Abstract
Purpose.
To characterize the 24-hour pattern of intraocular pressure (IOP) in untreated ocular hypertensive (OHTN) patients.
Methods.
IOP measurements were taken every 2 hours during a 24-hour period from 15 untreated OHTN patients (ages 41–77 years). Measurements were both sitting and supine (diurnal) and supine only (nocturnal). Mean diurnal and nocturnal IOPs in the OHTN group were compared to previously reported values in age-matched healthy and glaucomatous eyes. Post hoc analysis compared the 24-hour IOP pattern of the OHTN patients who converted to glaucoma and those who did not with that in the same healthy and glaucomatous eyes.
Results.
Mean sitting and supine IOPs were significantly higher in the OHTN group than in the healthy control but not the glaucoma group. Similar to the glaucoma group, the OHTN group demonstrated significant differences from healthy controls in diurnal IOP variation and IOP changes upon awakening in habitual and supine positions. The 24-hour IOP curve acrophases and amplitudes for OHTNs were closer to those of the glaucoma than the healthy control group in the habitual position. Thirty-three percent of OHTNs developed glaucoma during a mean follow-up period of 4.3 ± 3.8 years. Similar to findings in the glaucoma group, habitual IOP curve phase delay, habitual IOP variation, diurnal-to-nocturnal IOP changes, and IOP changes upon awakening of the converters were significantly different from those in healthy controls. There were no differences between nonconverters and other groups.
Conclusions.
Baseline 24-hour IOP pattern in OHTN patients is similar to that in glaucomatous patients. In contrast to nonconverters, OHTN patients who converted to glaucoma are significantly different from healthy controls.
The 24-hour IOP pattern in patients with untreated OHTN was similar to that of patients with glaucoma. OHTN patients who progressed to glaucoma demonstrated significantly different IOP patterns from healthy controls. Data may help distinguish OHTN patients at higher risk of developing glaucoma.
Introduction
Elevated intraocular pressure (IOP) is a major risk factor for the development of glaucoma. Therapeutic strategies designed to reduce IOP remain the principal method of treatment.1,2 In the United States, ocular hypertension (OHTN) has been estimated to affect approximately 5% of people over 40 years of age.3 However, this large OHTN patient population remains poorly characterized in terms of the standard quantitative measure of IOP.
Measurement of IOP, taken at a single time point during office hours, can render an incomplete picture of IOP due to the diurnal variability of IOP.4 While the diurnal IOP curve provides a better estimate of an individual's IOP variation than a single office reading,5 IOP data gathered over 24 hours are required to characterize IOP variation more completely.6–10 Indeed, most patients' peak IOPs have been reported outside of office hours.11,12 The pattern of 24-hour IOP for patients with early glaucomatous changes and for healthy controls has been described,7,10,11,13 but limited information is available about the 24-hour IOP variation in patients with OHTN.
The goal of the present study was to characterize the 24-hour IOP profile associated with untreated OHTN and compare this to previously reported 24-hour IOP patterns associated with an age-matched control group of individuals with healthy eyes and an age-matched group of patients with early-stage primary open angle glaucoma (POAG).7 To the best of our knowledge, this is the first study to compare the pattern of 24-hour IOP in patients with OHTN to both healthy controls and patients with early glaucomatous signs of visual field loss and/or optic nerve damage.
Methods
Ethics Statement
This study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at the University of California at San Diego (UCSD). Written informed consent was obtained from all patients after explanation of the nature and possible consequences of the study.
Study Subjects
Experimental subjects were recruited consecutively from patients referred to the Hamilton Glaucoma Center of UCSD with the diagnosis of OHTN in both eyes. Each patient underwent a complete ophthalmologic evaluation that included a review of relevant medical history, best corrected visual acuity, slit-lamp biomicroscopy, gonioscopy, Goldman applanation tonometry, dilated funduscopy, and visual field testing. Patients with IOP ≥ 21 mm Hg on two consecutive visits prior to the experiment were considered for inclusion in the study. Patients with abnormal or suspected abnormal optic discs or visual fields were excluded. Abnormal discs included excavation, rim defect, hemorrhage, notching, nerve fiber layer defect, or cup/disc asymmetry between two eyes ≥0.2. A normal visual field was determined by clinical review and by Statpac II criteria for abnormality (full-threshold 24-2 examination, Humphrey Field Analyzer; Zeiss-Humphrey, Dublin, CA). A visual field was considered within normal limits if the corrected pattern standard deviation had a P > 5% and the glaucoma hemifield test was within normal limits. Individuals with history of prior glaucoma medication use or laser trabeculoplasty, prior eye surgery, ocular inflammation or trauma, a narrow iridocorneal angle, or any other concomitant ocular conditions were excluded. Individuals who smoked or had an irregular daily sleep schedule were also excluded.
Fifteen patients who met the criteria described were recruited as the OHTN group. This included 8 males and 7 females who were 41 to 77 years of age (57 ± 10 years, mean ± SD). There were 13 Caucasians and 2 African Americans. Mean central corneal thickness (CCT) was 591 ± 40 μm (mean ± SD).
Twenty-Four–Hour IOP Measurement
Twenty-four–hour IOP measurements for all three groups were conducted contemporaneously. The experimental procedure employed and data generated from healthy and glaucoma groups were previously described by Liu et al.7 Briefly, experiments were conducted within a few weeks after recruitment. Subjects maintained a daily 8-hour sleep period for 7 days prior to the experiment. On the study day, they arrived at the laboratory at approximately 2 PM and stayed indoors for the next 24 hours. The 8-hour period of darkness in the subject's room and times for IOP measurements were adjusted to correspond to each individual's sleep period. Individualized corresponding clock times were subsequently normalized for data presentation as if each subject had a sleep period from 11 PM to 7 AM.
Over the 24 hours, IOP was measured in both eyes every 2 hours using a pneumatonometer (Model 30 Classic; Reichert, Depew, NY). Topical 0.5% proparacaine was applied as the local anesthetic. The pneumatonometer was calibrated against the manufacturer's verifier, and it was confirmed that different measurement angles produced the same IOP reading.
Before the sleep period, measurements were taken at 3:30 PM, 5:30 PM, 7:30 PM, and 9:30 PM. Diurnal IOPs were obtained in both the supine and sitting position following lying down in bed or sitting up for 5 minutes, respectively. Light intensity was kept at 500 to 1000 lux at eye level during this time. Lights in individual rooms were turned off at 11 PM. Nocturnal IOP measurements were taken in the supine position only at 11:30 PM, 1:30 AM, 3:30 AM, and 5:30 AM. If necessary, subjects were awakened briefly and a room light (less than 10 lux) was used to assist the nocturnal measurements. Daytime room lighting was restored at 7 AM, and diurnal IOP measurements were taken again at 7:30 AM, 9:30 AM, 11:30 AM, and 1:30 PM as previously described.
Data and Statistical Analysis
Sitting and supine IOP data obtained from the OHTN patient group were compared to contemporaneous data obtained under identical experimental conditions from an age-matched healthy control group of 10 males and 14 females (56 ± 9 years of age, mean ± SD) and a glaucoma group of 11 males and 13 females (59 ± 12 years of age, mean ± SD). The characteristics of these groups have been described in detail by Liu et al.7 The CCT was 543 ± 46 μm and 556 ± 54 μm in the healthy control and glaucoma groups, respectively.
For all patients, IOP values from the two eyes were averaged. For each group, mean IOPs were calculated for each time point and during the diurnal and nocturnal periods. IOP variation was calculated by subtracting the trough IOP from the peak IOP during each diurnal or nocturnal period for all patients. These differences were averaged within groups. Statistical comparisons of the means between the diurnal and the nocturnal periods or two time points (e.g., 5:30 AM and 7:30 AM) within a group were made using a paired two-tailed t-test. Statistical comparisons between the OHTN, glaucoma, and healthy control groups were performed using one-way analysis of variance (ANOVA). If ANOVA yielded a significant result (P < 0.05), this was followed by two-tailed t-test for two-group comparisons.
Estimation of 24-hour IOP rhythm was performed for habitual and supine body positions using best-fitting cosine curve. This was obtained for each subject using IOP data from the 12 time points. The peak of the curve (acrophase) represents the phase timing, while the amplitude (height of the curve) estimates magnitude of 24-hour IOP variation. The Rayleigh test was used to determine the presence of a significant synchronized 24-hour IOP rhythm in each group.7 Two-tailed t-test was used for statistical comparison of mean group acrophases and amplitudes.
Appropriate Bonferroni corrections were applied to all P values. All data are presented as mean ± SEM unless otherwise specified.
Results
No significant difference was found in age among the three groups (ANOVA P = 0.39). However, there was a significant difference in CCT (ANOVA P = 0.02), with the OHTN group significantly higher than the healthy control (P = 0.012) but not the glaucoma group. There was no significant correlation of CCT with mean diurnal supine, diurnal sitting, and nocturnal IOPs (P = 0.88, 0.40, 0.20, respectively) in the OHTN group.
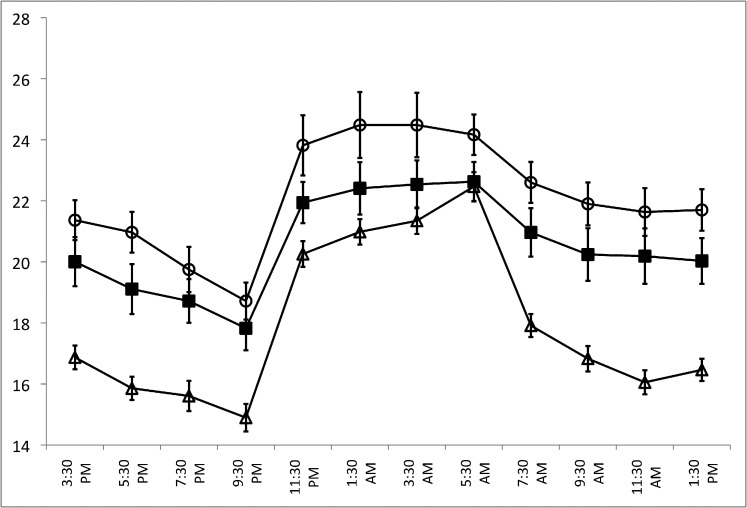
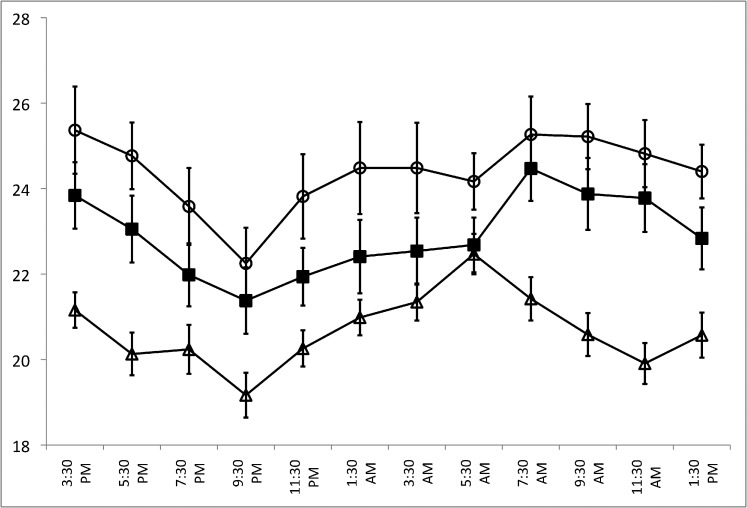
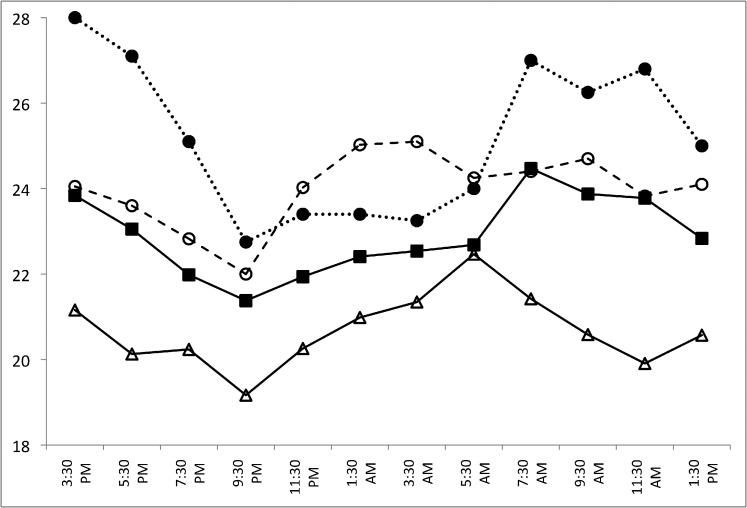
Figure 1 shows the 24-hour IOP patterns for the three subject groups in the habitual position (diurnal sitting, nocturnal supine), while Figure 2 shows the 24-hour IOP pattern for the supine position (diurnal supine, nocturnal supine). Sitting and supine IOPs at all time points were higher in the OHTN group compared to both the glaucoma and healthy control groups. In the OHTN group, similar to the other two groups, sitting and supine IOPs decreased progressively in the diurnal period while IOP increased during the nocturnal period. In the habitual position, the IOP peak occurred between 1:30 AM and 3:30 AM in the OHTN group (24.5 mm Hg) but at 5:30 AM in both the glaucoma and healthy control groups (22.6 and 22.5 mm Hg, respectively). The trough occurred at 9:30 PM for all three groups (18.7, 17.8, and 14.9 mm Hg for OHTN, glaucoma, and healthy control groups, respectively). In the supine position, the peak IOP occurred at 3:30 PM in the OHTN group (25.4 mm Hg), at 7:30 AM in the glaucoma group (24.5 mm Hg), and at 5:30 AM in the healthy control group (22.5 mm Hg). The trough occurred at 9:30 PM for all three groups (22.3, 21.4, and 19.2 mm Hg for OHTN, glaucoma, and healthy control groups, respectively).
Figure 1.
A comparison of 24-hour IOP patterns for the OHTN (open circles), healthy control (open triangles), and glaucoma (solid squares) groups in the habitual position (diurnal sitting, nocturnal supine). The OHTN group consisted of 15 untreated 41- to 77-year-old patients with IOP > 21 mm Hg on two consecutive visits and no evidence of glaucomatous changes. This group was compared with an age-matched control group with healthy eyes (n = 24) and an untreated glaucoma group (n = 24). Error bars denote SEM.
Figure 2.
A comparison of 24-hour IOP patterns for the OHTN (open circles), healthy control (open triangles), and glaucoma (solid squares) groups in the supine position (diurnal supine, nocturnal supine). Error bars denote SEM.
The mean diurnal and nocturnal IOPs for each group are summarized in Table 1. The mean diurnal and nocturnal IOPs were significantly higher in the OHTN group compared to the healthy control group (P < 0.001, P < 0.001, P = 0.015 for diurnal sitting, diurnal supine, and nocturnal IOP, respectively; ANOVA P < 0.001) but not the glaucoma group. In the habitual position for all three groups, the mean nocturnal IOP was significantly higher than their diurnal IOP (P = 0.003, P < 0.001, P < 0.001 for OHTN, glaucoma, and healthy controls, respectively). The diurnal-to-nocturnal change in IOP in the habitual position was smaller in both the OHTN and glaucoma groups compared to the control group. In the supine position, the nocturnal compared to the diurnal IOP decreased in both the OHTN and glaucoma groups while it increased in the healthy control group.
Table 1. .
Mean Diurnal and Nocturnal IOP
|
|
Diurnal Period (7 AM–11 PM) |
Nocturnal Period (11 PM–7 AM) |
Change |
| Habitual IOP, mm Hg | |||
| Healthy control | 16.3 ± 0.4 | 21.3 ± 0.4 | 5.0 ± 0.3* |
| Glaucoma | 19.6 ± 0.7 | 22.4 ± 0.7 | 2.7 ± 0.6* |
| OHTN | 21.1 ± 0.6 | 24.2 ± 0.9 | 3.2 ± 0.9† |
| Supine IOP, mm Hg | |||
| Healthy control | 20.4 ± 0.4 | 21.3 ± 0.4 | 0.9 ± 0.4† |
| Glaucoma | 23.1 ± 0.7 | 22.4 ± 0.7 | −0.8 ± 0.4 |
| OHTN | 24.4 ± 0.7 | 24.2 ± 0.9 | −0.2 ± 0.2 |
Data expressed as the mean ± SEM; N = 15 (OHTN group), 24 (healthy control group), and 24 (glaucoma group). *P < 0.01 and †P < 0.05; paired t-test between diurnal and nocturnal IOPs.
Significant differences in diurnal variation in both sitting (ANOVA P = 0.0012) and supine (ANOVA P = 0.0053) IOP were observed between groups. Diurnal IOP variation (Table 2) in both the OHTN (diurnal sitting and supine P = 0.036 and 0.021, respectively) and glaucoma (diurnal sitting and supine P = 0.0012 and 0.039, respectively) groups was significantly higher than that of the healthy control group. In the nocturnal period, no significant differences in supine IOP variation between the three groups were observed (ANOVA P = 0.46).
Table 2. .
Diurnal and Nocturnal IOP Variation in mm Hg (Peak IOP − Trough IOP)
|
|
Diurnal Sitting |
Diurnal Supine |
Nocturnal Supine |
| Healthy control | 4.0 ± 0.3 | 4.1 ± 0.3 | 3.2 ± 0.3 |
| Glaucoma | 5.8 ± 0.4 | 5.4 ± 0.4 | 2.9 ± 0.3 |
| OHTN | 5.6 ± 0.5 | 6.1 ± 0.6 | 3.5 ± 0.5 |
Data expressed as the mean ± SEM; N = 15 (OHTN group), 24 (healthy control group), and 24 (glaucoma group).
Table 3 summarizes changes in IOP upon awakening (5:30 AM–7:30 AM). In the habitual position, the IOP decreased for all groups upon awakening. This decrease was significantly smaller in both the OHTN and glaucoma groups compared to the healthy control group (P = 0.003 and P = 0.012, respectively). In the supine position, the IOP increased on awakening in the OHTN and glaucoma group while it decreased in the healthy control group. This IOP change in both the OHTN and glaucoma groups was significantly different from that in the healthy control group (P = 0.048 and P = 0.006, respectively).
Table 3. .
IOP Changes upon Awakening
|
|
5:30 AM |
7:30 AM |
Change |
| Habitual IOP, mm Hg | |||
| Healthy control | 22.4 ± 0.5 | 17.9 ± 0.4 | −4.6 ± 0.4* |
| Glaucoma | 22.6 ± 0.6 | 21.0 ± 0.8 | −1.7 ± 0.9 |
| OHTN | 24.2 ± 0.7 | 22.6 ± 0.7 | −1.6 ± 0.8 |
| Supine IOP, mm Hg | |||
| Healthy control | 22.4 ± 0.5 | 21.4 ± 0.5 | −1.0 ± 0.5† |
| Glaucoma | 22.7 ± 0.6 | 24.4 ± 0.7 | 1.8 ± 0.7† |
| OHTN | 24.2 ± 0.7 | 25.3 ± 0.9 | 1.1 ± 0.7 |
Data expressed as the mean ± SEM; N = 15 (OHTN group), 24 (healthy control group), and 24 (glaucoma group). *P < 0.01 and †P < 0.05; paired t-test 5:30 AM and 7:30 AM IOPs.
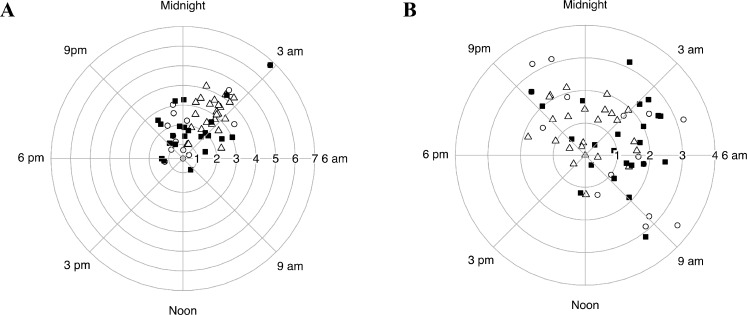
Figure 3 shows the clock hour of acrophases with amplitudes in radial scale for all three groups in the habitual (Fig. 3A) and supine (Fig. 3B) positions obtained from best-fitted cosine curve estimation of 24-hour IOP rhythm. In the habitual position, the Rayleigh test detected a 24-hour IOP rhythm in all subject groups (P < 0.001, P < 0.0001, P < 0.0001 for the OHTN, glaucoma, and healthy control groups, respectively). The acrophase occurred at 5:34 AM ± 0.9 hours in the OHTN group. This was closer to the time for the glaucoma group (5:16 AM ± 0.8 hours) than for the healthy control group (3:49 AM ± 0.2 hours). The amplitude in the OHTN group was 2.4 ± 0.5 mm Hg, which was again closer to that of the glaucoma group (2.3 ± 0.3 mm Hg) compared to healthy controls (3.0 ± 0.2 mm Hg). In the supine position, there was a 24-hour rhythm in the healthy control (P = 0.003) and glaucoma (P = 0.001) groups but not the OHTN group (P = 0.42).
Figure 3.
Estimated 24-hour rhythm of IOP in habitual (A) and supine (B) body positions. The clock time of acrophase with the corresponding amplitude on the radial scale is shown for OHTN (open circles), healthy control (open triangles), and glaucoma (solid squares) groups.
We had the opportunity to follow the clinical evolution of these OHTN patients and found that 5 of the 15 patients (33%) eventually developed glaucoma per clinical criteria previously outlined.7 The mean follow-up period for this group was 5.7 ± 2.9 years, while the average time to conversion was 4.3 ± 3.8 years. Following baseline measurements of 24-hour IOP patterns, all OHTN patients were treated as deemed necessary by the attending glaucoma specialist. During the follow-up period, patients who converted to glaucoma were on average treated with a larger number of pressure-lowering eye drops and were more likely to have received laser trabeculoplasty compared to nonconverters (2.2 ± 0.4 drops and 0.8 ± 0.3 drops, respectively, P = 0.02; 60% and 10% trabeculoplasty, respectively). One patient in each group had cataract extraction during this period.
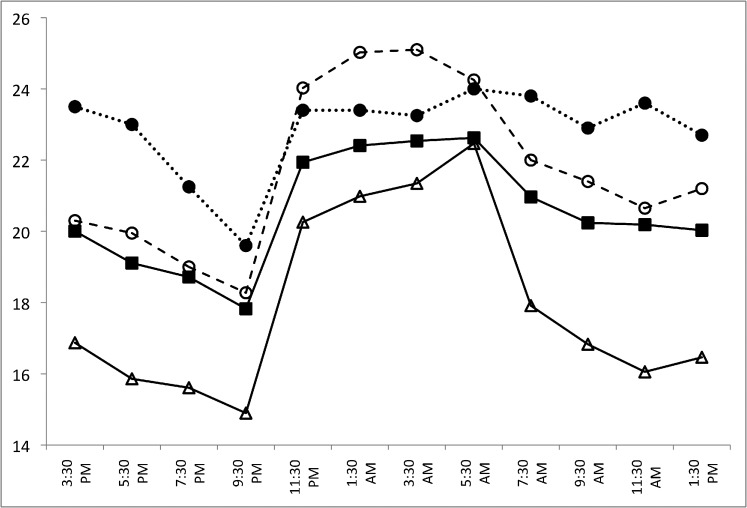
We performed a post hoc analysis of the baseline data from this small subset of patients. Figure 4 shows the 24-hour IOP patterns for the converter and nonconverter groups in the habitual position while Figure 5 shows the 24-hour IOP pattern for the supine position. All data are summarized in Table 4. In this converter group in the habitual position, the mean nocturnal IOP was significantly higher than their diurnal IOP (P = 0.02). This diurnal-to-nocturnal change in IOP was significantly smaller than in the healthy control group (P = 0.004) but not the glaucoma group. In the supine position, the nocturnal IOP decreased compared to the diurnal IOP. This decrease was again significantly different between the converter and the healthy control group (P = 0.004) but not the glaucoma group. Similarly to the glaucoma group, upon awakening, the IOP decreased in the habitual position and increased in the supine position in the converter group. These changes in IOP upon awaking were significantly different between the converter and healthy control groups only (P = 0.004, P = 0.012, habitual and supine positions, respectively). No significant differences were detected between the nonconverters and the glaucoma or healthy control groups.
Figure 4.
Twenty-four–hour IOP patterns for the OHTN patients who converted to glaucoma (converters, n = 5; dotted line, solid circles) and those who did not (nonconverters, n = 10; dashed line, open circles) in the habitual position (diurnal sitting, nocturnal supine). Healthy control (n = 24; open triangles) and glaucoma (n = 24; solid squares) groups are shown for comparison.
Figure 5.
Twenty-four–hour IOP patterns for the converter (n = 5; dotted line, solid circles) and nonconverter (n = 10; dashed line, open circles) groups in the supine position (diurnal supine, nocturnal supine). Healthy control (n = 24; open triangles) and glaucoma (n = 24; solid squares) groups are shown for comparison.
Table 4. .
IOP Parameters for the OHTN Patients Who Converted to Glaucoma (Converters) and Those Who Did Not (Nonconverters)
|
|
Converters |
Nonconverters |
| Mean IOP, mm Hg | ||
| Diurnal sitting | 22.5 ± 1.0 | 20.3 ± 0.6 |
| Nocturnal supine | 23.5 ± 1.3 | 24.6 ± 1.2 |
| Diurnal supine | 26 ± 1.0 | 23.7 ± 0.8 |
| IOP change on awakening | ||
| Habitual, mm Hg | ||
| 5:30 AM | 24 ± 1.2 | 24.3 ± 0.8 |
| 7:30 AM | 23.8 ± 1.3 | 22 ± 0.7 |
| Supine, mm Hg | ||
| 5:30 AM | 24 ± 1.2 | 24.3 ± 0.8 |
| 7:30 AM | 27 ± 1.6 | 24.4 ± 1.0 |
| Habitual IOP, mm Hg | ||
| Peak | 24 (5:30 AM) | 25.1 (3:30 AM) |
| Trough | 19.6 (9:30 PM) | 18.3 (9:30 PM) |
| Supine IOP, mm Hg | ||
| Peak | 28 (3:30 PM) | 25.1 (3:30 AM) |
| Trough | 22.8 (9:30 PM) | 22 (9:30 PM) |
| IOP variation, mm Hg | ||
| Diurnal sitting | 6.1 ± 1.1 | 5.3 ± 0.6 |
| Diurnal supine | 7.4 ± 1.4 | 5.5 ± 0.5 |
| Nocturnal supine | 2.5 ± 0.5 | 4.0 ± 0.7 |
Data expressed as the mean ± SEM; N = 5 (converter group) and 10 (nonconverter group).
In the habitual position, the Rayleigh test detected a 24-hour IOP rhythm for the nonconverter (P < 0.001) but not the converter group. The acrophase occurred at 7:29 AM ± 1.7 hours and at 4:37 AM ± 0.92 hours in the converter and nonconverter groups, respectively. The amplitude was 1.34 ± 0.24 mm Hg in the converter group and 2.88 ± 0.63 in the nonconverter group. The acrophase and amplitude were significantly different between the converter and healthy control (P < 0.001 and P < 0.001, respectively) groups only. In the supine position, the Rayleigh test did not detect a 24-hour IOP rhythm in either the converter or nonconverter group.
Discussion
To our knowledge, this is the first study reporting the 24-hour IOP pattern in patients with OHTN. In the habitual and supine positions, the general IOP pattern for the OHTN group closely resembled the pattern previously described for both healthy subjects and glaucoma patients.7 Similar to the current data, the 24-hour IOP rhythm that describes an early morning peak decreasing over the day to an evening trough has also been described previously by De Vivero et al.14 and, more recently, by Lee et al.15 in patients with normal-tension glaucoma. Our habitual diurnal data agree well with those reported by David et al.5 showing that the diurnal IOP levels gradually declined throughout the day from 7:45 AM to 7 PM.
Even though the IOP rhythm in the OHTN group is similar to that of the healthy control and glaucoma groups, the peak IOP occurred at different times compared to these groups, suggesting a phase shift in the OHTN IOP curve. In the habitual position, the IOP peak in OHTN patients occurred in the nocturnal period; in the supine position, the peak IOP occurred mid-afternoon in this group. This contrasts considerably with what is observed in glaucoma patients and healthy control subjects. The mechanism for this remains unknown and warrants further investigation. In both the habitual and supine positions, however, the trough IOP occurred at 9:30 PM, which is in agreement with data previously reported for healthy control and glaucoma groups.11
The current results suggest that the general 24-hour IOP pattern in patients with OHTN is similar to that of patients with POAG. The OHTN and glaucoma groups both demonstrated higher diurnal mean IOPs as well as greater diurnal IOP variation (maximum − minimum) compared to healthy controls. In addition, the variation in 24-hour IOP (amplitude of fitted cosine curve) in the habitual position was lower in both the OHTN and glaucoma groups compared to healthy controls. The OHTN and glaucoma groups also demonstrated similar phase delays in 24-hour IOP rhythms for the habitual position. This suggests a similar endogenous mechanism in OHTN and glaucoma.
In the healthy control group, we observed a greater nocturnal increase in mean IOP and a greater decrease in the IOP upon awakening (between 5:30 and 7:30 AM) in the habitual position compared to the supine position. Together, this suggests the effect of posture on IOP as described by Hara et al.16 In the habitual position, this diurnal-to-nocturnal change in mean IOP as well as the IOP decrease upon awakening was smaller in the OHTN and glaucoma groups. Possible explanations for these findings could be yet uncharacterized differences in postural regulation of IOP or simply higher daytime and nighttime IOPs in these groups. Accordingly, Liu et al. and Fan et al. have reported daytime versus nighttime differences in aqueous humor dynamics between OHTN and normotensive subjects,17,18 with significant reductions in nighttime outflow facility in normotensive but not OHTN subjects. This, in addition to the already low daytime outflow facility in OHTN patients,19 may partially explain the above findings. When we excluded the effect of posture, we found that the mean IOP levels changed in opposing directions: increasing in healthy controls and decreasing in the OHTN and glaucoma groups. Similarly, at awakening, we observed a decrease in the healthy control group but an increase in IOP in the OHTN and glaucoma groups. This suggests that regulation of IOP may be different in the OHTN and glaucoma groups compared to healthy controls. As previously suggested by Liu et al.,7 these observations may reflect various endogenous mechanisms yet to be identified that may be responsible for the IOP pattern in ocular hypertensives. One possibility is that early dysfunction in the trabecular meshwork in patients with OHTN,19 and the resultant reduction in pressure-sensitive aqueous outflow in addition to the increased diurnal aqueous production,20 may partially explain our findings.
In this study, we also examined serial testing data that we had collected over several years to follow the progression of our OHTN patients. The patients were divided into two subgroups: a population that did not progress to glaucoma (nonconverters, 10/15) and a population that did progress to glaucoma (converters, 5/15). Such data may address whether the 24-hour IOP pattern in the OHTN group is of use in providing additional insight into risk stratification of patients with OHTN. Our analysis revealed that the converter group demonstrated an IOP pattern very similar to that of the glaucoma group compared to the healthy controls. The converter and glaucoma groups demonstrated similar patterns of diurnal-to-nocturnal changes in IOP and IOP changes upon awaking in the habitual and supine positions.
These findings must be interpreted in view of several limitations of the study. First, we detected no differences between the nonconverters and either the glaucoma patients or healthy control subjects. This inability to differentiate the nonconverters from other groups is likely due to the small number of patients and variability of the data in this heterogeneous population. As demonstrated in the Ocular Hypertensive Treatment Study, a minority of OHTN patients will develop glaucoma; the percentage of these patients increases over time.3 This suggests that our nonconverter group may have contained both healthy individuals and individuals who may yet progress to glaucoma. Second, the presence of differences in CCT among the groups may have influenced our results. CCT is known to correlate positively with IOP and has been suggested as a risk factor in glaucoma development.12 Weighing against this possibility, however, is the fact that we found no correlation between mean IOP measurements and CCT in the OHTN group, and the mean CCT did not differ between the converter and nonconverter subgroups (P > 0.8).
Before more general recommendations can be made regarding the utility of the OHTN pattern in predicting progression, further study with a larger sample population is required. This might allow the differentiation of converter from nonconverter OHTN patients before they develop glaucoma for appropriate risk stratification and management.
Footnotes
Supported in part by NIH Grant EY07544.
Disclosure: T.M. Grippo, None; J.H.K. Liu, None; N. Zebardast, None; T.B. Arnold, None; G.H. Moore, None; R.N. Weinreb, None
References
- 1. Mansouri K, Weinreb R. Continuous 24-hour intraocular pressure monitoring for glaucoma--time for a paradigm change. Swiss Med Wkly. 2012; 142: w13545 [DOI] [PubMed] [Google Scholar]
- 2. Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011; 152: 340–344, e342 [DOI] [PubMed] [Google Scholar]
- 3. Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol. 2010; 128: 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006; 124: 793–797 [DOI] [PubMed] [Google Scholar]
- 5. David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992; 76: 280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003; 12: 232–236 [DOI] [PubMed] [Google Scholar]
- 7. Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003; 44: 1586–1590 [DOI] [PubMed] [Google Scholar]
- 8. Nakakura S, Nomura Y, Ataka S, Shiraki K. Relation between office intraocular pressure and 24-hour intraocular pressure in patients with primary open-angle glaucoma treated with a combination of topical antiglaucoma eye drops. J Glaucoma. 2007; 16: 201–204 [DOI] [PubMed] [Google Scholar]
- 9. Sit AJ. Continuous monitoring of intraocular pressure: rationale and progress toward a clinical device. J Glaucoma. 2009; 18: 272–279 [DOI] [PubMed] [Google Scholar]
- 10. Tajunisah I, Reddy SC, Fathilah J. Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 1851–1857 [DOI] [PubMed] [Google Scholar]
- 11. Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol. 2005; 139: 320–324 [DOI] [PubMed] [Google Scholar]
- 12. Mosaed S, Chamberlain WD, Liu JH, Medeiros FA, Weinreb RN. Association of central corneal thickness and 24-hour intraocular pressure fluctuation. J Glaucoma. 2008; 17: 85–88 [DOI] [PubMed] [Google Scholar]
- 13. Buguet A, Py P, Romanet JP. 24-hour (nyctohemeral) and sleep-related variations of intraocular pressure in healthy white individuals. Am J Ophthalmol. 1994; 117: 342–347 [DOI] [PubMed] [Google Scholar]
- 14. De Vivero C, O'Brien C, Lanigan L, Hitchings R. Diurnal intraocular pressure variation in low-tension glaucoma. Eye (Lond). 1994; 8 (pt 5): 521–523 [DOI] [PubMed] [Google Scholar]
- 15. Lee YR, Kook MS, Joe SG, et al. Circadian (24-hour) pattern of intraocular pressure and visual field damage in eyes with normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 881–887 [DOI] [PubMed] [Google Scholar]
- 16. Hara T, Hara T, Tsuru T. Increase of peak intraocular pressure during sleep in reproduced diurnal changes by posture. Arch Ophthalmol. 2006; 124: 165–168 [DOI] [PubMed] [Google Scholar]
- 17. Liu H, Fan S, Gulati V, et al. Aqueous humor dynamics during the day and night in healthy mature volunteers. Arch Ophthalmol. 2011; 129: 269–275 [DOI] [PubMed] [Google Scholar]
- 18. Fan S, Hejkal JJ, Gulati V, Galata S, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in volunteers with ocular hypertension. Arch Ophthalmol. 2011; 129: 1162–1166 [DOI] [PubMed] [Google Scholar]
- 19. Toris CB, Koepsell SA, Yablonski ME, Camras CB. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002; 11: 253–258 [DOI] [PubMed] [Google Scholar]
- 20. Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci. 1991; 32: 3145–3166 [PubMed] [Google Scholar]