhanced chest CT (figure 2A and 3A) showed she had a large intrathoracic mass which was heterogeneously enhancing and arising from the chest wall. No calcifications were identified in the mass on CT. The mass was biopsied during the admission and histology demonstrated small round blue cells consistent with Primitive neuroectodermal tumor (PNET) of the chest wall (figure 4). Additionally, immunohistochemical staining was positive for CD-99 (figure 4B). An F18-FDG PET study using coincidence imaging with gamma camera was then performed to further characterize the lesion which showed the lesion not to be hypermetabolic (figure 3B). An In111-pentetrotide (figure 2B) and Tc99m-MIBI (figure 5A & B ) exam was also performed and the lesion demonstrated avidity for both radiopharmaceuticals. After en bloc resection of the tumor and chemotherapy, a repeat contrast enhanced chest CT (figure 6A), In111-pentetreotide (figure 6B) and Tc99m-MIBI (figure 7 A & B) scan was performed about 4 months later and demonstrated resolution of disease.
Figure 1.
A 17-year-old girl with Askin tumor of the left chest wall. Chest radiograph performed during admission for a chief complaint of shortness of breath. A large mass (*) arises from the left lower chest wall and abuts the left heart border.
Figure 2.
A 17-year-old girl with Askin tumor of the left chest wall.
A) Contrast enhanced coronal chest CT performed for characterization of a left chest wall mass. An 11×13×15cm lobulated, heterogeneously enhancing soft tissue mass (*) is seen arising from the chest wall causing mass effect upon the heart and left lung.
B) Fused coronal In111-Pentetreotide SPECT images CT of the chest. The soft tissue mass shows uptake of In111- pentetreotide.
(Protocol: Coronal reformation from a CT of the chest. 140kVp, 1.4mm slice thickness, approximately 100mL Omnipaque 300, venous phase acquisition. Protocol: Coronal SPECT images from In111-pentetreotide. Six hours following IV injection of 6.4 mCi of In-111 pentetreotide, SPECT images of the chest were obtained. Images were fused using manual co-registration and Osirix software)
Figure 3.
A 17-year-old girl with Askin tumor of the left chest wall. A) Contrast enhanced coronal chest CT performed for characterization of a left chest wall mass. An 11×13×15cm lobulated, heterogeneously enhancing soft tissue mass (*) is seen arising from the chest wall causing mass effect upon the heart and left lung.
B) Fused coronal F18-FDG coincidence imaging with coronal CT of the chest. The left chest wall mass does not demonstrate increased uptake of F18-FDG.
(Protocol: Coronal reformation from a CT of the chest. 140kVp, 1.4mm slice thickness, approximately 100mL Omnipaque 300, venous phase acquisition. Protocol: Coronal F18-FDG coincidence images obtained on a gamma camera one hour after injection of 9.7 mCi of F18-FDG intravenously. Fusion was performed with manual co-registration and Osirix software).
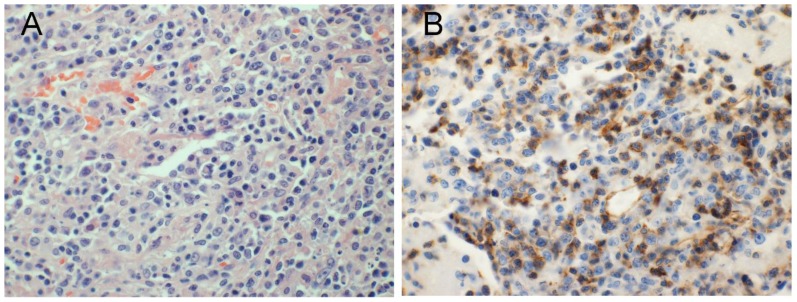
Figure 4.
A 17-year-old girl with Askin tumor of the left chest wall.
A) Hematoxylin and eosin stain; high power view (20× magnification) of a core biopsy of the chest wall mass showing small round blue cells.
B) High power (20× magnification) view of a core biopsy of the chest wall mass with CD-99 immunohistochemistry staining showing positive staining. Overall findings consistent are with primitive neuroectodermal tumor.
Figure 5.
A 17-year-old girl with Askin tumor of the left chest wall. Anterior (A) and posterior (B) Tc99m-MIBI planar images of the chest. Increased uptake of radiopharmaceutical is seen within the left chest wall mass (*).
(Protocol: Planar images were obtained 10 minutes after IV injection of 22.0 mCi of Tc99m-MIBI.)
Figure 6.
A 17-year-old girl with Askin tumor of the left chest wall after surgical resection.
A) Contrast enhanced coronal chest CT performed approximately 4 months after surgical resection of a left chest wall mass. A small pleural effusion (*) is seen in the surgical bed where the tumor was resected.
B) Fused coronal In111-Pentetreotide SPECT images CT of the chest. No radiotracer uptake is seen in the region of the surgical bed where only a small pleural effusion (*) is present.
(Protocol: Coronal reformation from a CT of the chest. 140kVp, 1.4mm slice thickness, approximately 100mL Omnipaque 300, venous phase acquisition. Protocol: Coronal SPECT images from In111-pentetreotide. Six hours following IV injection of 6.0 mCi of In-111 pentetreotide, SPECT images of the chest were obtained. Images were fused using manual co-registration and Osirix software).
Figure 7.
A 17-year-old girl with Askin tumor of the left chest wall after surgical resection. Anterior (A) and posterior (B) Tc99m-MIBI planar images of the chest obtained approximately 4 months after surgical resection of mass. There is resolution of the previously seen uptake.
(Protocol: Planar images were obtained 10 minutes after IV injection of 20.0 mCi of Tc99m-MIBI.)
DISCUSSION
Askin tumor of the chest wall is a rare malignancy that is considered to be a PNET, which itself is now considered to be part of a spectrum of soft tissue sarcomas in the Ewing sarcoma family of tumors (EFT) [1]. These tumors have also been shown to share similar immunohistochemical properties and characteristic chromosomal translocations and, therefore, are thought to have a common cell origin [2]. The chromosomal abnormality is a reciprocal translocation between chromosomes 11 and 22 t(11;22) [3]. Despite having a similar genetic mutation, varying imaging characteristics (especially in nuclear medicine) have been reported between EFT lesions of different patients [4,5]
EFT represents the second most common primary bone malignancy affecting children and adolescents after osteosarcoma [6]. The reported incidence is approximately 3 per million with both sexes almost equally affected and predominantly occurring in the 20–30 year old age range[7]. Six to 15% of EFT cases are found to arise from the chest wall [8].
Treatment for Askin tumor is generally a multidisciplinary endeavor with use of chemotherapy, surgery and external beam radiation therapy [7]. The prognosis of Askin tumor is poor with one case series of 104 patients with the disease that showed survival median survival at 28 months to be 45% [7]. The clinical features of Askin tumor include painless swelling, painful mass, dyspnea and chest wall pain without mass [7].
On imaging, Askin tumor can be seen on radiographs as a large unilateral extrapulmonary mass [9]. Contrast enhanced CT often shows a heterogeneous mass due to hemorrhage and necrosis with calcification being a rare finding [10]. On MRI, the lesion is often heterogeneous signal on T1 with hemorrhagic areas in the tumor demonstrating higher signal, and cystic or necrotic components in the tumor having higher signal on T2 weighting; there is often avid enhancement after IV gadolinium is administered [10]. In nuclear medicine imaging, a case report has described Askin tumor to be Ga-67 and Tl-201 avid prior to therapy with Tl-201 being a better marker for follow up exams to detect disease recurrence [11].
There is very little literature about nuclear medicine imaging of Askin tumor and, to our knowledge, no literature related to use of In111-pentetreotide imaging of the tumor prior to this paper. This case demonstrates nuclear scintigraphy imaging findings of Askin tumor that could help define future imaging guidelines for this rare malignancy.
In this case Tc99m-MIBI and In111-pentetrotide, scans were both positive prior to the patient receiving treatment. Subsequent Tc99m-MIBI and In111-pentetrotide scans were negative after the tumor was treated with chemotherapy and surgically resected. Previous use of Tc99m-MIBI and In111-pentetrotide to image Ewing sarcoma showed in a case report that metastatic lesions to the lung had uptake of Tc99m-MIBI and variable uptake of In111-pentetrotide [12]. Another case series showed that Tc99m-MIBI had variable uptake in Ewing sarcoma of the bone, and the presence or absence of uptake was not useful for prognostic value [13]. Specific to Askin tumor, a previous case report had shown that Thallium-201 and Gallium-67 were potential radiopharmaceutical agents that could potentially be used to image the lesion [11].
Interestingly, the tumor in this case did not show hypermetabolism on F18-FDG PET prior to treatment. The literature is more variable regarding the use of F18-FDG to image EFT lesions. A case of extraskeletal EFT in the upper extremity [5] reported F18-FDG hypometabolism while another article reported a case of Askin tumor which demonstrated F18-FDG hypermetabolism [4]. Others have reported F18-FDG hypermetabolism in PNET of the soft tissues of the lower extremity [14], skeleton [15], spinal cord [16], stomach [17], and retroperitoneum [18]. One hypothesis of FDG hypometabolism in EFT may be that some particular lines of the tumor may underexpresses GLUT 1 cell membrane transporters [5].
In addition to Askin tumor, the differential diagnosis for a chest wall mass includes neuroblastoma, soft tissue sarcoma, lymphoma and infection. Although the MRI and CT imaging of an Askin tumor and neuroblastoma may overlap, the majority of neuroblastomas demonstrate positivity on I123-MIBG scans which can potentially help differentiate the two. In a soft tissue sarcoma such as a rhabdomyosarcoma, the lesion should be isodense and isointense to muscle on CT and MRI respectively. Osteosarcomas should be F18-FDG positive and may have increased uptake on Tc99m-MIBI, Ga-67 and Tl-201 scinitigraphy as well. An infection would present with more classic clinical findings such as fever and leukocytosis. Lymphomas of the chest wall might also present with similar radiologic findings as an Askin tumor and would be difficult to differentiate based on imaging alone.
In conclusion, we report a case of Askin tumor in a 17 year old patient which demonstrates In111-pentetreotide and Tc99m-MIBI avidity prior to treatment and resolution of activity after treatment. Additionally, the lesion was F18-FDG hypometabolic.
TEACHING POINT
Askin tumors can be Tc99m-MIBI and In111-pentetrotide avid and use of these radiopharmaceuticals can also be used to assess for disease response to treatment. Despite the aggressive nature of Askin tumor, F18-FDG PET imaging is variable and does not necessarily show hypermetabolism in all cases.
Table 1.
Summary table for Askin tumor
| Etiology | Arises from the soft tissues of the chest wall probably from migrating embryonal cells of the neural crest. t(11;22) |
| Incidence | Rare; <3 per million/year |
| Gender ratio (M:F) | 1:1.3 |
| Age predilection | 20–30 |
| Risk factors | Possible association with radiation therapy for Hodgkin disease |
| Treatment | Surgical excision, radiation and chemotherapy |
| Prognosis | Overall survival poor. 2 year 38%; 6 year 14%--worse with metastatic disease. |
| Findings on imaging | Heterogeneously dense mass on CT. Increased signal on T1 & T2 depending on hemorrhage & cystic components. Variable hypermetabolism on F18-FDG scans. |
Table 2.
Differential table for Askin tumor
| Diagnosis | CT | MRI T1 | MRI T2 | Pattern of contrast enhancement | FDG PET | Scintigraphy |
|---|---|---|---|---|---|---|
| Askin Tumor | Heterogeneous density +/− necrosis | Heterogenous high signal (+/−hemorrhage) | Heterogeneous intermediate/high signal | Enhances | Variable | Reported to be Tl-201 and Ga-67 avid. |
| Neuroblastoma | Heterogeneous density +/− necrosis | May be increased due to hemorrhage | May be increased due to cystic changes | Enhances | Variable | 70% + I123- MIBG |
| Rhabdomyosarcoma | Isodense to muscle | Isointense to muscle | Hyperintense to muscle | Enhances | Hypermetabolic | Bone scan for skeletal metastasis |
| Osteosarcoma | Heterogeneous bone mass usually in the metaphysis of a long bone. | Non-osteoid portions of the tumor near isointense to skeletal muscle | Heterogeneous increased signal. Peritumoral edema | Enhances | Hypermetabolic | Tl-201, Tc99m- MIBI, Ga-67 increased uptake. |
| Lymphoma | Pleural mass extending into soft tissues of chest wall | Variable | High signal | Enhances | Hypermetabolic | Bone scan for skeletal metastasis |
| Infection | Periosteal reaction, bone destruction | Decreased signal in the marrow | Increased signal in the marrow | Enhances | Variable | 3-phase positive on Tc99m- MDP, Labeled leukocyte scan positive |
ACKNOWLEDGEMENTS
We would like to acknowledge James Lin, MD from the Department of Pathology of Harbor-UCLA Medical Center for obtaining the histopathology images.
ABBREVIATIONS
- CD-99
Cluster of differentiation 99
- CT
Computed tomography
- EFT
Ewing sarcoma family of tumors
- F18
Fluorine 18
- FDG
Fluorodeoxyglucose
- Ga-67
Gallium 67
- GLUT
Glucose transporter
- I123
Iodine 123
- In111
Indium 111
- IV
Intravenous
- MDP
Methylene diphosphonate
- MIBG
metaiodobenzylguanidine
- MIBI
methoxyisobutylisonitrile
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- PNET
Primitive neuroectodermal tumor
- SPECT
Single-photon emission computed tomography
- Tc99m
Technetium 99 metastable
- Tl-201
Thallium 201
REFERENCES
- 1.Grier HE. The Ewing family of tumors. Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997 Aug;44(4):991–1004. doi: 10.1016/s0031-3955(05)70541-1. Review. [DOI] [PubMed] [Google Scholar]
- 2.Delattre O, Zucman J, Melot T, Garau XS, Zucker J, Lenoir GM, Ambros PF, Sheer D, Turc-Carel C, Triche TJ, Aurias A, Thomas G. The Ewing family of tumors--a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 3.Xu Q, Xu K, Yang C, Zhang X, Meng Y, Quan Q. Askin tumor: four case reports and a review of the literature. Cancer Imaging. 2011 Nov 30;11:184–8. doi: 10.1102/1470-7330.2011.0025. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demir MK, Koşar F, Sanli Y, Esmaeilzadeh S, Urer HN. 18F-FDG PET-CT features of primary primitive neuroectodermal tumor of the chest wall. Diagn Interv Radiol. 2009 Sep;15(3):172–5. [PubMed] [Google Scholar]
- 5.Musana KA, Raja S, Cangelosi CJ, Lin YG. FDG PET scan in a primitive neuroectodermal tumor. Ann Nucl Med. 2006 Apr;20(3):221–5. doi: 10.1007/BF03027434. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006 Jun;11(6):590–601. doi: 10.1634/theoncologist.11-6-590. Review. [DOI] [PubMed] [Google Scholar]
- 7.Gurney JG, Swensen AR, Bulterys M. Malignant Bone Tumors. In: Ries LA, Smith MA, Gurney JG, editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. NCI, SEER Program; Bethesda, MD: 1999. [Google Scholar]
- 8.Laskar S, Nair C, Mallik S, Bahl G, Pai S, Shet T, Gupta T, Arora B, Bakshi A, Pramesh CS, Mistry R, Qureshi S, Medhi S, Jambhekar N, Kurkure P, Banavali S, Muckaden MA. Prognostic factors and outcome in Askin-Rosai tumor: a review of 104 patients. Int J Radiat Oncol Biol Phys. 2011 Jan 1;79(1):202–7. doi: 10.1016/j.ijrobp.2009.10.039. Epub 2010 Apr 14. [DOI] [PubMed] [Google Scholar]
- 9.Schulman H, Newman-Heinman N, Kurtzbart E, Maor E, Zirkin H, Laufer L. Thoracoabdominal peripheral primitive neuroectodermal tumors in childhood: radiological features. Eur Radiol. 2000;10(10):1649–52. doi: 10.1007/s003300000367. [DOI] [PubMed] [Google Scholar]
- 10.Winer-Muram HT, Kauffman WM, Gronemeyer SA, Jennings SG. Primitive neuroectodermal tumors of the chest wall (Askin tumors): CT and MR findings. AJR Am J Roentgenol. 1993 Aug;161(2):265–8. doi: 10.2214/ajr.161.2.8392786. [DOI] [PubMed] [Google Scholar]
- 11.Howman-Giles R, Uren RF, Kellie SJ. Gallium and thallium scintigraphy in pediatric peripheral primitive neuroectodermal tumor (Askin tumor) of the chest wall. J Nucl Med. 1995 May;36(5):814–6. [PubMed] [Google Scholar]
- 12.Giles FJ, Waxman AD, Nguyen KN, Fuerst MP, Kusuanco DA, Franco MM, Bierman H, Lim SW. Comparison of Technetium-99m sestamibi and indium-111 octreotide imaging in a patient with Ewing’s sarcoma before and after stem cell transplantation. Cancer. 1997 Dec 15;80(12 Suppl):2478–83. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2478::aid-cncr19>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Sever Z, Cohen IJ, Connolly LP, Horev G, Perri T, Treves T, Hardoff R. Tc-99m MIBI to evaluate children with Ewing’s sarcoma. Clin Nucl Med. 2000 Jun;25(6):410–3. doi: 10.1097/00003072-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Javery O, Krajewski K, O’Regan K, Kis B, Giardino A, Jagannathan J, Ramaiya NH. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol. 2011 Dec;197(6):W1015–22. doi: 10.2214/AJR.11.6667. Review. [DOI] [PubMed] [Google Scholar]
- 15.Györke T, Zajic T, Lange A, Schäfer O, Moser E, Makó E, Brink I. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun. 2006 Jan;27(1):17–24. doi: 10.1097/01.mnm.0000186608.12895.69. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer CC, Townsend DW, Kottapally S, Jadali F. FDG imaging of spinal cord primitive neuroectodermal tumor. J Nucl Med. 1998 Jul;39(7):1207–9. [PubMed] [Google Scholar]
- 17.Czekalla R, Fuchs M, Stölzle A, Nerlich A, Poremba C, Schaefer KL, Weirich G, Höfler H, Schneller F, Peschel C, Siewert JR, Schepp W. Peripheral primitive neuroectodermal tumor of the stomach in a 14-year-old boy: a case report. Eur J Gastroenterol Hepatol. 2004 Nov;16(12):1391–400. doi: 10.1097/00042737-200412000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe N, Kawano M, Takada M, Iwamoto S, Shimizu M, Kawabe H, Kamisaki Y, Noguchi K, Seto H. F-18 FDG PET imaging in a primitive neuroectodermal tumor. Clin Nucl Med. 2006 Aug;31(8):484–5. doi: 10.1097/01.rlu.0000227414.09097.49. [DOI] [PubMed] [Google Scholar]