Abstract
The effects of neural activity on cerebral hemodynamics underlie human brain imaging with functional magnetic resonance imaging and positron emission tomography. However, the threshold and characteristics of the converse effects, wherein the cerebral hemodynamic and metabolic milieu influence neural activity, remain unclear. We tested whether mild hypercapnia (5% CO2) decreases the magnetoencephalogram response to auditory pattern recognition and visual semantic tasks. Hypercapnia induced statistically significant decreases in event‐related fields without affecting behavioral performance. Decreases were observed in early sensory components in both auditory and visual modalities as well as later cognitive components related to memory and language. Effects were distributed across cortical regions. Decreases were comparable in evoked versus spontaneous spectral power. Hypercapnia is commonly used with hemodynamic models to calibrate the blood oxygenation level‐dependent response. Modifying model assumptions to incorporate the current findings produce a modest but measurable decrease in the estimated cerebral metabolic rate for oxygen change with activation. Because under normal conditions, low cerebral pH would arise when bloodflow is unable to keep pace with neuronal activity, the cortical depression observed here may reflect a homeostatic mechanism by which neuronal activity is adjusted to a level that can be sustained by available bloodflow. Animal studies suggest that these effects may be mediated by pH‐modulating presynaptic adenosine receptors. Although the data is not clear, comparable changes in cortical pH to those induced here may occur during sleep apnea, sleep, and exercise. If so, these results suggest that such activities may in turn have generalized depressive effects on cortical activity. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: cerebral blood flow, cerebral metabolic rate for oxygen, blood oxygenation level‐dependent response, functional magnetic resonance imaging, magnetoencephalography, temporal lobe, prefrontal cortex, occipital lobe, carbon dioxide, arterial spin labeling
INTRODUCTION
The use of functional magnetic resonance imaging to localize brain activation during cognition rests on the relationship between neural activity and the blood oxygenation level‐dependent (BOLD) response. According to the standard model [Buxton et al., 2004], neural activity uses energy and thus increases oxidative metabolism and the cerebral metabolic rate for oxygen (CMRO2). The increase in CMRO2 is accompanied by an increase in cerebral blood flow (CBF) and cerebral blood volume that is at least twice as large [Davis et al., 1998; Fox and Raichle, 1986; Hoge et al., 1999]. Through this decoupling, the resulting net decrease in deoxyhemoglobin concentration produces the increased BOLD signal.
The characteristics of the converse effect, of changes in cerebral hemodynamics and other aspects of the metabolic and ionic milieu on neuronal activity, are less clear [Moore and Cao, 2008]. One method to explore this issue is to induce hypercapnia by breathing air enriched in CO2. Carbon dioxide acts as a vasodilator and increases CBF by about 50% at 5% CO2 concentrations [Kim et al., 1999], probably via its effects in decreasing intracerebral pH although the mechanisms are not fully understood [Ainslie and Duffin, 2009]. Clinically, it has long been known that decreasing arterial CO2 levels through hyperventilation can provoke seizures in epileptic patients [Foerster, 1924], whereas hypercapnia can suppress them [Gibbs and Gibbs, 1950]. A more direct measure of the effects of hypercapnia on brain electrical activity is provided by the electroencephalogram (EEG), which is generated by transmembrane currents due to synaptic activity and related active membrane channels [Murakami et al., 2003; Niedermeyer and Lopes da Silva, 1999]. Aram and Lodge [ 1987] showed that in‐slice preparations of rat cingulate cortex, which increased the levels of CO2, caused a pH‐mediated reduction in spontaneous EEG. In awake humans, the total power of spontaneous EEG across frequencies from 1 to 64 Hz has been reported to be reduced at moderate levels of hypercapnia [Bloch‐Salisbury et al., 2000]. Similarly, in humans under general anesthesia, Kalkman et al. [ 1991] found significant increases in the alpha (8–12 Hz) and beta (13–30 Hz) bands of the spontaneous EEG after hyercapnic challenge (PaCO2 ∼ 50 mm Hg), but not in the delta (1–4 Hz) or theta (5–7 Hz) frequency ranges. However, under apparently similar levels of hypercapnia, Halpern et al. [ 2003] reported alpha band power increases and delta (1–4 Hz) decreases, whereas in animals under chronic hypercapnia, Matakas et al. [ 1978] reported significant alpha decreases.
Most EEG power is in the lower frequency bands, which often are dominated by alpha and other rhythms that are inversely correlated with alertness, arousal, and BOLD signal [Feige et al., 2005; Goldman et al., 2002]. Thus, they are problematic as indicators of activation. Recently, Zappe et al. [ 2008], working in anesthetized monkeys, found that moderate levels of hypercapnia induced by 6% CO2 resulted in decreased spontaneous beta and gamma activity and multiunit activity, whereas activity in the theta and alpha remained unchanged. Simultaneous BOLD and blood volume measurements showed an increase during hypercapnia. Administration of 3% CO2 produced only a trend toward reduced spontaneous activity. They note that 3% CO2 in the artificially ventilated anesthetized monkey may produce cerebrovascular effects more comparable to 5% CO2 in the freely breathing unanesthetized human.
Event‐related potentials (ERPs), time‐locked averages of EEG with respect to a stimulus, are typically well correlated with the local BOLD response [Arthurs et al., 2006] and thus may provide a less ambiguous measure of the neural response to hypercapnia. ERPs are volume‐conducted local field potentials, which are closely correlated with the BOLD response in many but not all experimental situations [Devor et al., 2003; Logothetis and Wandell, 2004]. In apparently the only study thus far published of ERPs in humans during moderate hypercapnia, no significant change was found [Bloch‐Salisbury et al., 2000]. In contrast, intracranial recordings from human patients during presurgical evaluation for epilepsy found substantial effects of mild hypercapnia on the firing rate of medial temporal lobe neurons [Halgren et al., 1977]. These results may have been influenced by pathology or medication effects. However, they are consistent with recent recordings of depressed monosynaptic responses in rat hippocampal slices under mild hypercapnia [Dulla et al., 2005]. Similarly, Jones et al. [ 2005] examined the response of the rat barrel cortex to electrical stimulation of the whisker pad under urethane anesthesia. Compared to an average of the pre‐ and posthypercapnic periods, 5% CO2 evoked a nonsignificant 6% decrease in both MUA and CSD, whereas 10% CO2 evoked a 35% decrease in CSD and a 35% decrease in MUA, both highly significant. Because the hypercapnic challenge for BOLD calibration is usually 5%, these data could be taken to support the assumptions of that technique. However, it is unclear whether anesthesia, species, or cortical location might result in a different threshold for hypercapnic effects on neuronal processing.
In addition to its critical relevance for modeling the neural basis of the BOLD response [Chiarelli et al., 2007b], the effects of mild hypercapnia on neural activity are also important in understanding the effects of many medical conditions such as sleep apnea and emphysema, where paCO2 may be considerably removed from the norm (paCO2 ∼ 40 mm Hg) [Kaila and Ransom, 1998]. Even such mundane activities as sleeping, exercise, and travel to high altitudes may alter paCO2 [Ainslie and Duffin, 2009; Phillipson and Bowes, 1986; Richerson, 2004]. Indeed, the potential neural effects of very mild hypercapnia suggest that during cognitive and sensory processing, neural activity may not only influence hemodynamics but also those hemodynamic effects may feed back to influence neural activity [Moore and Cao, 2008].
In the current study, we recorded the magnetoencephalogram (MEG) to test the hypothesis that hypercapnia, as induced by breathing 5% CO2, affects neural activity in conscious task‐performing humans. MEG is similar to EEG in being directly related to transmembrane currents, but has higher spatial resolution and has complementary sensitivity to cortical generators [Cohen and Cuffin, 1983; Murakami et al., 2003]. Specifically, we tested if early sensory evoked activity in visual and auditory cortices and late cognitive activity in fronto‐temporal association cortices during language processing were decreased by mild hypercapnia. We also tested if mild hypercapnia decreases evoked or spontaneous spectral power in different frequency bands. Across measures, locations, and latencies, mild hypercapnia was found to have a consistent depressive effect on neural activity. We interpret these findings as reflecting a homeostatic mechanism and evidence of modulation of neural activity by hemodynamics.
METHODS
Participants and Recording procedures
Seven healthy right‐handed volunteers free of neurological conditions (4 females, mean age: 25.4 years, standard deviation: 3.8 years) served as subjects. MEG signals were recorded from 306 channels at 0.1–1000 Hz using a Neuromag‐Vectorview Instrument (Elekta, Stockholm) with a magnetometer and orthogonal pairs of planar gradiometers at each of 102 locations over the entire scalp.
Subjects were fitted with a breathing mask (H. Rudolph, Missouri, USA), which covered the entire nose and was fitted with a mechanism that prevented rebreathing of exhaled air. A pneumatic switching valve alternates breathing air between normal room air and humidified medical grade air with 21% O2, 5% CO2, and 74% N2 through a low‐resistance valve connected to a Douglas bag. Respiration and end‐tidal CO2 (ETCO2) were recorded throughout the experiment (CardioPulmonary Technologies, Sussex, WI). Hypercapnic‐breathing blocks consisted of subjects breathing a 5% CO2 air mixture during a 90‐s rise time and a steady‐state CO2 level for 120 s (see Fig. 1A). Under these conditions, the ETCO2 plateau saturation in humans is reached after ∼30 s of hypercapnia [Cohen et al., 2002].
Figure 1.
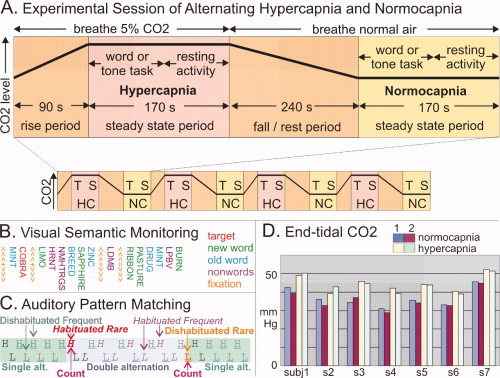
Methods. A: Experimental session. A typical 48‐min session comprising four hypercapnic (HC) and four normocapnic (NC) periods, with task‐evoked (T) and spontaneous (S) MEG collected in each period. B: Example sequence from the Visual Semantic Monitoring Task. The subject detected animal names (targets) randomly interspersed among rapidly presented (SOA = 400 ms) words (either presented once, new, or presented many times after pre‐exposure, old), nonpronounceable nonwords (npnw, consonant strings), or fixation (≪+≫). C: Example sequence from Auditory Pattern Monitoring Task. The subject counts sequence changes between single and double alternation of high (H, 500 Hz) and low (L, 250 Hz) 50 ms tones, SOA = 500 ms. D: Endtidal CO2 levels obtained in the seven subjects during two cycles of normo‐ and hypercapnic conditions. Endtidal CO2 concentrations increased by an average of ∼8 mm Hg during HC. Task performance was >90% in all conditions and did not differ between HC and NC.
During these periods, subjects were tested on the sensory and cognitive tasks, after which a 240‐s rest period of normocapnic breathing followed to attain baseline CO2 levels. Alternating normocapnic‐breathing blocks followed the same pattern with the exception that subjects breathed ambient room air. To avoid order effects, three subjects were studied under normocapnic conditions first, and four subjects were tested under hypercapnic conditions first. After each experiment, subjects were debriefed and asked if they were able to tell normocapnic from hypercapnic conditions. No subject reported a noticeable difference. This design allowed us to measure the effects of increased CO2 levels in the blood on neuronal population activity across a wide range of sensory and cognitive tasks.
Stimuli and Task
During repeated, counter‐balanced HC/NC cycles with steady‐state ETCO2 levels, subjects were tested on visual semantic monitoring (N = 7) and auditory pattern monitoring (N = 5) while recording 306 channels of MEG. As is shown in Figure 1A, a given session consisted of four presentations of a single task during HC/NC/HC/NC or NC/HC/NC/HC periods. Fifteen seconds of spontaneous activity during rest with eyes open and fixated on a target were collected at the end of each task period.
Visual Semantic Monitoring
During the visual task, subjects were presented with a continuous string of visual novel words, repeated words, nonpronounceable consonant strings, and fixation crosses at 400‐ms stimulus onset asynchrony (see Fig. 1B). Four hundred words from each category were presented to ensure a high‐signal‐to‐noise ratio. The sequence of stimulus conditions was balanced, so that each condition was preceded by every other condition with equal likelihood. In addition, 80 target words referring to animals were presented, and subjects were instructed to make a semantic decision by pressing a response button. Previous studies have shown that similar tasks evoke an N400 component that is associated with lexicosemantic word encoding [Kutas and Van Petten, 1994]. The N400m is maximal in gradiometers over the left anterior temporal and posteroventral prefrontal cortices [Dale et al., 2000] where it is generated [Halgren et al., 1994a, b]. The N440m is larger to novel words, which require more sustained processing [Dale et al., 2000]. Visual words also evoke an M100 over occipital areas related to sensory processing.
Auditory Pattern Discrimination
Subjects engaged in an auditory task (Fig. 1C) where they counted shifts in the ongoing pattern of tone sequences. All tones were either high (H; 500 Hz) or low (L; 250 Hz) pitched. Sequences were either single‐alternation (i.e., H‐L‐H‐L‐…) and double‐alternation (i.e., H‐H‐L‐L‐…). The tones had a duration of 50 ms and were presented at an SOA of 500 ms. A total of 528 tones were presented during each capnic condition. Unlike the typical “auditory oddball task,” this design allows the effects of rare sequence shifts to be observed unconfounded by stimulus characteristics or dishabituation [Buxton, 2002]. Previous work demonstrates that rare pattern shifts evoke a sequence of fields from 200 to 400 ms mainly generated in temporal and frontal association areas and corresponding to the cognitive N2, P3a, and P3b components that have been extensively studied in scalp and intracranial ERPs [Halgren et al., http://www.ncbi. nlm.nih.gov/pubmed/20665718].
Spectral Analysis
Artifact rejection of non‐neuronal sources was performed on all single trials independent of experimental or capnic condition. Spectral analysis was performed on individual trials and spontaneous data (sampling frequency: 250 Hz, highpass filter: 0.1, and lowpass filter: 100 Hz) using the FieldTrip toolbox (F.C. Donders Centre: http://www.ru.nl/fcdonders/fieldtrip). Time‐frequency responses were estimated with Morlet wavelets (width = 5) from 1 to 100 Hz at a 1‐Hz frequency resolution and divided into theta (5–7 Hz), alpha (8–12 Hz), beta (13–24 Hz), low gamma (25–55 Hz), and high‐gamma (65–95 Hz) frequency bands. All spectral analyses were performed on single trials and single subjects before creating averages and grand‐average.
RESULTS
We examined the effects of hypercapnia on evoked and spontaneous MEG activity.
Endtidal CO2
Mean endtidal CO2 values were calculated for each individual from the presentation of the first stimulus to the last stimulus during HC and NC conditions (see Fig 1D). The average NC–HC difference (8 mmHG) is similar to other reports involving a 5% CO2 challenge in humans [Howden et al., 2004].
Behavioral
There was no significant difference between hypercapnia and normocapnia for either accuracy or reaction time in the visual semantic‐monitoring task and no significant difference for accuracy in the auditory pattern matching task. Reaction time in the visual semantic‐monitoring task under normocapnia was 655 ± 22 ms (mean ± standard deviation) and under hypercapnia was 690 ± 49 ms, a difference that was not significant (two‐tailed, paired t‐test; p = 0.137). Response accuracy on this task was 82% ± 13% under normocapnia and 89% ± 5% under hypercapnia, again a difference that was not significant (two‐tailed, paired t‐test; p = 0.125). Accuracy on the auditory pattern‐matching task was 96% ± 2% under normocapnia and 94% ± 1.5% under hypercapnia, a difference that was not significant (two‐tailed, paired t‐test; p = 0.173). Breathing rate per minute during normocapnia was 17.2 ± 1.9 and during hypercapnia 17.7 ± 1.3 (mean ± standard deviation), a difference that was not significant (two‐tailed, paired t‐test; p = 0.278).
Hypercapnia Attenuates MEG Fields Evoked by Visual Words
Analyses were performed on both sensory (M100) and cognitive (N400m) components of the averaged event‐related field (ERF). A grand average of magnetoencephalogram (MEG) activity to novel words is shown in Figure 3A. Visually presented words evoked a strong dipolar pattern peaking at about 100 ms over the occipital lobe in all subjects (Fig. 2A). This pattern, the M100 evoked field, is typically observed in visual experiments with MEG. To quantify the effects of capnic state on neural response in different brain areas, an equivalent current dipole was fitted to the occipital field of the M100 at its maximal amplitude on the combined data from the normocapnic and hypercapnic conditions in each of the seven subjects. Examples from two subjects are shown in Figure 2B. Goodness of fit for the M100 response was 88% (std.dev. 4%). Dipole strength was then estimated separately in the normocapnic and hypercapnic conditions. Hypercapnia significantly decreased dipole strength by 20.3% (s.d. 5.5) (Table I) (p < 0.005, paired t‐test, two‐tailed).
Figure 3.
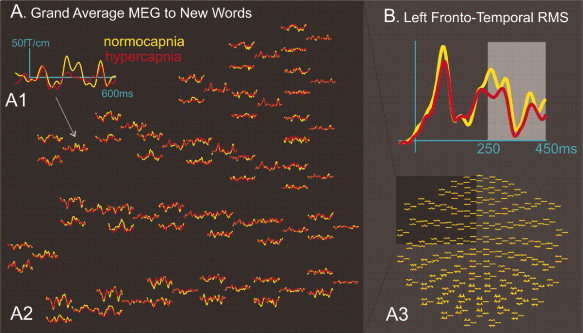
Effects of hypercapnia on late frontotemporal MEG fields evoked by words. A: Grand average across seven subjects of response to all novel words. An example left frontotemporal sensor (A1) is shown, drawn from the entire set of left frontotempoal sensors (A2), which are in turn drawn from the entire sensor array (A3). B: Root mean square activity averaged across all left frontotemporal sensors. Late activity between 250 and 450 ms after word onset was chosen for further statistical analysis (see text).
Figure 2.
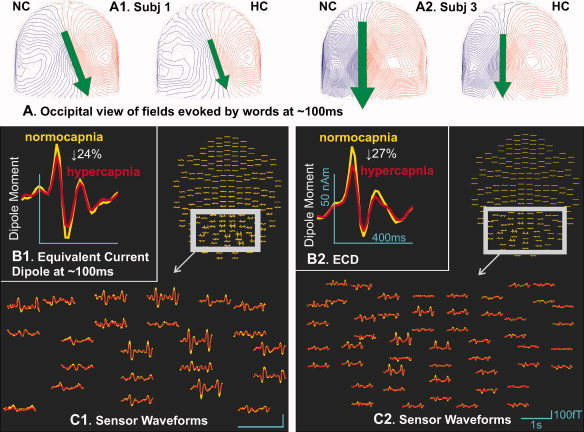
Effects of hypercapnia on early occipital MEG fields evoked by words. A: Occipital view of MEG fields evoked by visual words during semantic monitoring at ∼100 ms after word onset. Iso‐field lines for inward flux are blue; outward flux are red visual (B). Equivalent current dipole fits to the occipital field at its maximal amplitude in the Visual Semantic Monitoring task, ∼100 ms after stimulus onset. Hypercapnia significantly decreased dipole strength by ∼20%. C: Average waveforms at the 204 MEG gradiometers to visual words during normocapina (yellow) and hypercapnia (red). All trials except fixation are included in these averages. Data from two subjects (numbers 1 and 3) are displayed.
Table I.
Effects of hypercapnia on MEG fields evoked by visual words
| Subject | Normocapnic | Hypercapnic | HC‐induced decrease in dipole moment (NC–HC)/NC (%) | ||
|---|---|---|---|---|---|
| Dipole moment (nA m) | Goodness of fit (%) | Dipole moment (nA m) | Goodness of fit (%) | ||
| 1 | 37 | 82 | 28 | 82 | 24 |
| 2 | 30 | 85 | 26 | 84 | 14 |
| 3 | 45 | 96 | 33 | 91 | 27 |
| 4 | 36 | 92 | 27 | 87 | 26 |
| 5 | 27 | 93 | 22 | 87 | 18 |
| 6 | 13 | 89 | 10 | 84 | 21 |
| 7 | 20 | 88 | 18 | 85 | 13 |
Late peaks in the MEG fields appear larger under normocapia than hypercapnia. Data were combined across channels and subjects in order to quantify this effect. Because the fields can be either positive or negative, the root mean square (RMS) was calculated across gradiometer sensors before averaging. Figure 3B shows the time course of the RMS averaged across the left frontotemporal sensors and all subjects. Our results show that the RMS calculated at 250 and 450 ms after word onset is consistently larger during normocapnia compared to hypercapnia [t(6) = 2.1, p < 0.001].
Hypercapnia Attenuates MEG Fields Evoked by Tone Pattern Shifts
Five of the subjects performed a pattern detection task where rapidly presented tones occasionally shifted between single and double alternation (Fig. 1C). RMS of differential magnetoencephalogram (MEG) activity evoked by rare tone pattern shifts when compared with control stimuli was calculated from individual trials for each subject across all 204 gradiometer sensors at each latency and then averaged across subjects. As is shown in Figure 4A, the RMS activity during the normocapnic periods was compared to the hypercapnic periods at all latencies. RMS activity was also averaged in each gradiometer channel between 100 and 500 ms after tone onset. As is shown in Figure 4B, RMS showed a significantly higher amplitude during the normocapnic when compared with the hypercapnic periods (n = 5, paired t‐test, two‐tailed, p < 0.01).
Figure 4.
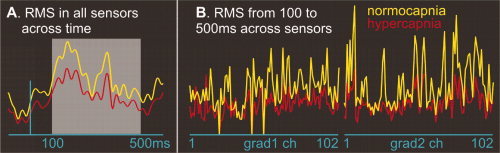
Effects of hypercapnia on MEG fields evoked by tone pattern shifts. Root mean square of differential MEG activity evoked by rare tone pattern shifts when compared with control stimuli was averaged across five subjects. A: RMS activity was averaged across all sensors at each latency from 100 ms before stimulus onset to 600‐ms poststimulus. Note the increased amplitude during the normocapnic when compared with the hypercapnic periods at all latencies. B: Activity in each gradiometer channel was averaged between 100 and 500 ms after tone onset. Note the increased amplitude during the normocapnic when compared with the hypercapnic periods at all 204 sensors. The difference between normocapnic and hypercapnic activity was highly significant (see text).
Hypercapnia attenuates evoked and spontaneous MEG spectral activity
Spectral power was averaged across 20 sensors at the top of the head where contamination of the MEG with muscle activity was not a concern. Estimates of power were calculated for evoked activity in specific frequency bands to novel words in the visual word‐processing task from stimulus onset to 600‐ms poststimulus. One‐way ANOVA showed a significant effect for capnic condition, F(1,47) = 20.7, p < 0.001. Post hoc t‐tests identified significantly lower levels of power in the alpha [8–12 Hz; t(6) = 2.1, p < 0.05], and beta [9–12 Hz; t(6) = 2.4, p < 0.05] bands for hypercapnia compared to normocapnia. No such effects were observed for the low [25–55 Hz; t(6) = 0.24, p > 0.05] and high [65–95 Hz; t(6) = 0.7, p > 0.05] gamma bands.
Spectral MEG power was also measured during resting (Figure 5). One‐way ANOVA did not reveal a main effect of capnic condition [F(1,46) = 0.4, p = 0.53]. Hypercapnia attenuated power in all bands (theta by 7%, alpha by 15%, beta by 10%, and gamma by 11%), but post hoc t‐tests showed that only the gamma decrease was significant [t(5) = 3.9, p < 0.02].
Figure 5.
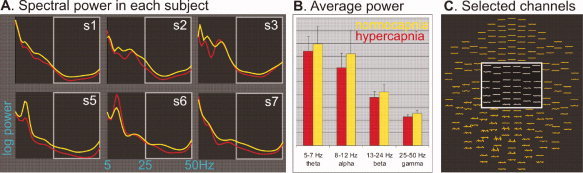
Hypercapnia attenuates spontaneous resting MEG fields. A: Spectral power in each of six subjects is plotted against frequency from 5 to 50 Hz. Power is generally greater under normocapnia in most subjects and frequencies, with most variability in the alpha band. During this recording, subjects were resting quietly in a quiet room and may have experienced different levels of alpha activity. The gamma band is indicated by the light gray box. B: Power is averaged across subjects for each frequency band, as indicated. Hypercapnia attenuated power in all bands (theta by 7%, alpha by 15%, beta by 10%, and gamma by 11%) but only the gamma decrease was significant (paired t‐test, two‐tailed P < 0.02). C: Spectral power was measured in 40 sensors at the top of the head (white box) where contamination with EMG was not a concern.
DISCUSSION
Across both auditory and visual modalities, hypercapnia induced significant decreases in ERFs. Amplitude reductions were observed in early sensory components as well as later cognitive components and extended across many cortical regions. Hypercapnia also showed a tendency to decrease spectral power during rest in all frequency bands, an effect that reached significance in the gamma band. These results demonstrate that increased CO2 levels attenuate the event‐related cortical neuromagnetic response in the auditory and visual systems during both early sensory as well as later cognitive processing. This effect is prevalent across widespread cortical areas and affects spontaneous brain activity during rest. Neurohemodynamic modeling procedures that assume that neural activity, and thus CMRO2, is constant during hypercapnia may need to be modified to reflect hypercapnia‐induced changes in neural activity.
It is unlikely that attentional factors due to hypercapnic breathing can explain the observed effects. The experimental setup minimized any perceived difference between normal and CO2‐enriched air, and no subject reported any noticeable difference between conditions. Attention can modulate early sensory components of the evoked potential. However, such effects generally appear in paradigms with rapidly presented competing stimuli. In previous studies, the stimuli showing decreased responses when they are unattended are relatively weak, task‐irrelevant stimuli that need to be suppressed in order to efficiently process the task‐relevant stimuli [Hillyard and Anllo‐Vento, 1998]. This is not the case in the tasks reported here, which measured the responses to strong task‐relevant stimuli. If there is any effect of distraction, it might be expected to make the task more difficult, which would tend to increase the size of the N400, whereas the opposite pattern was observed.
Comparison with Previous Human Studies
Previous examinations of spontaneous EEG spectral power during hypercapnia either combined the low frequency with the high or only looked at power in the low frequencies. Because most EEG power is in the lower frequency bands, most measures that combine all frequencies are insensitive to changes in higher frequencies. Furthermore, the lower frequency bands are often dominated by alpha and other rhythms that are inversely correlated with alertness, arousal, and BOLD [Feige et al., 2005; Goldman et al., 2002]. Thus, low frequency power is problematic as an indicator of activation. In contrast, high gamma power may be a good indicator of neural activation [Logothetis et al., 2001; Miller et al., 2009].
A previous ERP study [Bloch‐Salisbury et al., 2000] found no effects of hypercapnia on the N1, P2, or P3 ERP components evoked by a simple auditory signal‐detection task. Because MEG sensors have smaller lead fields than EEG, consistent MEG deflections directly comparable to these components cannot be reliably identified. Furthermore, although the generators underlying these ERP components would be expected to also participate in generating the MEG signals evoked by rare tones in our auditory task, they may not be identical [Hari et al., 1982]. When we examined differential RMS power of broad band MEG to rare tone shifts, we found a significant decrease under hypercapnia. Furthermore, we found a significant decrease in the visual sensory response M100 as well as later cognitive responses in the visual word semantic task. Without simultaneous EEG recordings, it is not possible to interpret the differences between our findings and those of Bloch‐Salisbury [ 2000]. However, it is clear from our data that there are reliable decreases in both auditory and visual components at both early and longer latencies involving both sensory and cognitive processing.
It should be noted that possible factors affecting the amplitude, though not the spatial distribution, of EEG and MEG signals may include gray and white‐matter anisotropy [Hauseisen et al., 2002] and intracellular and extracellular pH‐concentration [Niedermeyer and Lopes da Silva, 1999]. The within‐subject design of the current experiment controls for potential anisotropy effects, whereas conductivity changes due to extracellular pH alterations are expected to affect the EEG to a much higher degree than MEG. Intracellular pH, on the other hand, is highly buffered and may show only minimal changes in the current experiment [Deitmer and Chesler, 2008].
Implications for Calibrated‐BOLD Studies
The calibrated‐BOLD method was introduced by Davis and colleagues [ 1998] as a method to use combined measurements of the CBF and BOLD responses to activation to estimate the corresponding fractional change in CMRO2 (%ΔCMRO2). The method is based on the idea that the BOLD response depends on both the change in CBF and CMRO2, and so by properly combining BOLD and CBF response measurements with an appropriate model for the BOLD response, %ΔCMRO2 can be estimated. The model for the BOLD signal (fractional signal change s) proposed by these authors is as follows:
| (1) |
where f is the ratio of CBF between the active state and the baseline state and r is the ratio of CMRO2 between these two states. The parameter M represents a scaling factor specific to that brain region and depends on baseline state and also on the MRI acquisition (magnetic field strength and echo time). Based on a combination of experimental results and numerical simulations, the parameter α is assumed to be equal to 0.38 [Grubb et al., 1974], while β is taken to be 1.5 [Boxerman et al., 1995; Davis et al., 1998]. Although α and β are taken to be known parameters, the scaling parameter M must be estimated in each experiment. This is done by measuring CBF and BOLD responses to mild hypercapnia, assuming that hypercapnia increases CBF but does not change CMRO2. With this assumption (r = 1 for hypercapnia), M is calculated with Eq. (1) from the measured responses f and s in hypercapnia. This value of M is then combined with the measured responses f and s to a neural stimulus to estimate the CMRO2 ratio r for the neural stimulus. The estimated CMRO2 change with neural activation is often expressed as %ΔCMRO2 = 100(r − 1).
The assumption that mild hypercapnia does not affect CMRO2 remains controversial [Zappe et al., 2008]. High levels of hypercapnia usually have been found to depress CMRO2 in animal studies [Kliefoth et al., 1979; Sicard and Duong, 2005], although one study found increased CMRO2 after several hours of sustained hypercapnia [Yang and Krasney, 1995]. However, the central question here is whether the mild hypercapnia associated with an awake human subject breathing a 5% CO2 gas mixture also reduces CMRO2. Early human studies using the nitrous oxide method are usually taken to support this assumption [Kety and Schmidt, 1948; Novack et al., 1953]. However, these studies lacked the sensitivity to detect all but the largest changes in CMRO2. Other animal studies [Nilsson and Siesjo, 1976; Sicard and Duong, 2005] support the assumption that mild hypercapnia does not substantially change CMRO2. Nevertheless, the widely divergent conditions of these experiments (species, anaesthesia, level of hypercapnia, and method used to induce hypercapnia) render questionable their significance for small hypercapnic changes in normal behaving humans. For these reasons, the validity of the assumption remains an open question. Conflicting effects of mild hypercapnia on behavioral performance have also been reported, with some reporting no effect on cognitive and psychomotor tasks [Bloch‐Salisbury et al., 2000], while others report psychomotor slowing [Fothergill et al., 1991]. As noted earlier, the literature is unclear whether hypercapnia at the levels used for BOLD calibration affects neuronal processing in sensory or association areas of the unanesthetized human cortex. The current results indicate that this is the case.
A useful index of the coupling of flow and metabolism changes is the ratio %ΔCMRO2/%ΔCBF. A number of studies have used the calibrated‐BOLD approach to estimate this ratio and have found values in the approximate range 0.2–0.6 [Ances et al., 2008, 2009; Chiarelli et al., 2007a, b; Davis et al., 1998; Hoge et al., 1999; Kastrup et al., 2002; Kim et al., 1999; Leontiev and Buxton, 2007; Leontiev et al., 2007; Perthen et al., 2008; Stefanovic et al., 2004, 2005] (Note that the changes in blood oxygenation that underlie the BOLD effect occur, because this ratio is less than one.) However, these estimates assume that there is no change in CMRO2 with hypercapnia. In the current study, we found that the power in the gamma band of the MEG signal was reduced by 11% when subjects inhaled a 5% CO2 gas mixture. If we assume that CMRO2 decreases by the same amount, we can use the framework of the calibrated‐BOLD approach to estimate the size of the error produced in the calculated CMRO2 change or the CMRO2/CBF coupling ratio. For the hypercapnia experiment, we now take r = 1 + δCO2, where δCO2 is the fractional change in CMRO2 induced by hypercapnia: in the standard analysis δCO2 = 0, but if CMRO2 decreases by 11%, δCO2 = −0.11. Table II shows the results of calculating %ΔCMRO2 and the CMRO2/CBF coupling ratio for both δCO2 = 0 and δCO2 = −0.11 using mean measured CBF and BOLD responses to activation and hypercapnia from several recent studies [Ances et al., 2008; Leontiev et al., 2007; Perthen et al., 2008] that used the same gas inhalation set‐up as the current study. In each case, the neglect of a CMRO2 decrease with hypercapnia leads to an overestimate of %ΔCMRO2 with activation. The CMRO2/CBF coupling ratios, originally estimated to fall in the range 0.26–0.64, are reduced to the range 0.17–0.61 (the reduction varies from 0.03 to 0.1 for the different sets of data). These estimates of the potential errors due to the assumption of constant CMRO2 with mild hypercapnia are similar to previous estimates [Ances et al., 2008].
Table II.
Errors in the estimation of activation %ΔCMRO2 with calibrated‐BOLD, using recent experimental measurements of CBF and BOLD responses to hypercapnia and activation
| Study | Brain region | Hypercapnia | Activation | Estimated ΔCMRO2 (%)a | %ΔCMRO2 %ΔCBF | ||||
|---|---|---|---|---|---|---|---|---|---|
| ΔCBF (%) | ΔBOLD (%) | ΔCBF (%) | ΔBOLD (%) | δCO2 = 0 | δCO2 = −0.11 | δCO2 = 0 | δCO2 = −0.11 | ||
| Perthen et al., 2008 | Visual cortex | 48 | 2.6 | 48 | 1.2 | 18.5 | 14.0 | 0.39 | 0.29 |
| Ances et al., 2008 | Visual cortex | 71 | 2.08 | 78 | 0.89 | 32.5 | 28.4 | 0.42 | 0.36 |
| Ances et al., 2008 | Basal ganglia | 42 | 1.52 | 32 | 0.13 | 20.3 | 19.6 | 0.64 | 0.61 |
| Leontiev et al., 2007 | Visual cortex | 66 | 2.52 | 82 | 1.81 | 21.5 | 13.6 | 0.26 | 0.17 |
Calculated from the Davis model [Eq. (1)] with the assumption that the fractional change in CMRO2 with hypercapnia is δCO2. The standard assumption is δCO2 = 0, and the calculations were repeated assuming that the fractional decrease in CMRO2 is identical to the observed fractional decrease in MEG power in the gamma band (δCO2 = −0.11).
In summary, if the change in power of the fluctuations of the MEG signal with hypercapnia is an accurate quantitative reflection of the associated change in CMRO2, then studies using the calibrated‐BOLD method with hypercapnic calibration are overestimating the CMRO2 change with activation. However, the errors are modest, with the corrected ratios of the fractional changes in CMRO2 and CBF still falling in the approximate range 0.2–0.6. Recently, an alternative hyperoxia calibration method has been proposed [Chiarelli et al., 2007b], and this method potentially could avoid the errors of the hypercapnia calibration.
Possible Mechanisms
The MEG is generated by transmembrane currents due to synaptic activity and related active membrane channels [Murakami et al., 2003; Niedermeyer and Lopes da Silva, 1999]. The broad hypercapnia‐induced decrease in cortical activity, across different locations, at different latencies, in different sensory modalities as well as cognitive processing, argues for a very general mechanism. Recent work in animals suggests that a mechanism involving pH and adenosine provides a path whereby increased CO2 levels may dampen overall neuronal activity. In rodent hippocampal slices, increasing CO2 from 2 to 5% attenuates the CA1 field EPSP response to stimulation of the Schaffer collaterals by 18% [Dulla et al., 2005], close to the 20% decrease found in the current study. The mechanism of this suppression was shown to involve modulation via pH of presynaptic adenosine A1 receptors, thus decreasing gluatminergic transmitter release. Another potential mechanism is related to temperature. As reviewed by Moore & Cao [ 2008], hypercapnia‐induced changes in vasodilation cause an increase in the rate of arterial blood flow, which, in monkeys, has shown to reduce local brain temperature, which in turn can suppress neuronal activity, consistent with our observations. In either case, hypercapnia would induce a global decrease in synaptic currents and thus link intraneuronal currents, the likely generator of MEG signals.
Homeostatic Function
The large CBF change associated with hypercapnia increases tissue oxygenation [Tachtsidis et al., 2009], consistent with a large BOLD response reflecting an associated increase of blood oxgenation [Perthen et al., 2008]. For this reason, a shortage of O2 cannot account for the observed changes in neural activity. However, considering that low‐cerebral pH could arise due to excessive accumulation of lactate or CO2, this could serve as a signal independent of O2 level itself that CBF is unable to keep pace with neuronal activity. The results observed in the present study may reflect a homeostatic mechanism by which neuronal activity is adjusted to a level that can be sustained by available blood flow. This is consistent with the idea of oxygen conformance, the reduction in the demand for O2 as a way to cope with reduced availability of O2 [Hochachka et al., 1996]. Energy use is tightly related to synaptic activity [Attwell and Laughlin, 2001]. By downregulating presynaptic release, the proposed mechanism attenuates the entire cascade of transmitter reuptake and resynthesis as well as postsynaptic depolarization, modulation of intrinsic currents, and consequent cell‐firing. Because inhibitory neuronal activation is tightly coupled to excitatory through feedforward and feedback circuits [Buzsáki, 1984], inhibitory cells would also be expected to decrease in a proportional manner.
According to this hypothesis, cerebral acidosis is taken as a sign by the vasculature that neuronal activity is too great to be served by existing blood flow. The well‐known increase in CBF is one solution to this problem. The greater blood flow not only restores normal pH, but also other resources that would be expected to be depleted by high activity. We hypothesize that the phenomenon reported here, wherein cerebral acidosis suppresses neuronal activity, serves the same purpose as the increased blood flow. Both seek to correct the imbalance between neuronal activity and blood flow. Note that this hypothesis addresses why acidosis suppresses cortical activity when it is generated by neuronal activity; breathing CO2 reproduces the acidosis and its consequent increase of CBF, but that increase will not correct cortical pH under these artificial circumstances.
The universal nature of the suppression may help explain why, despite its large and widespread effects on neural activity, hypercapnia did not affect performance speed or accuracy on either task. Similar results have previously been obtained with a more extensive neuropsychological battery at moderate levels of hypercapnia that significantly affected spontaneous EEG [Bloch‐Salisbury et al., 2000]. It seems plausible that a universal suppression of neurotransmission might provide an optimal strategy for reducing energy use while maintaining function when compared with a selective suppression of limited aspects of neural activity.
CONCLUSION
We propose that hypercapnia has a multivalent response on both the vasculature and neural activity. This multivalent response can be understood as an adaptive mechanism that interprets lowered pH as a sign that the rCBF is unable to maintain homeostasis, and consequently acts to both decrease neural activity, and increases rCBF in an effort to restore balance. Under this proposal, hypercapnia would also have an indirect effect of reducing rCBF via its effect on neural activity.
REFERENCES
- Ainslie PN, Duffin J ( 2009): Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495. [DOI] [PubMed] [Google Scholar]
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB ( 2008): Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: Implications for BOLD‐fMRI. Neuroimage 39: 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB ( 2009): Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp 30: 1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aram JA, Lodge D ( 1987): Epileptiform activity induced by alkalosis in rat neocortical slices: Block by antagonists of N‐methyl‐d‐aspartate. Neurosci Lett 83: 345–350. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Donovan T, Spiegelhalter DJ, Pickard JD, Boniface SJ ( 2007): Intracortically distributed neurovascular coupling relationships within and between human somatosensory cortices. Cereb Cortex 17: 661–668. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB ( 2001): An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21: 1133–1145. [DOI] [PubMed] [Google Scholar]
- Bloch‐Salisbury E, Lansing R, Shea SA ( 2000): Acute changes in carbon dioxide levels alter the electroencephalogram without affecting cognitive function. Psychophysiology 37: 418–426. [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM ( 1995): MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 34: 555–566. [DOI] [PubMed] [Google Scholar]
- Buxton R ( 2002): Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. Cambridge, UK: Cambridge University. [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT ( 2004): Modeling the hemodynamic response to brain activation. Neuroimage 23( Suppl 1): S220–S233. [DOI] [PubMed] [Google Scholar]
- Buzsáki G ( 1984): Feed‐forward inhibition in the hippocampal formation. Prog Neurobiol 22: 131–153. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Gallichan D, Piechnik SK, Wise R, Jezzard P ( 2007a) Flow‐metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med 57: 538–547. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Piechnik S, Jezzard P ( 2007b) Sources of systematic bias in hypercapnia‐calibrated functional MRI estimation of oxygen metabolism. Neuroimage 34: 35–43. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN ( 1983): Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol 56: 38–51. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG ( 2002): Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level‐dependent fMRI response. J Cereb Blood Flow Metab 22: 1042–1053. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E ( 2000): Dynamic statistical parametric mapping: Combining fMRI and MEG for high‐resolution imaging of cortical activity. Neuron 26: 55–67. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR ( 1998): Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM ( 2003): Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39: 353–359. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA ( 2005): Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron 48: 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E ( 2005): Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol 93: 2864–2872. [DOI] [PubMed] [Google Scholar]
- Foerster O ( 1924): Hyperventilationsepilepsie. Deutsche Ztschr Nervenheilk 83: 186–188. [Google Scholar]
- Fothergill DM, Hedges D, Morrison JB ( 1991): Effects of CO2 and N2 partial pressures on cognitive and psychomotor performance. Undersea Biomed Res 18: 1–19. [PubMed] [Google Scholar]
- Fox PT, Raichle ME ( 1986): Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs FA, Gibbs EL ( 1950): Atlas of Electroencephalography. Cambridge: Addison‐Wesley Press. [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr, Cohen MS ( 2002): Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13: 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Raichle ME, Eichling JO, Ter‐Pogossian MM ( 1974): The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 5: 630–639. [DOI] [PubMed] [Google Scholar]
- Halgren E, Babb TL, Crandall PH ( 1977): Responses of human limbic neurons to induced changes in blood gases. Brain Res 132: 43–63. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM ( 1994a) Spatio‐temporal stages in face and word processing. I. Depth‐recorded potentials in the human occipital, temporal and parietal lobes. J Physiol 88: 1–50. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K ( 1994b) Spatio‐temporal stages in face and word processing. I. Depth‐recorded potentials in the human occipital, temporal and parietal lobes. J Physiol (Paris) 88: 1–50. [DOI] [PubMed] [Google Scholar]
- Halgren E, Sherfey J, Irimia A, Dale AM, Marinkovic K: Sequential temporo‐fronto‐temporal activation during monitoring of the auditory environment for temporal patterns. Hum Brain Mapp. 2010. Jul 27. [Epub ahead of print] PubMed PMID: 20665718; PubMed Central PMCID: PMC2970649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern P, Neufeld MY, Sade K, Silbiger A, Szold O, Bornstein NM, Sorkine P ( 2003): Middle cerebral artery flow velocity decreases and electroencephalogram (EEG) changes occur as acute hypercapnia reverses. Intensive Care Med 29: 1650–1655. [DOI] [PubMed] [Google Scholar]
- Hari R, Kaila K, Katila T, Tuomisto T, Varpula T ( 1982): Interstimulus interval dependence of the auditory vertex response and its magnetic counterpart: Implications for their neural generation. Electroencephalogr Clin Neurophysiol 54: 561–569. [DOI] [PubMed] [Google Scholar]
- Hillyard S, Anllo‐Vento L ( 1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA 95: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ, Land SC ( 1996): Unifying theory of hypoxia tolerance: Molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 93: 9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB ( 1999): Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA 96: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Lightfoot JT, Brown SJ, Swaine IL ( 2004): The effects of breathing 5% CO2 on human cardiovascular responses and tolerance to orthostatic stress. Exp Physiol 89: 465–471. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Hewson‐Stoate N, Gias C, Mayhew J ( 2005): The effect of hypercapnia on the neural and hemodynamic responses to somatosensory stimulation. Neuroimage 27: 609–623. [DOI] [PubMed] [Google Scholar]
- Kaila K, Ransom BR, editors ( 1998): pH and Brain Function. Chichester: Wiley. [Google Scholar]
- Kalkman CJ, Boezeman EH, Ribberink AA, Oosting J, Deen L, Bovill JG ( 1991): Influence of changes in arterial carbon dioxide tension on the electroencephalogram and posterior tibial nerve somatosensory cortical evoked potentials during alfentanil/nitrous oxide anesthesia. Anesthesiology 75: 68–74. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Neumann‐Haefelin T, Glover GH, Moseley ME ( 2002): Changes of cerebral blood flow, oxygenation, and oxidative metabolism during graded motor activation. Neuroimage 15: 74–82. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF ( 1948): The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB ( 1999): Determination of relative CMRO2 from CBF and BOLD changes: Significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med 41: 1152–1161. [DOI] [PubMed] [Google Scholar]
- Kliefoth AB, Grubb RL, Jr. , Raichle ME ( 1979): Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem 32: 661–663. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C ( 1994): Psycholingusistics electrified: Event‐related brain potential investigations In: Gernsbacher M, editor. Handbook of Psycholinguistics. New York: Academic; pp 83–143. [Google Scholar]
- Leontiev O, Buxton RB ( 2007): Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated‐BOLD fMRI. Neuroimage 35: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB ( 2007): CBF/CMRO2 coupling measured with calibrated BOLD fMRI: Sources of bias. Neuroimage 36: 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA ( 2004): Interpreting the BOLD signal. Annu Rev Physiol 66: 735–69. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Matakas F, Birkle J, Cervos‐Navarro J ( 1978): The effect of prolonged experimental hypercapnia on the brain. Acta Neuropathol 41: 207–210. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG ( 2009): Decoupling the cortical power spectrum reveals real‐time representation of individual finger movements in humans. J Neurosci 29: 3132–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Cao R ( 2008): The hemo‐neural hypothesis: On the role of blood flow in information processing. J Neurophysiol 99: 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Hirose A, Okada YC ( 2003): Contribution of ionic currents to magnetoencephalography (MEG) and electroencephalography (EEG) signals generated by guinea‐pig CA3 slices. J Physiol 553( Pt 3): 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva F ( 1999): Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia: Lippincott, Williams & Wilkins. [Google Scholar]
- Nilsson B, Siesjo BK ( 1976): A method for determining blood flow and oxygen consumption in the rat brain. Acta Physiol Scand 96: 72–82. [DOI] [PubMed] [Google Scholar]
- Novack P, Shenkin HA, Bortin L, Goluboff B, Soffe AM ( 1953): The effects of carbon dioxide inhalation upon the cerebral blood flow and cerebral oxygen consumption in vascular disease. J Clin Invest 32: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB ( 2008): Caffeine‐induced uncoupling of cerebral blood flow and oxygen metabolism: A calibrated BOLD fMRI study. Neuroimage 40: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson EA, Bowes G ( 1986): Control of breathing during sleep In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, Section 3. The Respiratory System, Vol. 2: Control of Breathing. Bethesda, MD: American Physiological Society; pp 649–689. [Google Scholar]
- Richerson GB ( 2004): Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ ( 2005): Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus‐evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage 25: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB ( 2004): Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22: 771–778. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Kobayashi E, Bagshaw AP, Hawco C, Dubeau F, Gotman J, Pike GB ( 2005): Hemodynamic and metabolic responses to activation, deactivation and epileptic discharges. Neuroimage 28: 205–215. [DOI] [PubMed] [Google Scholar]
- Tachtsidis I, Tisdall MM, Leung TS, Pritchard C, Cooper CE, Smith M, Elwell CE ( 2009): Relationship between brain tissue haemodynamics, oxygenation and metabolism in the healthy human adult brain during hyperoxia and hypercapnea. Adv Exp Med Biol 645: 315–320. [DOI] [PubMed] [Google Scholar]
- Yang SP, Krasney JA ( 1995): Cerebral blood flow and metabolic responses to sustained hypercapnia in awake sheep. J Cereb Blood Flow Metab 15: 115–123. [DOI] [PubMed] [Google Scholar]
- Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK ( 2008): The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex 18: 2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]


