Abstract
Sinonasal tumors may grow to considerable size before presentation and in view of their relation to the base of skull, orbit, cranial nerves and vital vessels; a precise diagnostic and therapeutic planning is needed to achieve the optimal results. We report a case who presented with unilateral nasal blockage, rhinorrhea and episodes of epistaxis which diagnosed as sinonasal inverted papilloma and angiofibroma.
Key words: sinonasal tumors, inverted papilloma, angiofibroma.
1. INTRODUCTION
Whereas most of neoplasms in sinuses are malignant, there is a fairly even distribution between benign and malignant tumors in the nasal cavity. Due to the contiguity of the nasal cavity with sinuses, it is often difficult to identify the specific site of origin of large sinonasal tumors.
Both inverted papilloma and angiofibroma are considered as benign sinonasal tumors which are sometimes difficult to be differentiated. Therefore, precise histopathological examination is needed to achieve the right diagnosis.
2. CASE REPORT
Thirty seven years old male with no known chronic medical illness presented with history of four months left sided nasal blockage and discharge associated with left facial pain and two episodes of large amount of epistaxis from left nostril which stopped spontaneously. No history of ear symptoms, blurring of the vision and neck swelling noted.
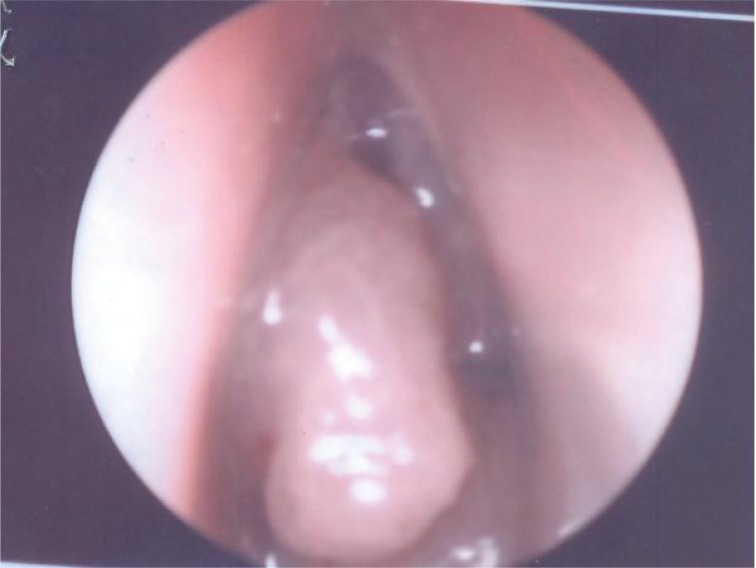
On endoscopic examination, the left nasal cavity was occupied by the mass but did not extend to nasopharynx (Figure 1). The mass was polypoidal and pinkish in appearance and bled minimally with touch and suction. Both fossa of Rosen Muller were clear. Moderate deviation of nasal septum to the right side noted but no mass seen in right nasal cavity.
Figure 1.
Preoperative endoscopic examination showing the mass occupying the left nasal cavity.
Punch biopsy from two different parts of the mass performed for histopathological examination and reported as inverted papilloma. Also CT scan done and reported as followings: heterogeneous soft tissue mass seen in left maxillary sinus and nasal cavity with no extension to nasopharynx (Figure 2). Erosion of medial and posterolateral wall of left maxillary sinus noted. No intra cranial or orbital extension reported. Posterior extension to the left infratemporal space with intact pterygopalatine fossa was seen. The features were consistent with aggressive left maxillary soft tissue tumor such as inverted papilloma.
Figure 2.
Ct Scan showing the mass in the left nasal cavity and left maxillary sinus.
Operation was decided and done as left open partial maxillectomy and complete excision of the tumor. After incision of the skin and raising the flap, osteotomy was performed as removal of frontal process of maxilla and medial wall of maxillary sinus. Then the lacrimal sac was exposed and distal end of lacrimal duct was cut. Intraoperatively, a big mass seen that occupied whole left nasal cavity and maxillary sinus. During the first step of resection of the tumor, bleeding occurred and was secured by packing for about half an hour. The tumor was removed from posterolateral wall of maxilla to the left infratemporal fossa and from posterior wall of maxilla until left pterygopalatine fossa. Inferiorly, the mass was resected from floor of the maxillary sinus and erosion of hard palate was noted. In addition, superior turbinate was removed and ethmoidectomy and sphenoidectomy conducted. There was no tumor seen inside the sphenoid sinus. The left frontal sinus was opened as well and clear antrum with polypoidal mucosa was noted.
Finally, the bleeding secured. The amount of intraoperative blood loss was estimated as 700 ml. The cavity was packed by PIPP that was kept for one week duration. The patient was discharged 9 days after operation. Post-operative histopathology report was: co-existing of inverted papilloma and angiofibroma.
Currently, patient is asymptomatic with no sign of recurrence noted clinically (Figure 3) and in post-operative CT scan. He is under regular three monthly follow up.
Figure 3.
Postoperative endoscopic examination showing a disease free nasal cavity.
3. DISCUSSION
Inverted papilloma is a benign sinonasal tumor with undefined etiology and also called as Schneider papilloma, Ewing papilloma, transitional cell papilloma and papillomatos. It is a rare nasal tumor with incidence of 0.5% to 4% of all primary nasal tumors (1, 2, 3). Inverted papilloma is 4-5 times more frequent in males and is unilateral in more than 90% of the cases (4).
It is named as inverted papilloma due to endophilic growth of superficial epithelium to inside the adjacent stoma (3, 5, 6). It originates from lateral wall of the nasal cavity and the primary involvement of paranasal sinuses is very rare (5%), but they can be affected secondarily (6, 7, 8).
Although it is benign, it has aggressive growth, high potential for invasion (4, 9), great recurrence rate (3, 6) and malignization rate of 1-13% (6, 9).
Usually inverted papilloma presents as unilateral nasal blockage in about 98% of the cases, rhinorrhea (17%), epistaxis (6%),anosmia (4%), headache and facial pain. Regarding the extension of the tumor, it usually resides in nasal cavity in 93% of the inverted papilloma, but in 7% of the cases, it may extend outside of nasal cavity. The involvement of nasopharynx has been noted in 3% and in less than 2% it extends to pterygopalatine and intracranial fossa (1, 2, 5).
Inverted papilloma is diagnosed by precise history, meticulous examination and imaging. CT scan, MRI and histopathological examination confirm the diagnosis. Certainly, the surgeon must be patient about the vascular tumors such as angiofibroma or the masses extending to cranial fossa like meningocele (5).
The differential diagnoses of inverted papilloma include antrochoanal polyps, nasal cavity squamous polyp, angiofibroma, meningocele, meningoencephalocele, fibrous dysplasia, gigantic cells granuloma and other neoplasia (5, 6, 8).
The gold standard treatment for inverted papilloma is surgical excision. Post-operative recurrence usually occurs within two years; though there is a possibility of 17% to happen six year after excision. Subsequently, the patients must be followed up for at least six years post-operatively (3, 10).
Juvenile nasopharyngeal angiofibroma (JNA) is a rare benign tumor that mainly happens in prepubertal and adolescent males. If JNA is seen in females, the chromosomal testing must be done to rule out the genetical disorders. Onset of the disease is ordinarily in the second decade and it rarely occurs in the age of more than 25 years. It is a highly vascularized tumor and has a tendency to bleed that is sometimes disastrous (9).
The tumor usually originates from sphenopalatine foramen and has a slow growth. It commonly spreads to the nasopharynx, pterygopalatine fossa, nasal cavities and paranasal sinuses. It also may extend to the orbit and intracranial fossa (11). A retrospective review by Cruz et al. (12) demonstrated that the most common area for extension of the JNA is pterygopalatine fossa (100%), nasal cavity (94.7%), sphenoid sinus (84.2%), nasopharynx (73.7%) and orbit (31.6%).
The clinical presentation of the JNA includes nasal obstruction, epistaxis, headache and facial swelling. The nasal blockage is the most frequent symptom, particularly in early stages of the tumor and it is usually unilateral. The nasal bleeding is commonly unilateral and recurrent. It is sometimes severe and needs medical consideration. Unilateral rhinorrhea, impairment of smell, otalgia and swelling of the soft palate are the other clinical manifestations of the JNA (12, 13).
The diagnosis is made by history, endoscopic nasal examination and imaging. CT scan is used to determine the extension of the mass, bony erosion of skull base and invasion of the pterygopalatine and infratemporal fossae. MRI is indicated to demarcate the intracranial extension of the tumor. Angiography confirms the diagnosis and shows the blood supply of the tumor (14).
The differential diagnoses of JNA are nasal polyposis, antrochoanal polyp, teratoma, encephalocele, meningoencephalocele, dermoids, inverted papilloma, rhabdomyosarcoma and squamous cell carcinoma (15).
Several nonsurgical ways have been recommended for treatment of the JNA such as hormone therapy and radiotherapy, but surgery is the universally accepted choice for treatment of JNA. Numerous surgical approaches have been defined such as transpalatal, lateral rhinotomy and midfacial degloving procedures. It is recommended to do embolization prior to operation to reduce the intraoperative bleeding. Preoperative embolization of tumor supplying vessels has been recommended in the literature to decrease intraoperative blood loss. Despite some risks, the preoperative embolization is effective in reducing intraoperative blood loss (9).
Conflict of interest
None declared.
REFERENCES
- 1.Vrabec DP. The inverted Schneiderian papilloma: 25-year study. Laryngoscope. 1994;104:582–608. doi: 10.1002/lary.5541040513. [DOI] [PubMed] [Google Scholar]
- 2.Tsue TT, Bailet JW, Barlow DW, Makielski KH. Bilateral sinusal papilloma in aplastic maxillary sinuses. Am J Otolaryngol. 1997;18(4):263–268. doi: 10.1016/s0196-0709(97)90007-7. [DOI] [PubMed] [Google Scholar]
- 3.Weissler MC, Montgomery WW, Montgomery SK. Inverted papiloma. Ann Otol Rhinol Laryngol. 1986;95:215–221. doi: 10.1177/000348948609500301. [DOI] [PubMed] [Google Scholar]
- 4.Oikawa K, Furuta Y, Oridate N, Nagahashi T, Homma A, Ryu T, Fukuda S. Preoperative staging of sinonasal inverted papilloma by magnetic resonance imaging. Laryngoscope. 2003;133(11):1983–1987. doi: 10.1097/00005537-200311000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Alegre ACM, Ramos AHC, Voegels RL, Romano F. Papiloma e Papiloma invertido Em: Campos CA, Costa HOO. Tratado de Otorrinolaringologia. 2003:126–32. [Google Scholar]
- 6.Myers EN, Fernau JL, Johnson JT. Management of inverted papilloma. Laryngoscope. 1990;100:481–490. doi: 10.1288/00005537-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Krouse JH. Development of a staging system for inverted papilloma. Laryngoscope. 2000;110:965–968. doi: 10.1097/00005537-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Vrabec DP. The inverted Schneiderian papilloma: A clinical and pathological study. Laryngoscope. 1975;85:186–221. doi: 10.1288/00005537-197501000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Alves FRA, Granato L, Maia MS, Lambert E. Surgical Approaches to Juvenile Nasopharyngeal Angiofibroma: Case Report and Literature Review. Arq. Int. Otorrinolaringol/Intl. Arch. Otorhinolaryngol. 2006;10(2):162–166. [Google Scholar]
- 10.Atlung T, Sunar A, Bilgin H. Inverted papilloma. Report of a multicentric case. Rev Laryngol. 1989;110:299–301. [PubMed] [Google Scholar]
- 11.Scholtz AW, Appenroth E, Kamen JK, Scholtz LU, Thumfart WF. Juvenile nasopharyngeal angiofibroma: management and therapy. Laryngoscope. 2001;111:6817. doi: 10.1097/00005537-200104000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Cruz AAV, Atique JMC, Melo-Filho FV, Elias JJ. Orbital Involvement in Juvenile Nasopharyngeal Angiofibroma: Prevalence and Treatment. Ophthal Plast Reconstr Surg. 2004;20(4):296–300. doi: 10.1097/01.iop.0000132163.00869.44. [DOI] [PubMed] [Google Scholar]
- 13.Tang IP, Shashinder S, Gopala Krishnan G, Narayanan P. Juvenile nasopharyngeal angiofibroma in a tertiary centre: ten-year experience. Singapore Med J. 2009;50(3):261–264. [PubMed] [Google Scholar]
- 14.Szymanska A, Golabek W, Siwiec H, Pietura R, SzczerboTrojanowska M. Juvenile angiofibroma: the value of CT and MRI for treatment planning and followup. Otolaryngol Pol. 2005;59(1):8590. [PubMed] [Google Scholar]
- 15.Hoover LA, Hanafee WN. Differential Diagnosis of Nasopharyngeal Tumors by Computed Tomography Scanning. Arch Otolaryngol Head Neck Surg. 1983;109(1):43–47. doi: 10.1001/archotol.1983.00800150047009. [DOI] [PubMed] [Google Scholar]