Abstract
Purpose.
To evaluate the safety and efficacy of topical pazopanib in the treatment of corneal neovascularization (CNV).
Methods.
Twenty eyes of 20 patients with stable CNV were enrolled in a prospective, open label, noncomparative study and treated with topical pazopanib 0.5% for 3 weeks, and followed for 12 weeks. The primary endpoint was to determine the tolerability and safety of topical pazopanib in the treatment of CNV defined by the occurrence of ocular and systemic adverse events during the study. The secondary endpoint was to evaluate the effect of topical pazopanib on the reduction of (1) neovascular area (NA), defined as the area of the corneal vessels themselves, (2) invasion area (IA), defined as the fraction of the total cornea into which the vessels extend, (3) vessel length (VL), defined as the mean measurement of the extent of vessels from end to end, and (4) vessel caliber (VC), defined as the mean diameter of the corneal vessels.
Results.
There were no severe adverse events following the use of topical pazopanib. Compared with the baseline visit, NA and VL showed a statistically significant decrease at week 3 (P = 0.02 and 0.01, respectively); and NA, IA, and VL statistically significantly decreased at week 12 (P = 0.03, 0.04, and <0.01, respectively). Visual acuity maintained without changes after the 12 week follow-up.
Conclusions.
This preliminary study suggests that topical treatment with pazopanib 0.5% is safe, well tolerated, and may have a role as an alternative for the treatment of CNV (ClinicalTrials.gov number, NCT01257750).
Treatment with topical pazopanib 0.5%, a combined inhibitor of VEGF receptor and PDGF receptor, was safe and significantly reduced corneal neovascularization. Topical pazopanib may be an alternative to treat corneal neovascularization.
Introduction
Corneal neovascularization (CNV) as a result of vessel invasion from the limbal arcade to the normally avascular cornea is caused by a wide variety of common pathologic conditions such as infection, trauma, and loss of the limbal stem cell barrier.1,2 CNV plays an important role in induction of allo-immunity3 and leads to the loss of the ocular immune privilege; one of the strongest risk factors worsening the prognosis of penetrating keratoplasty.4–6 Moreover, postoperative ingrowth of neovessels into the avascular recipient bed is a strong trigger to prompt graft rejection.7
Neovascularization is mediated by a diverse array of cellular and molecular factors.8–10 Members of VEGF-A, B, C, and D are key mediators in the development of neovessels.11 In the cornea, the main sources of VEGF are epithelial cells, vascular endothelial cells, macrophages, and fibroblasts.12 VEGF expression is significantly upregulated in the inflamed and vascularized corneas. VEGF-triggered effects mediated via tyrosine kinase receptors (VEGFR1, 2, 3) increase growth, migration, and survival of the endothelial cells.13,14 VEGF blockade inhibits CNV and promotes corneal graft survival.15–17 Platelet-derived growth factor (PDGF), a dimeric glycoprotein, is also an important mediator in neovascularization (NV). PDGF, via tyrosine kinase PDGF receptors (α and β), stimulates VEGF transcription and also plays an important function in pericyte recruitment to neovessels. Sprouting endothelial cells secrete PDGF, and pericytes express PDGFR-β; thus, inhibition of the PDGF signaling pathway disrupts pericyte recruitment. This is important, since endothelial cells without pericyte support and VEGF signaling undergo apoptosis.17–24
The critical role of non-VEGF factors in suppressing NV has indirectly been supported by previous studies, which have shown that anti-VEGF therapy alone is not very effective in regression of mature vessels that have developed as a result of pathologic angiogenesis.25,26
These observations have led to the hypothesis that the combination of VEGF and PDGF signaling inhibition may be maximally effective in promoting vessel regression in pathologic ocular NV.27
Pazopanib (GlaxoSmithKline, King of Prussia, PA), is a small multi-tyrosine kinase inhibitor (TKI) that targets VEGF and PDGF receptors and inhibits angiogenesis.28,29 Pazopanib was first approved by the Food and Drug Administration (FDA) for the treatment of advanced renal cell carcinoma and more recently for treatment of soft tissue sarcoma.30,31 Preclinical data indicate that pazopanib may have potential ocular applications. Inhibition of laser-induced choroidal NV has been observed following oral administration of pazopanib, consistent with inhibition of the VEGF and PDGF pathways.32 Formulated as an eye drop, pazopanib has been shown to inhibit laser-induced choroidal NV,33 diabetic retinal vascular leukostasis, and leakage in experimental models,34 and is under clinical investigation for the treatment of neovascular AMD.
In this study, we evaluated the clinical safety and efficacy of topical pazopanib for the treatment of established CNV. To our knowledge, this is the first study reporting the effects of topical pazopanib on CNV.
Methods
Design
Prospective, open label, noncomparative, phase I/II study.
Setting
This study was approved by the institutional review board and conducted at the Cornea Service of the Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, and adhered to the tenets of the Declaration of Helsinki.
Patient Selection
Twenty eyes of 20 with CNV were included in the study. Only patients with clinically stable CNV that extended at least 1-mm beyond the limbus were considered for enrollment. If both eyes were eligible for the study, the most affected eye was selected. Clinically stable CNV was established by excluding patients with: (1) ocular infection in the previous month, (2) ocular surgery on the studied eye within the previous 3 months, (3) contact lens use within the past 2 weeks, (4) persistent corneal epithelial defect (>1 mm) within the past 3 months that lasted 14 days or longer, and (5) full thickness or lamellar keratoplasty within the past 3 months. Additional exclusion criteria are listed in Table 1. All patients provided written informed consent before the start of the study.
Table 1. .
Exclusion Criteria
| • Uncontrolled hypertension* (systolic blood pressure of ≥150 mm Hg or diastolic blood pressure of ≥90 mm Hg). |
| • History of clotting disorder* (including predisposition to hypercoagulation or any previous thromboembolic event). |
| • Uncontrolled diabetes mellitus* (hemoglobin A1c >7%). |
| • Renal, liver, and coagulation abnormalities including current anticoagulation medications other than aspirin. |
| • Current or recent (≤1 month) systemic corticosteroid therapy or periocular corticosteroid injections to the study eye. |
| • Recent (2 week) change in dose and frequency of topical steroids, nonsteroidal anti-inflammatory agents, or received intravitreal or periocular steroids within 4 weeks prior to study entry. |
| • Ocular or periocular malignancy. |
| • Women 45 years of age or younger that are of child bearing potential. |
| • Full thickness or lamellar keratoplasty within 90 days prior to study entry. |
| • Recent (≤3 months) or planned surgery. |
| • Persistent epithelial defect (>1 mm and ≥14 days duration) within 2 weeks prior to study entry. |
| • Corneal or ocular surface infection within 30 days prior to study entry. |
| • Received any other investigational therapy or treatment with anti-VEGF agents (intraocular or systemic) within 60 days prior to study entry. |
| • Soft contact lens (excluding bandage contact lens) use within 2 weeks prior to study entry. |
| • Any condition (including language barrier) that precludes patient's ability to comply with study requirements including completion of study. |
To minimize the risk of potential adverse events.
Treatment Protocol
All patients were provided with pazopanib 0.5% solution (GlaxoSmithKline) and were instructed to instill one drop four times per day in the study eye for 3 weeks. Patients were instructed to continue with their usual ophthalmic medication regimens. The superior and inferior puncta of the study eye were plugged for the duration of the treatment to minimize systemic drug absorption.
Follow-up Protocol
Study visits were scheduled at baseline and weeks 1, 3, 7, and 12. A detailed review of the medical history and a complete ocular examination that included Snellen visual acuity measurement, and slit-lamp biomicroscopy were performed during each visit. Digital corneal photography (IM-900; Haag-Streit, Koeniz, Switzerland) was performed at baseline and weeks 3, 7, and 12. Systolic and diastolic blood pressures were recorded at every visit. Blood samples were taken during screening and week 3 to monitor liver function, complete cell blood counts, and thyroid stimulating hormone.
Outcome Measures
Safety.
Drug safety was evaluated by closely monitoring the occurrence of ocular and systemic adverse events throughout the study period. Ocular adverse events were assessed through complete ocular examinations that included measurement of visual acuity, IOP, and pachymetry. Systemic adverse events were evaluated with patient questioning, vital signs monitoring (blood pressure and heart rate), and laboratory testing: complete blood count, thyroid-stimulating hormone, and liver function tests (AST, ALT) on the scheduled visits.
Efficacy.
The primary efficacy outcome measures were the size and extent of CNV. The efficacy of topical pazopanib treatment on CNV was evaluated by comparing corneal photographs acquired at baseline with photographs acquired on the follow-up visits. Secondary efficacy outcome measures of pazopanib were central corneal thickness and best-corrected visual acuity. Size and extent of CNV was investigated by four primary metrics: (1) neovascular area (NA), the area of the corneal vessels when they are projected into the plane of a photograph, (2) invasion area (IA), the fraction of the total corneal area into which the vessels extend, (3) vessel length (VL), the total length of the vessels projected into the cornea, and (4) vessel caliber (VC), the mean diameter of the corneal vessels.
Our approach to NV quantification was detailed previously,35 and only slightly modified. Briefly, using graphics editing software Photoshop CS2 (Adobe Systems Inc., Berkeley, CA) and a plug-in written in Java for ImageJ 10.2 (National Institutes of Health, Bethesda, MD), we delimited the corneal area and enhanced blood vessel visibility using color filters, then manually traced and pixelated them for quantification. Invasion area was assessed connecting the end of all vascular sprouts in order to define the contour of the corneal area taken by neovessels. The measured invasion area was expressed as a ratio of the total corneal area. Total vessel length and mean caliber were calculated using the skeletonization function in Amira 5 (Visage Imaging, San Diego, CA). This function automatically extracts the pixel-skeletons of the vessels preserving the extent and connectivity of the vascular structures, then calculating the distance mapping of the vessels (total length) and average vessel thickness (vessel caliber).
Statistical Analyses.
To assess changes in the parameters measured we considered all time points: (1) the initial (screening) visit, (2) the 3 week visit (end of treatment), (3) the 7 week visit, and (4) the last follow-up visit (12 week). Double-tailed Wilcoxon matched-pairs signed rank test or paired t-tests were used to compare the baseline values with each time point. The hypothesis for safety was that topical pazopanib does not induce significant changes on baseline parameters. The hypothesis for the efficacy metrics was that topical pazopanib reduces the scores of a given metric from the baseline time point to the subsequent time points in a statistically significant (defined as P < 0.05) manner.
Results
Twenty eyes of 20 patients with stable CNV were included in the study. Patients (14 males and 6 females) had a mean age of 54.2 ± 4.3 years at baseline. Table 2 shows the demographic characteristics of the studied cohort. For safety (primary) outcome measures, 20 patients were included in the analysis. For efficacy (secondary) outcome measures, photographs from only 18 patients were included in the statistical analysis, since two patients experienced unexpected clearing of corneal exudates that unveiled central CNV that had not been visible below the previous exudates in the baseline photographs. Values are presented as mean ± SE.
Table 2. .
Study Population Demographics
|
Patient |
Sex |
Age, y |
Diagnosis |
Additional Medications |
| HSV/HZO Keratitis | ||||
| 1 | M | 66 | HZO keratitis | 1, 7 |
| 2 | F | 80 | HZO keratitis | 2, 3 |
| 3 | F | 72 | HSV keratitis | 1, 4 |
| 4 | M | 35 | HSV keratitis, corneal ulcer | 1, 2, 7 |
| 5 | M | 24 | HSV keratitis | 1, 2, 3, 7 |
| 6 | F | 71 | HSV keratitis | None |
| 7 | F | 74 | HZO keratitis, bacterial ulcer | 1, 2, 5 |
| Keratoplasty/Failed Graft | ||||
| 8 | M | 59 | Aphakic bullous keratopathy, PKP | 2, 5 |
| 9 | F | 66 | Traumatic cataract, PBK | 1, 2, 3, 5, 7 |
| 10 | M | 41 | Fungal keratitis, PKP | 3, 6 |
| 11 | M | 41 | Microbial ulcer, PKP | 3, 7 |
| 12 | M | 38 | Microphthalmia, sclero-cornea | 5 |
| 13 | F | 56 | Corneal perforation, keratoconus | 1 |
| Limbal Stem Cell Deficiency | ||||
| 14 | M | 79 | Limbal stem cell deficiency | 1, 2, 5, 6 |
| Pterygium | ||||
| 15 | M | 77 | MGD, rosacea, DES, recurrent pterygium | 1 |
| Trauma | ||||
| 16 | M | 44 | Trauma, corneal scarring | 1 |
| 17 | M | 68 | Traumatic corneal perforation | 5 |
| 18 | M | 35 | SLK | 1 |
| 19 | M | 29 | Stevens-Johnson syndrome | 1, 3 |
| 20 | M | 27 | Rosacea, chronic blepharitis | 2 |
HZO, herpes zoster ophthalmicus; HSV, herpes simplex virus; PKP, penetrating keratoplasty; PBK, Pseudophakic bullous keratopathy; MGD, meibomian gland dysfunction; DES, dry eye syndrome; SLK, superior limbic keratoconjunctivitis; 1, Artificial tears; 2, Topical steroid; 3, Topical antibiotic; 4, Oral antiviral; 5, Topical antiglaucoma; 6, Topical cyclosporine; 7, Oral antibiotics.
Primary Outcomes
Safety.
No unexpected ocular or systemic adverse events were reported during the treatment or follow-up periods, including eye examinations, vital signs, or patients' reports. All laboratory parameters remained unchanged from baseline to the 3 week visit. Mean arterial pressure (MAP; [(2 × diastolic blood pressure) + systolic blood pressure] ÷ 3) remained unchanged at all follow-up visits when compared with baseline. MAP was 92.0 ± 1.7 mm Hg at baseline, 92.27 ± 2.0 mm Hg at week 1 (P = 0.91), 90.7 ± 1.4 mm Hg at week 3 (P = 0.44), 92.2 ± 1.8 mm Hg (P = 0.92) at week 7, and 93.3 ± 2.0 (P = 0.56) at week 12. Other safety parameters, including heart rate, pachymetry, and IOP, did not experience significant changes (Table 3).
Table 3. .
Safety Parameters before and at the Time of Treatment Discontinuation
|
|
Baseline, mean ± SD |
End of Treatment, mean ± SD |
P
Value of Change |
| Systemic | |||
| Mean arterial pressure, mm Hg | 92.0 ± 1.7 | 90.7 ± 1.4 | 0.44 |
| Heart rate, bpm | 70.4 ± 8.1 | 68.7 ± 7.6 | 0.46 |
| Ocular | |||
| IOP, mm Hg | 15.7 ± 5.9 | 16.9 ± 5.3 | 0.23 |
| Pachymetry, microns | 572 ± 135 | 590 ± 162 | 0.89 |
| Visual acuity, decimal | 0.55 | 0.65 | 0.74 |
The most commonly reported adverse event was unilateral tearing secondary to punctal occlusion in 10 (50%) patients. Adverse events directly related to drug instillation included transitory stinging in four patients (22%), dryness sensation in one patient (6%), and blurred vision after drug instillation in one patient (6%). None of the patients reported these events in more than two visits.
Visual Acuity.
To analyze the effect of pazopanib on visual acuity, the Snellen visual acuity values were converted to their LogMAR equivalents. At the baseline visit the mean corrected LogMAR visual acuity was 1.03 ± 0.25, 1.22 ± 0.29 at week 3, 1.14 ± 0.27 at week 7, and 1.08 ± 0.23 at week 12. Compared with the baseline visit, there were no statistically significant changes at any of studied time points (P = 0.74, 0.73, and 0.19, respectively).
Secondary Outcomes
Efficacy.
Neovascular Area.
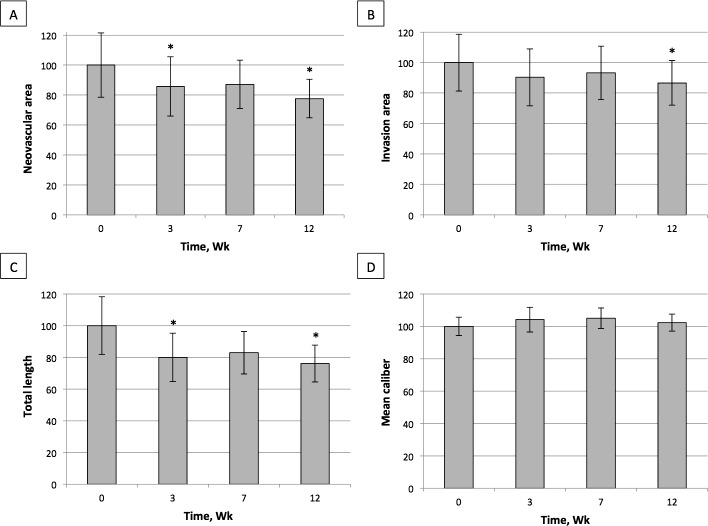
At week 3 (end of treatment) there was a statistically significant reduction in the NA of 14.3% (1.0 ± 0.5 mm2; P = 0.02) compared with baseline. At week 7 a reduction of 12.9% (0.9 ± 0.7 mm2; P = 0.32) was noted. By week 12 (end of the follow-up period) there was a statistically significant reduction of 22.4% (1.5 ± 0.8 mm2; P = 0.03; Fig. 1A). Overall, 67% of patients experienced some degree of response to therapy, with a mean decrease of 37.5% of the NA in this subgroup.
Figure 1. .
Percentage of change in neovascular area (A), invasion area (B), total vessel length (C), and average vessel caliber (D) in response to topical pazopanib at the studied time points. At the end of follow-up period the mean reduction was 22.4% (mean ± SE [1.5 ± 0.8 mm2; P = 0.03]) for neovascular area, 13.3% (3.9 ± 1.8 mm2; P = 0.04) for invasion area, 23.9% (38.0 ± 17.2 mm; P < 0.01) for vessel length, and 2.3% (0.0003 ± 0.0005 mm; P = 0.62) for vessel caliber. Reduction of neovascularization was statistically significant for all metrics except for vessel caliber. Wk, weeks.
Invasion Area.
When compared with baseline, IA contracted 9.7% (2.8 ± 1.7 mm2; P = 0.09) and 6.7% (2.0 ± 1.4 mm2; P = 0.18) at weeks 3 and 7, respectively. By week 12, a statistically significant reduction of 13.3% (3.9 ± 1.8 mm2; P = 0.04) was achieved (Fig. 1B). In this metric 72% of the patients responded to treatment, with a mean contraction of IA of 23.1%.
Vessel Length.
At the 3 week visit there was a reduction of 20.0% in VL (32.9 ± 13.8 mm; P = 0.01) as compared with baseline. At week 7 VL reduced by 17.0% (27.1 ± 16.5 mm; P = 0.11) and, by week 12 a statistically significant reduction of 23.9% (38.0 ± 17.2 mm; P < 0.01) was achieved (Fig. 1C). At the end of the follow-up period 72% of the patients showed some degree of response to therapy, with a mean VL decrease of 35.7%.
Vessel Caliber.
Vessel caliber did not experience significant changes at any of the time points studied: 4.1% at week 3 (0.0005 ± 0.0004 mm; P = 0.32), 5.0% (0.0006 ± 0.0004 mm; P = 0.26) at week 7, and 2.3% (0.0003 ± 0.0005 mm; P = 0.62) by week 12 (Fig. 1D). At the end of the follow-up period 33% of the patients showed some reduction of VC, with a mean decrease of 15.0%.
Conclusions
VEGF and PDGF play critical roles in corneal neovascularization. These molecules activate homologous receptors to VEGFRs and PDGFRs.11–14,17–22 Blockade of VEGF signaling suppresses vascular endothelial cell migration, proliferation, and survival.36 Pazopanib, a multi-targeted receptor kinase inhibitor, blocks phosphorylation signaling induced by ligand binding to these receptors.27 The present study shows that topical pazopanib is safe and well tolerated, and can effectively reduce established CNV as measured by invasion area, vessel length, and neovascular area.29
Treatment with topical pazopanib led to a 22% decrease of corneal neovessels (NA) and NA is a function of vessel caliber and length. In this study reduction in NA and VL were equivalent; however, reduction in VC was not equivalent to reduction in either NA or VL. Our results show that VC changed minimally after treatment and remained stable throughout the study. Based on this, we could postulate that treatment with topical pazopanib is more effective at reducing vessel length than vessel caliber, as opposed to treatment with topical bevacizumab as reported by us.35
VEGFR blockade disrupts endothelial cell migration and proliferation.36 In CNV, pericyte recruitment lags days to weeks behind establishment of new vessels.37 PDGF receptor-signaling blockade impairs pericyte recruitment and results in pericyte detachment from new coated vessels.38 Pericyte detachment makes neovessels more susceptible to downregulation of angiogenic responses (VEGFR blockade by pazopanib) and leads to regression of “naked” vessels. Therefore, the early (week 3) reduction in NA, IA, and VL seen with pazopanib treatment may be due to the effect of VEGF-R blockade and subsequent regression of new immature and naked vessels. The delayed response (week 12) may be the result of impaired proliferation and pericyte detachment in stable vessels.
Measurement of vessel leakage is a subjective parameter, and is mostly evaluated clinically. In this study, two patients showed clinically significant reduction in corneal exudates throughout the study. This finding is similar to those reported by Thakur et al.34 in the retina after treatment with pazopanib. We excluded these 2 patients from the statistical analysis since we considered them to be clinically improved, although new vessels were revealed (which were obscured previously) in the areas with reduced exudates after the treatment.
It has been reported that patients receiving oral pazopanib are more likely to develop mild to moderate increases in blood pressure and liver function tests, transaminase or thyroid stimulating hormone levels.39 In order to minimize the risk of systemic drug absorption lacrimal puncta were occluded in all participants in our study. We did not observe severe ocular or systemic adverse events during the study. Systemic blood pressure and heart rate, parameters of clinical interest when using anti-VEGF agents, remained stable throughout the study. Topical pazopanib was well tolerated as measured by patients' symptoms, and there were no episodes of corneal epitheliopathy, thinning, or other ocular side effects.
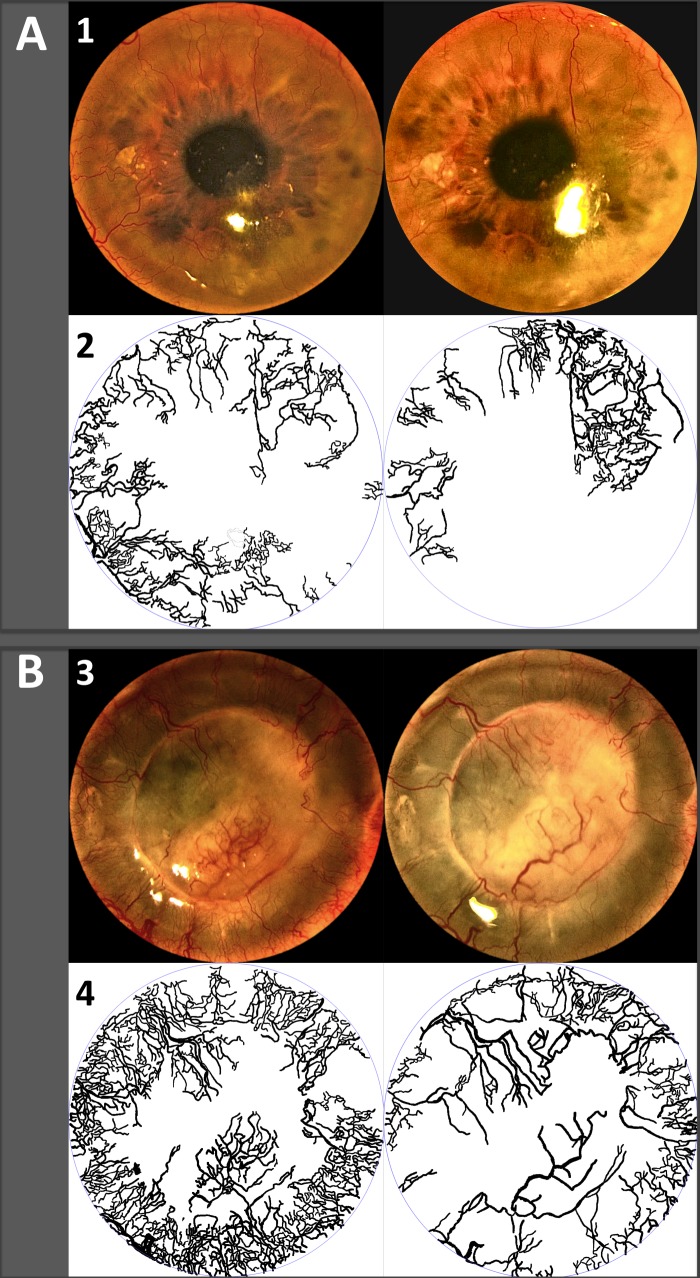
A limiting factor of this pilot study, whose main objective was to assess safety, included the lack of a control group and randomization. Another limitation was the heterogeneity of baseline CNV in our cohort. We included patients with different degrees of CNV from different causes, which may have limited our ability to identify the effect of topical pazopanib in specific clinical scenarios. This factor may also have limited the capacity of pazopanib to achieve higher average NV reduction (i.e., lack of change in very severe or chronic CNV cases). Overall, patients with newer or less severe CNV responded more favorably to treatment (Figs. 2A–B), while some severe and chronic cases experienced only a modest response (Fig. 3). Since the purpose of this study was mainly recognition of possible adverse effects of topical pazopanib, we prioritized the number of subjects over the specific characteristics of the population. A longer follow-up after treatment may have helped us to recognize further reduction in CNV, as shown by our group with the use of topical bevacizumab.35
Figure 2. .
Effect of topical pazopanib before (left) and after treatment (right). Superior panels (1, 3) show the clinical images, and the inferior panels (2, 4) depict neovessels traced from the images above. (A) A 35-year-old man with history of recurrent herpetic keratouveitis complicated with corneal neovascularization. (B) A 41-year-old man with history of firework injury and corneal transplantation complicated with fungal keratitis and extensive corneal neovascularization.
Figure 3. .
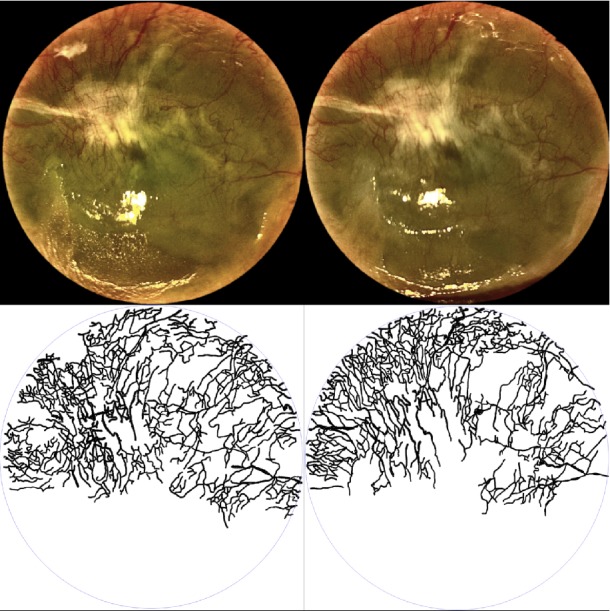
Effect of topical pazopanib before (left) and after treatment (right). Superior panel shows the clinical images and inferior panel depicts neovessels traced from the images above. A 29-year-old man with chronic Stevens-Johnson syndrome and severe dry eye complicated with corneal neovascularization showing only a modest response to treatment with topical pazopanib.
In summary, the results of this study indicate that topical treatment with pazopanib 0.5% leads to a statistically significant reduction in CNV based on decrease in NA, VL, and IA. Our results also suggest that CNV can remain stable or even continue regressing after discontinuation of the treatment. In this study, there were no reports of severe adverse effects in treatment with topical pazopanib that would preclude continued investigation. This leads us to conclude that topical pazopanib, at the dose and duration used in this study, is safe, well tolerated, and may have a role as an adjunctive approach to conventional therapy for the treatment of CNV. Additional prospective, randomized trials may be needed to further evaluate the safety and efficacy of topical pazopanib and to determine the ideal dosage and duration of therapy.
Footnotes
Supported by grants from the National Institutes of Health (K24 EY019098) and by GlaxoSmithKline Laboratories.
Disclosure: F. Amparo, None; Z. Sadrai, None; Y. Jin, None; B. Alfonso-Bartolozzi, None; H. Wang, None; H. Shikari, None; J.B. Ciolino, None; J. Chodosh, None; U. Jurkunas, None; D.A. Schaumberg, None; R. Dana, GlaxoSmithKline Laboratories (F, C)
References
- 1. Chang, Gabison E, Kato T, Azar D. Corneal neovascularization. Curr Opin Ophthamol. 2001; 12: 242–249 [DOI] [PubMed] [Google Scholar]
- 2. Epstein RJ, Stulting RD, Hendricks RL, Harris DM. Corneal neovascularization. Pathogenesis and inhibition. Cornea. 1987; 6: 250–257 [DOI] [PubMed] [Google Scholar]
- 3. Hajrasouliha AR, Funaki T, Sadrai Z, Hattori T, Chauhan SK, Dana R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2012; 53: 1244–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niederkorn J. Immune privilege and immune regulation in the eye. Adv Immunol. 1990; 48: 191–226 [DOI] [PubMed] [Google Scholar]
- 5. Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996; 37: 2485–2494 [PubMed] [Google Scholar]
- 6. Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994; 101: 1536–1547 [DOI] [PubMed] [Google Scholar]
- 7. Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008; 126: 71–77 [DOI] [PubMed] [Google Scholar]
- 8. Risau W. Mechanisms of angiogenesis. Nature. 1997; 386: 671–674 [DOI] [PubMed] [Google Scholar]
- 9. Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992; 270: 469–474 [DOI] [PubMed] [Google Scholar]
- 10. Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005; 4: 3–8 [DOI] [PubMed] [Google Scholar]
- 11. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1995; 1: 27–31 [DOI] [PubMed] [Google Scholar]
- 12. Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006; 104: 264–302 [PMC free article] [PubMed] [Google Scholar]
- 13. Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998; 39: 18–22 [PubMed] [Google Scholar]
- 14. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000; 41: 2514–2522 [PubMed] [Google Scholar]
- 15. Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004; 45: 2666–2673 [DOI] [PubMed] [Google Scholar]
- 16. Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010; 51: 2411–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRh+ perivascular progenitor cells in tumours regulates pericyte differentiation and vascular survival. Nat Cell Biol. 2005; 7: 870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finkenzeller B, Technau A, Marme D. Platelet-derived growth factor induced transcription of the vascular endothelial growth factor gene is mediated by protein kinase C. Biochem Biophys Res Commun. 1995; 208: 432–439 [DOI] [PubMed] [Google Scholar]
- 19. Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003; 112: 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003; 17: 1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jain RK, Booth MF. What brings pericytes to tumor vessels? J Clin Invest. 2003; 112: 1134–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasumi Y, Kłosowska-Wardega A, Furuhashi M, Ostman A, Heldin CH, Hellberg C. Identification of a subset of pericytes that respond to combination therapy targeting PDGF and VEGF signaling. Int J Cancer. 2007; 121: 2606–2614 [DOI] [PubMed] [Google Scholar]
- 23. Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004; 18: 338–340 [DOI] [PubMed] [Google Scholar]
- 24. Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003; 111: 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003; 162: 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helfrich I, Scheffrahn I, Bartling S, et al. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010; 207: 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jo N, Mailhos C, Ju M, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006; 168: 2036–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris PA, Boloor A, Cheung M, et al. Discovery of 5-[[4-[(2, 3-dimethyl-2Hindazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide (pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. 2008; 51: 4632–4640 [DOI] [PubMed] [Google Scholar]
- 29. Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic–pharmacodynamics correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007; 6: 2012–2021 [DOI] [PubMed] [Google Scholar]
- 30. Food and Drug Administration FDA labeling information. FDA website. [ online]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022465lbl.pdf. Accessed June 19, 2012 [Google Scholar]
- 31. van der Graaf WTA, Blay JY, Chawla SP, et al. Pazopanib in metastatic soft tissue sarcoma (PALETTE): a randomized, double-blind, placebo controlled phase 3 trial. Lancet. 2012; 379: 1879–1886 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi K, Saishin Y, Saishin Y, King AG, Levin R, Campochiaro PA. Suppression and regression of choroidal neovascularization by the multitargeted kinase inhibitor pazopanib. Arch Ophthalmol. 2009; 127: 494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yafai Y, Yang XM, Niemeyer M, et al. Anti-angiogenic effects of the receptor tyrosine kinase inhibitor, pazopanib, on choroidal neovascularization in rats. Eur J Pharmacol. 2011; 666: 12–18 [DOI] [PubMed] [Google Scholar]
- 34. Thakur A, Scheinman RI, Rao VR, Kompella UB. Pazopanib, a multitargeted tyrosine kinase inhibitor, reduces diabetic retinal vascular leukostasis and leakage. Microvasc Res. 2011; 82: 346–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009; 127: 381–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008; 105: 7738–7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cursiefen C, Hofmann-Rummelt C, Küchle M, Schlötzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003; 87: 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaoran Z, Zhirong L, Gezhi X. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves the antiangiogenic efficacy for advanced stage mouse corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 1493–1501 [DOI] [PubMed] [Google Scholar]
- 39. Nieto M, Borregaard J, Ersbøll J, et al. The European Medicines Agency review of pazopanib for the treatment of advanced renal cell carcinoma: summary of the scientific assessment of the committee for Medicinal Products for Human Use. Clin Cancer Res. 2011; 17: 6608–6614 [DOI] [PubMed] [Google Scholar]