Abstract
APS (adaptor molecule containing PH and SH2 domains) is an intracellular adaptor protein that forms an adaptor family along with Lnk and SH2-B. While experiments using cultured cell lines have demonstrated that APS is phosphorylated in response to various stimuli, its in vivo functions remain unclear. We attempted to determine the physiological roles of APS by generating APS-deficient (APS−/−) mice. APS−/− mice were viable and fertile and showed no abnormalities or growth retardation. Immunologically, APS−/− mice showed normal development and distribution of lymphocytes and myeloid cells, except for increased numbers of B-1 cells in the peritoneal cavity. APS−/− mice exhibited an enhanced humoral immune response against trinitrophenol-Ficoll, a thymus-independent type 2 antigen, while APS−/− B-2 cells exhibited normal proliferative responses and tyrosine phosphorylation of intracellular proteins upon B-cell receptor (BCR) cross-linking. APS colocalized with filamentous actin (F-actin) accumulated during the capping of BCRs in APS-transgenic B cells. After BCR stimulation, F-actin contents were lower in APS−/− B-1 cells than in wild-type B-1 cells. Our results indicate that APS might have a novel regulatory role in actin reorganization and control of B-1 cell compartment size.
B cells differentiate from progenitor cells to form a diverse repertoire reactive to almost all potential pathogens. During this differentiation, a series of developmental programs and checkpoints regulate the production of functionally mature B cells. The pre-B-cell receptor (pre-BCR) shares many signaling components with the BCR and transmits critical signals that allow the selection of precursors that express productive immunoglobulin heavy chains (29). Self-antigens presented on stromal cells trigger BCRs, which then provide signals that help determine the fate of the lymphoid precursors (13). Various growth factors and cytokines, such as stem cell factor, Flt3-L, and interleukin 7 (IL-7), assist in the regulation of lymphoid precursor expansion by binding to c-Kit, Flk2, and IL-7 receptors, respectively (3).
Developing B-cell precursor populations, as well as peripheral B-cell subpopulations, can be characterized according to their expression of BCRs and various other surface markers (9). Immature B cells generated in the bone marrow (BM) emigrate to the periphery and give rise to a heterogeneous peripheral B-cell population, consisting of recirculating cells located in spleen and lymph node follicles and nonrecirculating cells mainly localized to the splenic marginal zone (MZ). The majority of splenic B cells in the adult mouse are follicular (FO) B cells, with MZ B cells representing only 5 to 10% (26, 34). B-1 cells are another self-renewing B-cell subset, but they do not develop in the BM. B-1 cells predominate in the peritoneal and pleural cavities (21). While MZ B cells and B-1 cells produce natural antibodies and provide a first line of defense against antigens, FO B cells are involved in thymus-dependent (TD) antibody responses, from which memory and plasma cells are generated (26).
The binding of extracellular ligands to cell surface polypeptide receptors, such as antigen receptors and growth factor receptors, initiates a cascade of events through the activation of intracellular protein kinases (2, 40). The phosphorylation events catalyzed by these kinases both modulate the catalytic activity of effector enzymes and mediate the protein-protein interactions that juxtapose critical signal transduction elements. While the details of how signaling molecules are activated or recruited to receptors have yet to be completely elucidated, recent studies have defined an array of adaptor proteins that integrate and regulate multiple signaling events (22, 25, 37). Adaptor proteins lack kinase, phosphatase, and transcriptional domains and instead contain multiple binding sites, such as SH2, SH3, or PH domains, that mediate protein-protein or protein-lipid interactions. The importance of adaptor proteins has been demonstrated in various signaling pathways. For example, mice lacking the adaptor protein SLP-76 (SH2 domain-containing leukocyte protein of 76 kDa) or LAT (linker for activation of T cells) show severe defects in T-cell development due to impaired pre-T-cell receptor signaling during T lymphopoiesis (4, 38, 52). Similarly, mice lacking BLNK/SLP-65/BASH manifest severe defects in the maturation of pro-B cells to pre-B cells (11, 20, 36).
Together with Lnk and SH2-B, APS forms an adaptor protein family that shares a homologous N-terminal region with proline-rich stretches, PH and SH2 domains, and a conserved C-terminal tyrosine phosphorylation site (19, 35, 43, 50). It has previously been demonstrated that Lnk plays a critical role in the regulation of B-cell precursor and hematopoietic progenitor cell production. Mutant mice lacking Lnk show enhanced B-cell production due to the hypersensitivity of B-cell precursors to stem cell factor, a ligand for c-Kit (43). In addition, lnk−/− mice exhibit increased numbers of hematopoietic progenitors in the BM, and the ability of hematopoietic progenitors to repopulate irradiated host animals was greatly enhanced by the absence of Lnk (42). It has also been reported that SH2-B is an important signaling molecule in the insulin-like growth factor I-mediated reproductive pathway. SH2-B−/− mice displayed slight growth retardation and impaired fertility due to insufficient gonadal tissue maturation (33). A third member of the Lnk protein family was also isolated by using a homologous region conserved between Lnk and SH2-B as a probe (19). This protein was the murine homologue of APS that had already been cloned as a possible candidate substrate for the c-Kit tyrosine kinase receptor. Other studies have since shown that APS is phosphorylated upon stimulation with various growth factors, including insulin, nerve growth factor (NGF), and BCR cross-linking (19, 31, 39, 50). Nonetheless, the physiological role of APS has not been properly evaluated in vivo.
To investigate the in vivo function of APS in the immune system, we generated mutant mice that lacked APS (APS−/− mice). APS−/− mice were viable, fertile, and showed no abnormalities or growth retardation. Although lymphoid- and myeloid-cell developments were not severely affected, B-1 cell numbers in the peritoneal cavity were increased and humoral immune responses to thymus-independent type 2 (TI-2) antigen were significantly enhanced in the absence of APS. APS colocalized with filamentous actin (F-actin) during the capping of receptors of stimulated B cells overexpressing APS, which showed increased F-actin contents upon BCR cross-linking. In contrast, F-actin content was lower in stimulated APS−/− B-1 cells than in wild-type B-1 cells. These results indicated that APS might regulate the function and maintenance of B-1 cells and modulate the response against TI-2 antigen via controlling actin reorganization.
MATERIALS AND METHOD
Mice
Genomic fragments containing coding exons of the APS gene were isolated as described previously (19). The G418 resistance cartridge from pMC1neo (Stratagene, La Jolla, Calif.) was inserted between the 3.6-kb EcoRI fragment 5′ of the first coding exon and the 2.4-kb PCR-amplified fragment containing the seventh coding exon, and the herpes simplex virus tk gene was ligated to the 3′ end for selection against nonhomologous recombination. Transfection into AK7 embryonic stem (ES) cells (8) and the screening of ES clones carrying the targeted allele were performed as described previously (43). The generation of chimeric mice from APS+/− ES cells and germ line transmission of the mutant allele were confirmed by Southern blot analysis of tail DNA obtained from progeny mice. Experiments were performed with 129/Sv-C57BL/6 mixed-background mice. All mice were housed under specific-pathogen-free conditions and were analyzed between 6 and 12 weeks of age.
The generation of lymphocyte-specific APS-transgenic (APS-Tg) mice will be described elsewhere (M. Iseki and S. Takaki, unpublished data). Briefly, mouse APS cDNA (19) was inserted into the p1026x vector that contained the murine lck proximal promoter, immunoglobulin intronic heavy-chain enhancer Eμ, and the human growth hormone (GH) gene cassette (18, 44), and fragments containing the transgene were injected into mouse zygote pronuclei.
Flow cytometry
Single-cell suspensions of lymphocytes from BM, spleen, or the peritoneal cavity were prepared, stained, and analyzed as previously described (43). The following monoclonal antibodies were used: fluorescein isothiocyanate (FITC)-conjugated anti-Mac-1 (M1/70), phycoerythrin (PE)-conjugated and biotin-conjugated anti-CD3ɛ (145-2C11), PE- and biotin-anti-CD43 (S7), PE-anti-Gr-1 (RB6-8C5), FITC-anti-CD21/CD35 (7G6), PE- and biotin-anti-CD23 (B3B4), PE- and biotin-anti-CD5 (53-7.3) (all purchased from BD PharMingen, San Diego, Calif.), FITC- and PE-anti-B220 (RA3-6B2) (Caltag Laboratories, Burlingame, Calif.), biotin-anti-IL-5 receptor alpha (IL-5Rα) (T21) (15), and biotin-anti-immunoglobulin D (IgD) (CS/15; a gift from K. Miyake, University of Tokyo). FITC- and PE-conjugated F(ab′)2 polyclonal anti-IgM fragments were purchased from Caltag Laboratories, and PE- and TRI-COLOR-conjugated streptavidins (Caltag Laboratories) were used to reveal biotin-coupled antibody staining.
Proliferation assays
Splenic B cells were purified by using a magnetic cell sorting system (Miltenyi Biotec, Bergisch Gladbach, Germany) following incubation with biotin-conjugated anti-CD43 (BD PharMingen) and streptavidin-coupled microbeads (Miltenyi Biotec). B cells (2 × 105) were cultivated in 0.2 ml of RPMI 1640 medium supplemented with 8% fetal calf serum, 50 μM 2-mercaptoethanol, 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a 96-well plate. The cells were stimulated with F(ab′)2 of goat anti-mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, Pa.), IL-4 (PeproTech EC, London, United Kingdom), anti-CD40 (HM40-3; BD PharMingen), or lipopolysaccharide (LPS) (Difco Laboratories, Detroit, Mich.). The cells were pulse-labeled with [3H]thymidine (0.2 μCi per well) during the last 16 h of a 66-h culture period, and the incorporated [3H]thymidine was measured with a MATRIX 96 direct beta counter (Packard, Meriden, Conn.).
Immunization and ELISA
Concentrations of each immunoglobulin isotype in serum were determined for 6-week-old mice by isotype-specific enzyme-linked immunosorbent assay (ELISA) (46). To measure the production of the antibodies against a TI antigen, the mice were intraperitoneally injected with 10 μg of trinitrophenol (TNP)-LPS (TI-1 antigen) or 10 μg of TNP-Ficoll (TI-2 antigen) in normal saline and bled on days 7 and 14 after injection. Serial dilutions of serum were analyzed for TNP-specific immunoglobulin isotypes by ELISA with 2,4-dinitrophenol-coupled bovine serum albumin (which cross-reacts with anti-TNP antibodies) as a capture reagent. To examine the response against a TD antigen, mice were immunized intraperitoneally with 10 μg of TNP-keyhole limpet hemocyanin (KLH) in a 1:1 homogenate of incomplete Freund's adjuvant (Nacalai Tesque, Kyoto, Japan) and normal saline. A booster dose of 10 μg of TNP-KLH in normal saline was given on day 21. The mice were bled on days 7, 14, 28, and 35, and the presence of anti-TNP antibodies of each immunoglobulin subclass was determined by using a TNP-specific ELISA.
Immunoprecipitation and immunoblotting
Purified splenic B cells were stimulated with 30 μg of F(ab′)2 goat anti-mouse IgM/ml and lysed with lysis buffer (19). Cell lysates were subjected to immunoprecipitation and immunoblot analysis as previously described (19). Briefly, lysates were incubated with anti-APS-N antibodies and protein G Sepharose 4 Fast Flow (Amersham Pharmacia Biotech) at 4°C for 1 h. Then, the Sepharose beads were washed five times with 1% NP-40-phosphate-buffered saline (PBS). The precipitated proteins were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis under reducing conditions and transferred to a polyvinylidene difluoride membrane (Immobilon; Millipore, Bedford, Mass.). After blocking with 5% bovine serum albumin or low-fat dry milk-Tris-buffered saline (pH 7.6), blots were probed with anti-APS (19) and anti-phosphotyrosine monoclonal antibody (4G10; Upstate Biotechnology, Lake Placid, N.Y.).
F-actin measurement
Purified B cells or antibody-stained splenocytes or peritoneal cells were stimulated with 30 μg of F(ab′)2 goat anti-mouse IgM/ml, washed with PBS, and then fixed in 3.7% formaldehyde for 30 min on ice. Following permeabilization with 0.2% Triton X-100 in PBS for 30 min, the cells were stained with rhodamine-phalloidin (Molecular Probes, Eugene, Oreg.) for 1 h and washed three times. Fluorescence intensity was measured by flow cytometry.
Confocal microscopy
Purified B cells stimulated with F(ab′)2 goat anti-IgM antibody were deposited on microscope slides by using a Cytospin 3 centrifuge (Shandon Scientific, Cheshire, England) and fixed in 3.7% formaldehyde. The cells were permeabilized with 0.2% Triton X-100 in PBS and stained with anti-APS and FITC-conjugated anti-rabbit IgG (for APS detection) and rhodamine-phalloidin (for actin detection). After washing with PBS, coverslips were mounted and the slides were observed with a laser scanning confocal microscope (Olympus, Tokyo, Japan).
RESULT
Generation of APS−/− mice
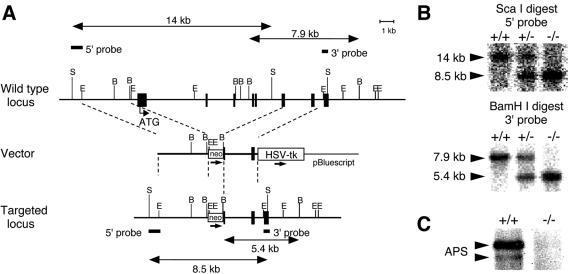
To generate APS-null mice, we deleted six exons of the APS gene, from the first methionine residue to the middle of the SH2 domain, and replaced this region with the neomycin phosphotransferase cassette (Fig. 1A). The successful disruption of the APS gene and the absence of intact or partial APS proteins were confirmed by Southern blot and immunoblot analyses (Fig. 1B and C). APS−/− mice were produced in a Mendelian ratio from heterozygous crossbreeding (+/+, 22.3%; +/−, 48.8%; and −/−, 28.9% [n = 121]) and were indistinguishable from littermates with respect to appearance, general behavior, body and organ size, and fertility.
FIG. 1.
Generation of APS-deficient mice. (A) Schematic representations of wild-type and mutant APS gene loci and the targeting vector. Exons are represented by black boxes. The neomycin resistance gene (neo) and the gene encoding herpes simplex virus thymidine kinase (HSV-tk) are indicated. The 5′ and 3′ probes used for Southern blotting are indicated. Restriction site abbreviations: B, BamHI; S, ScaI; E, EcoRI. (B) Representative Southern blot analysis. Tail genomic DNA obtained from +/+, +/−, and −/− mice was probed with 5′ or 3′ probes. (C) Lack of APS protein expression in APS−/− mice. Splenocytes obtained from +/+ or −/− mice were lysed, and APS expression was determined by immunoprecipitation followed by immunoblot analysis with anti-APS C-terminal antibodies. APS proteins were detected as doublet bands (arrowheads).
B-1 cell numbers were increased in APS−/− mice
APS is highly expressed in the spleen, lymph nodes, and BM, as well as in the brain and kidney (19). It has also been shown that APS is expressed by B cells but not by T cells in the spleen and is tyrosine phosphorylated upon BCR cross-linking in B-cell lines (19). Based on these observations, we examined lymphocyte and myeloid-cell development in APS−/− mice. As expected from the undetectable APS expression in wild-type T-lineage cells, thymocytes developed normally in APS−/− mice, and subpopulations of peripheral CD4+ and CD8+ T cells and CD122 (IL-2Rβ)+ NK T cells were not altered in the absence of APS (data not shown).
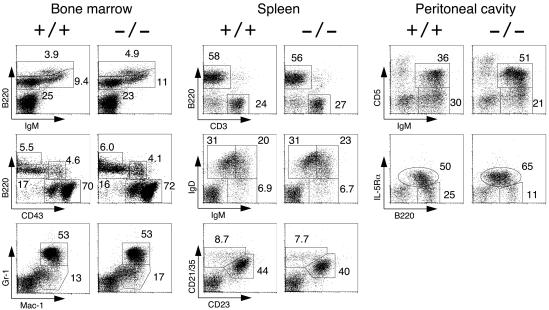
The lymphocyte and myeloid-cell numbers in APS−/− mouse BM, spleen, and lymph nodes were also normal. B-cell precursor fractions, such as pro-B cells (B220+ CD43+), pro-B and pre-B cells (B220+ IgM−), immature B cells (B220+ IgM+), recirculating mature B cells (B220high IgM+), and myeloid cells (Mac-1+ Gr-1+) from APS−/− BM were also comparable to those from wild-type BM (Fig. 2). In the spleen, the ratio of B (B220+) to T cells (CD3+) and the percentages of B-cell subpopulations, such as immature (IgMhigh IgDlow), transitional (IgMhigh IgDhigh), mature (IgMlow IgDhigh), FO (CD21/35+ CD23+), and MZ (CD21/35high CD23−) cells, were not altered by APS deficiency (Fig. 2). In addition, we observed no abnormalities in terms of splenic architecture in APS−/− mice, and B-cell follicles, MZs, and T-cell areas were all well maintained (data not shown).
FIG. 2.
Lymphocyte development in APS−/− mice. Representative two-color fluorescence plots show BM cell expression of B220 and IgM, B220 and CD43, and Gr-1 and Mac-1 (left panels); the splenocyte expression of B220 and CD3, IgD and IgM, and CD21/35 and CD23 (middle panels); and the peritoneal cavity lymphocyte expression of IgM and CD5 and of B220 and IL-5Rα (right panels). Numbers represent the percentages of cells that fall into the indicated boxes of total gated live cells. CD5+ and IL-5Rα+ B-1 cell numbers were increased in the peritoneal cavities of APS−/− mice.
As B-1 cells have characteristics that are distinct from those of BM-derived B-2 cells in terms of development, anatomic localization, and function, we next examined peritoneal cavity B-1 cells. The total number of peritoneal lavage cells was slightly increased in APS−/− mice (Table 1). As shown in Fig. 2, IgM+ CD5+ B-1a cells, as well as total B220+ IL-5Rα+ B-1 cells, were overrepresented in APS−/− mice, and the number of IgM+ CD5+ B-1a cells was nearly twofold higher in APS−/− mice than in wild-type mice, while IgM+ CD5− B-2 cell numbers were unchanged (Table 1). Thus, our results indicated that, while APS appeared not to be essential for the development of most lymphoid and myeloid cells, APS deficiency did result in a significant increase in B-1 cell numbers.
TABLE 1.
Increase in B-1 cell numbers in the peritoneal cavities of APS−/− micea
| Cells | Mean no. of B-1 cells (106) ± SEM in mice with genotype (n)b:
|
|
|---|---|---|
| +/+ (13) | −/− (17) | |
| Total | 5.1 ± 0.31 | 6.1 ± 0.52 |
| IgM+ CD5+ | 0.48 ± 0.069 | 0.90 ± 0.13c |
| IgM+ CD5− | 0.41 ± 0.040 | 0.62 ± 0.089 |
Cells obtained from the peritoneal cavity were labeled with the indicated antibodies and analyzed by flow cytometry.
Statistical analysis was carried out by using Student's t test. n, number of mice.
Significantly different from the value for +/+ mice at a P value of <0.02.
B-cell responses in the absence of APS
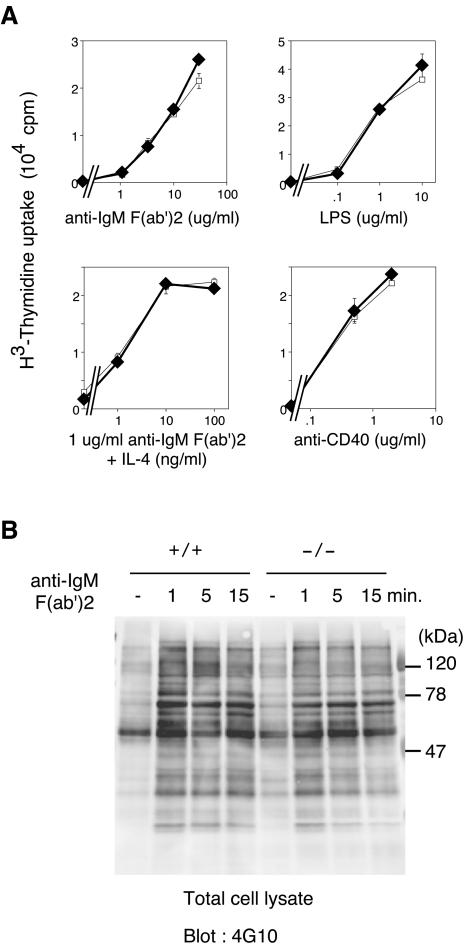
We then studied B-cell responses against various mitogenic stimuli to determine whether B-cell activation was affected by the absence of APS. Upon anti-IgM stimulation, APS−/− splenic B cells exhibited proliferation that was comparable to that of control B cells (Fig. 3A). APS−/− B cells also proliferated to the same extent as APS+/+ cells in response to LPS, anti-CD40, and IL-4 (Fig. 3A). The patterns of total cellular protein tyrosine phosphorylation and BCR-induced calcium influx were not significantly different in APS−/− splenic B cells and splenic B cells from APS+/+ littermates (Fig. 3B and data not shown). Thus, the biological and biochemical responses of splenic B cells in vitro were not significantly altered in the absence of APS.
FIG. 3.
Proliferative responses and protein tyrosine phosphorylation of cellular proteins in APS−/− B cells. (A) Proliferation of splenic B cells induced by anti-IgM F(ab′)2, LPS, anti-IgM F(ab′)2 plus IL-4, and anti-CD40. Splenic B cells from +/+ (□) or −/− (⧫) mice were treated with the indicated stimuli, and proliferation was measured on day 3 by [3H]thymidine incorporation. The values are the mean counts per minute ± standard deviations of triplicate determinations. Representative results of three independent experiments are shown. (B) Phosphorylation of intracellular proteins induced by anti-IgM F(ab′)2 stimulation of splenic B cells from +/+ or −/− mice. Purified splenic B cells were stimulated with 30 μg of anti-IgM F(ab′)2 antibody/ml for the indicated times. The total cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was probed with an anti-phosphotyrosine antibody, 4G10, and visualized by enhanced chemiluminescence.
The finding of increased B-1 cell numbers in the peritoneal cavity prompted us to examine the humoral immune responses of APS−/− mice. The immunoglobulin isotype levels in serum were all within their normal ranges, except for IgG3, which was slightly increased in APS−/− mice (Tables 2 and 3). APS−/− mice mounted normal humoral immune responses against the TD antigen TNP-KLH, and time courses of antibody production by APS−/− and APS+/+ mice after primary and secondary immunization were indistinguishable (Table 3 and data not shown). APS−/− mice also showed normal responses against the TI-1 antigen, TNP-LPS. However, APS−/− mice displayed significantly increased IgM and IgG3 isotype antibody production after immunization with the TI-2 antigen, TNP-Ficoll (Table 3).
TABLE 2.
Antibody production in APS−/− mice
| Genotype (n)a | Mean serum Ig level (μg/ml) ± SEM forb:
|
|||||
|---|---|---|---|---|---|---|
| IgM | IgG1 | IgG2a | IgG2b | IgG3 | IgA | |
| +/+ (9) | 120 ± 10 | 180 ± 67 | 90 ± 30 | 260 ± 42 | 49 ± 4.3 | 85 ± 9.5 |
| −/− (12) | 140 ± 13 | 150 ± 46 | 77 ± 17 | 200 ± 36 | 79 ± 6.9c | 84 ± 7.1 |
n, number of mice.
Concentrations of immunoglobulin subclasses in serum as determined by isotype-specific ELISA. Statistical analysis was carried out by using Student's t test.
Significantly different from the value for +/+ mice at a P value of <0.005.
TABLE 3.
Antibody responses against the TI-1, TI-2, and TD antigens
| Antigen and genotype (n)a | Mean antibody production (relative titer [102]) ± SEMb
|
||||||
|---|---|---|---|---|---|---|---|
| IgM | IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgA | |
| TI-1c | |||||||
| +/+ (8) | 11 ± 1.6 | 0.66 ± 0.15 | |||||
| −/− (7) | 16 ± 2.6 | 0.89 ± 0.21 | |||||
| TI-2d | |||||||
| +/+ (9) | 11 ± 1.1 | 4.2 ± 1.1 | |||||
| −/− (10) | 16 ± 1.7e | 9.5 ± 2.0e | |||||
| TDf | |||||||
| +/+ (3) | 4.7 ± 0.32 | 93 ± 6.8 | 16 ± 5.5 | 74 ± 22 | 9.4 ± 2.3 | 8.9 ± 2.2 | |
| −/− (5) | 3.9 ± 0.80 | 120 ± 33 | 11 ± 3.1 | 46 ± 14 | 7.9 ± 1.7 | 10 ± 2.8 | |
n, number of mice.
Statistical analysis was carried out by using Student's t test.
Mice were injected with the TI-1 antigen TNP-LPS, and the levels of hapten-specific IgM and IgG antibodies were measured at day 14 by ELISA.
Mice were injected with the TI-2 antigen TNP-Ficoll, and the levels of hapten-specific IgM and IgG3 antibodies were measured at day 14 by ELISA.
Significantly different from the value for +/+ mice at a P value of <0.05.
Mice were immunized with the TD antigen TNP-KLH, and the TNP-specific immunoglobulin subclasses were measured 7 days after the second immunization.
APS amounts affected actin assembly in activated B cells
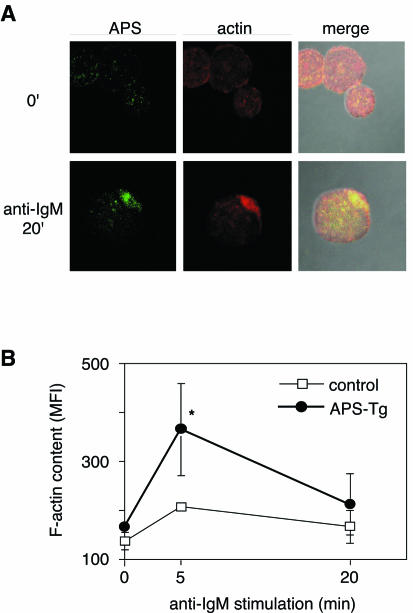
Recently, it was proposed that Lnk family proteins contribute to the regulation of the actin cytoskeleton and that SH2-B is involved in GH-induced actin reorganization (14). The actin-binding protein ABP-280 associates with Lnk (12), and Lnk overexpression in fibroblasts results in extensive actin reorganization (S.-M. Kwon and S. Takaki, unpublished data). Therefore, we examined the roles of APS in actin reorganization in primary B cells. Because of difficulty in the detection of APS by immunostaining in wild-type primary B cells, we employed B cells from lymphocyte-specific APS-Tg mice. Staining with rhodamine-phalloidin and anti-APS antibodies revealed that APS colocalized with F-actins accumulated during the capping of the BCR complex in B cells stimulated by BCR cross-linking (Fig. 4A). F-actin contents increased after BCR stimulation in primary B cells, and the increase in F-actin contents was more prominent in APS-Tg B cells than in control B cells (Fig. 4B).
FIG. 4.
APS colocalizes with actin and regulates its reorganization. (A) APS colocalizes with polymerized actin fibers. APS-Tg B cells stimulated with goat anti-IgM F(ab′)2 fragments for 20 min were fixed, permeabilized, and stained with rabbit anti-APS and FITC-conjugated anti-rabbit IgG (left column) or rhodamine-phalloidin (center column). Merged images of anti-APS staining, actin staining, and a phase-contrast image are also shown (right column). (B) Analysis of F-actin content by flow cytometry. Purified splenic B cells were stimulated with anti-IgM, fixed, permeabilized, stained with rhodamine-phalloidin, and analyzed by flow cytometry. The average F-actin content is represented as the mean fluorescence intensity (MFI) ± standard deviation of results from three independent experiments at each time point, as shown on the lower axis. Statistical analysis was carried out by using Student's t test. *, statistically significant at a P value of <0.05 compared with control.
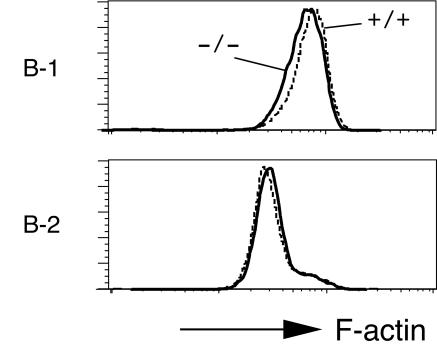
With the results observed for APS-Tg B cells in mind, we examined actin assembly induced by BCR cross-linking in APS−/− B cells. F-actin contents in peritoneal B-1 cells and splenic B-2 cells were analyzed by flow cytometry after rhodamine-phalloidin staining. Unstimulated APS−/− B cells tended to show slightly decreased F-actin contents compared to those of wild-type B cells (Table 4). After BCR cross-linking, APS−/− B-1 cells showed significantly reduced F-actin contents compared to those of wild-type B-1 cells (Fig. 5 and Table 4). The reduction in F-actin contents after BCR cross-linking was less significant in APS−/− B-2 cells. Thus, APS amounts affected actin assembly in activated B cells, and F-actin contents decreased in the absence of APS, especially in B-1 cells.
TABLE 4.
Decreased F-actin contents in APS−/− B-1 cells
| Cell type | Anti-IgM | Mean F-actin content (%) ± SEMa
|
|
|---|---|---|---|
| +/+ | −/− | ||
| B-1 | − | 100 | 92 ± 4.5 |
| + | 100 | 88 ± 2.8b | |
| B-2 | − | 100 | 97 ± 1.9 |
| + | 100 | 96 ± 2.9 | |
Antibody stained splenocytes or peritoneal cells were stimulated with anti-IgM (+) or left unstimulated (−), fixed, permeabilized, stained with rhodamine-phalloidin, and analyzed by flow cytometry. The average F-actin contents for −/− mice measured by fluorescence intensity for rhodamine-phalloidin are represented as the values relative to those for +/+ mice from four independent experiments. Statistical analysis was carried out by using Student's t test.
Significantly different from the value for +/+ cells at a P of <0.01.
FIG. 5.
Reduced F-actin contents in APS−/− B-1 cells stimulated by BCR cross-linking. Antibody-stained splenocytes or peritoneal cells were stimulated with 30 μg of F(ab′)2 goat anti-mouse IgM/ml, washed, fixed, permeabilized, stained with rhodamine-phalloidin, and analyzed by flow cytometry. The F-actin contents of anti-IgM-stimulated B-1 or B-2 cells from +/+ (dashed line) or −/− (solid line) mice are shown. Representative results of four independent experiments are shown.
DISCUSSION
APS deficiency results in increase of B-1 cells
In this study, we investigated the in vivo functions of APS, with a particular focus on the immune system, by generating APS−/− mice. Our results showed that, while the development and distribution of B-2 cells was normal, the numbers of B-1 cells in the peritoneal cavity and the humoral immune responses against a TI-2 antigen were significantly enhanced in APS−/− mice. Although various cellular responses induced by BCR stimulation were normal, assembled F-actins in activated B-1 cells were significantly reduced in the absence of APS. These results indicate that APS appears to play a role in B-1 cell development, maintenance, or activation and that APS may regulate actin reorganization and modulate the number of B-1 cells.
B-1 cells undergo positive selection and, compared to B-2 cells, require stronger BCR-mediated signaling or higher BCR expression levels for development and/or maintenance (10). A deficiency of signaling molecules that attenuate BCR signals often results in the selective reduction of B-1 cells in mice. For example, mice lacking the p85α subunits of phosphatidylinositol 3-kinase, Btk, or phospholipase C-γ2 all show severely reduced B-1 cell numbers, while B-2 cell numbers remain relatively normal (7, 23, 24, 41, 48). APS transcripts were comparably expressed in both B-1 and B-2 cells (data not shown). Our cellular and biochemical analyses of APS−/− splenic B cells failed to detect any obvious enhancement of BCR-mediated responses. However, it may be hypothesized that B-1 cells, which are more sensitive to BCR-mediated signal strength than B-2 cells, are likely to be selectively expanded in APS−/− mice due to the high level of BCR expression combined with slightly enhanced BCR-mediated signaling in the absence of APS. The reduction of actin assembly in stimulated B-1 cells might provide a certain advantage in signaling through BCRs. Alternatively, APS deficiency or the resulting reduced F-actin contents after BCR cross-linking may provide better signaling through growth factor or cytokine receptors that mediate the growth or survival responses of B-1 cells.
APS in signals mediated through growth factor receptors
It has been demonstrated that APS can be phosphorylated by various tyrosine kinase receptors, including c-Kit, the NGF receptor, the platelet-derived growth factor receptor, cytokine receptors such as IL-3R, and the GH receptor (19, 39, 50, 51). Indeed, APS was originally isolated as a potent c-Kit substrate and has been shown to interact with c-Kit in a tyrosine phosphorylation-dependent manner (50). It has been shown that Lnk, another member of the adaptor protein family, negatively regulates c-Kit signaling (32, 42, 43). However, APS−/− mice showed neither developmental abnormalities nor growth retardation. In addition, APS−/− mice manifested no anemia, and mast cells established from APS−/− BM cells displayed normal c-Kit-mediated proliferation and adhesion (C. Kubo, M. Iseki, and S. Takaki, unpublished data). Despite APS phosphorylation induced via receptors for growth factors and cytokines, we found no evidence that APS functions in c-Kit, NGF receptor, platelet-derived growth factor receptor, IL-3R, or GH receptor signaling. Thus, APS may be dispensable for signals mediated through tyrosine receptor kinases or cytokine receptors.
Independent from its identification as a possible c-Kit substrate, APS has also been isolated by virtue of its ability to associate with a cytoplasmic domain of the insulin receptor (31). APS binds to phosphotyrosines within the activation loop of the insulin receptor via the SH2 domain and undergoes phosphorylation of a tyrosine residue upon stimulation with insulin. Interestingly, APS−/− mice exhibited increased sensitivity to insulin and enhanced glucose tolerance (30). Although the mechanisms that lead to insulin hypersensitivity are not clear at present, it does appear that APS plays a negative regulatory role in insulin function and glucose metabolism. As discussed above, it cannot be ruled out at this point that an increase of peritoneal B-1 cells is a secondary effect of insulin hypersensitivity.
Possible molecular mechanisms for APS to function in B-lineage cells
Despite the observed interaction between APS and c-Cbl in transfected cell lines (1, 47, 51), APS probably exerts its function in a c-Cbl-independent manner. We found no association between APS and c-Cbl in wild-type B cells (data not shown). The time courses of phosphorylation for various cellular proteins were not affected in APS−/− B cells. Interestingly, it has been shown that Lnk does not down-regulate or affect c-Kit expression levels but negatively regulates c-Kit-mediated growth signals by attenuating Gab2 phosphorylation following activation of the mitogen-activated protein kinase pathway in a mast cell line (42). The C-terminal tyrosine residue of Lnk that corresponds to the c-Cbl binding site of APS is not required for the inhibitory function of Lnk either in the mast cell line or in B progenitors in BM (42, 44). Thus, both APS and Lnk function as negative regulators of signaling in B-lineage cells and work in a c-Cbl-independent manner, although they seem to act at different stages of lymphocyte development and in different signaling pathways. In contrast, SH2-B seems to act as a positive regulator of signal transduction. SH2-B−/− mice show mild growth retardation and infertility due to impaired gonad organ maturation (33). Despite the significant structural similarities between APS, Lnk, and SH2-B, these proteins appear to have diverse functions. However, possible overlapping functions of these adaptor proteins in vivo should be examined by generating mutant mice that lack various combinations of APS, Lnk, and SH2-B.
Vav3 has been shown to interact with APS (49) and to play a role in BCR signaling (17). Vav1 and Vav2 are expressed in B cells and regulate BCR signals (5, 45). Although we found no clear evidence for a relationship between APS and Vav family proteins, it is still possible that Vav proteins are involved in the signal transduction through APS. Vav1, the hematopoietic-specific guanine nucleotide exchange factor for Rho/Rac/Cdc42, controls the actin cytoskeleton in primary T cells (6, 16). F-actin contents were reduced in activated APS−/− B-1 cells. The involvement of microfilament assemblies in BCR signaling events that lead to mitogenesis or apoptosis has been demonstrated previously (27, 28). Although the molecular mechanisms that connect APS with actin polymerization remain to be elucidated, it is possible that APS may regulate the strength of signals by controlling the functions of Vav family proteins and actin assemblies.
In conclusion, our studies describe a novel regulatory role for APS in controlling B-1 cell compartment size in vivo. B-1 cells were increased and antibody production against a TI-2 antigen was enhanced in APS−/− mice. APS colocalized with F-actin, and actin reorganization was augmented in B cells overexpressing APS. In contrast, F-actin contents were decreased in APS−/− B cells, especially in B-1 cells. We propose that APS may modulate the strength of the signals, which control B-1 cell development or maintenance by controlling actin polymerization.
Acknowledgments
We are grateful to K. Miyake for providing antibody and A. Imamoto for providing the ES cell line. We thank our colleagues for helpful discussions, technical advice, and critical reading of the manuscript.
This work was performed with Special Coordination Funds for Promoting Science and Technology and with Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese Government.
REFERENCE
- 1.Ahmed, Z., B. J. Smith, and T. S. Pillay. 2000. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475:31-34. [DOI] [PubMed] [Google Scholar]
- 2.Alberola-Ila, J., S. Takaki, J. D. Kerner, and R. M. Perlmutter. 1997. Differential signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 15:125-154. [DOI] [PubMed] [Google Scholar]
- 3.Baird, A. M., R. M. Gerstein, and L. J. Berg. 1999. The role of cytokine receptor signaling in lymphocyte development. Curr. Opin. Immunol. 11:157-166. [DOI] [PubMed] [Google Scholar]
- 4.Clements, J. L., B. Yang, S. E. Ross-Barta, S. L. Eliason, R. F. Hrstka, R. A. Williamson, and G. A. Koretzky. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281:416-419. [DOI] [PubMed] [Google Scholar]
- 5.Doody, G. M., S. E. Bell, E. Vigorito, E. Clayton, S. McAdam, R. Tooze, C. Fernandez, I. J. Lee, and M. Turner. 2001. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2:542-547. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, K. D., Y. Y. Kong, H. Nishina, K. Tedford, L. E. Marengere, I. Kozieradzki, T. Sasaki, M. Starr, G. Chan, S. Gardener, M. P. Nghiem, D. Bouchard, M. Barbacid, A. Bernstein, and J. M. Penninger. 1998. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8:554-562. [DOI] [PubMed] [Google Scholar]
- 7.Fruman, D. A., S. B. Snapper, C. M. Yballe, L. Davidson, J. Y. Yu, F. W. Alt, and L. C. Cantley. 1999. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 283:393-397. [DOI] [PubMed] [Google Scholar]
- 8.Guris, D. L., J. Fantes, D. Tara, B. J. Druker, and A. Imamoto. 2001. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat. Genet. 27:293-298. [DOI] [PubMed] [Google Scholar]
- 9.Hardy, R. R., C. E. Carmack, S. A. Shinton, J. D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy, R. R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595-621. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi, K., R. Nittono, N. Okamoto, S. Tsuji, Y. Hara, R. Goitsuka, and D. Kitamura. 2000. The B cell-restricted adaptor BASH is required for normal development and antigen receptor-mediated activation of B cells. Proc. Natl. Acad. Sci. USA 97:2755-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He, X., Y. Li, J. Schembri-King, S. Jakes, and J. Hayashi. 2000. Identification of actin binding protein, ABP-280, as a binding partner of human Lnk adaptor protein. Mol. Immunol. 37:603-612. [DOI] [PubMed] [Google Scholar]
- 13.Healy, J. I., and C. C. Goodnow. 1998. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16:645-670. [DOI] [PubMed] [Google Scholar]
- 14.Herrington, J., M. Diakonova, L. Rui, D. R. Gunter, and C. Carter-Su. 2000. SH2-B is required for growth hormone-induced actin reorganization. J. Biol. Chem. 275:13126-13133. [DOI] [PubMed] [Google Scholar]
- 15.Hitoshi, Y., N. Yamaguchi, S. Mita, E. Sonoda, S. Takaki, A. Tominaga, and K. Takatsu. 1990. Distribution of IL-5 receptor-positive B cells. Expression of IL-5 receptor on Ly-1(CD5)+ B cells. J. Immunol. 144:4218-4225. [PubMed] [Google Scholar]
- 16.Holsinger, L. J., I. A. Graef, W. Swat, T. Chi, D. M. Bautista, L. Davidson, R. S. Lewis, F. W. Alt, and G. R. Crabtree. 1998. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8:563-572. [DOI] [PubMed] [Google Scholar]
- 17.Inabe, K., M. Ishiai, A. M. Scharenberg, N. Freshney, J. Downward, and T. Kurosaki. 2002. Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J. Exp. Med. 195:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iritani, B. M., K. A. Forbush, M. A. Farrar, and R. M. Perlmutter. 1997. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16:7019-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iseki, M., S. Takaki, and K. Takatsu. 2000. Molecular cloning of the mouse APS as a member of the Lnk family adaptor proteins. Biochem. Biophys. Res. Commun. 272:45-54. [DOI] [PubMed] [Google Scholar]
- 20.Jumaa, H., B. Wollscheid, M. Mitterer, J. Wienands, M. Reth, and P. J. Nielsen. 1999. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11:547-554. [DOI] [PubMed] [Google Scholar]
- 21.Kantor, A. B., and L. A. Herzenberg. 1993. Origin of murine B cell lineages. Annu. Rev. Immunol. 11:501-538. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, M. E., and A. C. Chan. 2000. Regulation of B cell function by linker proteins. Curr. Opin. Immunol. 12:267-275. [DOI] [PubMed] [Google Scholar]
- 23.Kerner, J. D., M. W. Appleby, R. N. Mohr, S. Chien, D. J. Rawlings, C. R. Maliszewski, O. N. Witte, and R. M. Perlmutter. 1995. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity 3:301-312. [DOI] [PubMed] [Google Scholar]
- 24.Khan, W. N., F. W. Alt, R. M. Gerstein, B. A. Malynn, I. Larsson, G. Rathbun, L. Davidson, S. Muller, A. B. Kantor, L. A. Herzenberg, et al. 1995. Defective B cell development and function in Btk-deficient mice. Immunity 3:283-299. [DOI] [PubMed] [Google Scholar]
- 25.Koretzky, G. A., and P. S. Myung. 2001. Positive and negative regulation of T-cell activation by adaptor proteins. Nat. Rev. Immunol. 1:95-107. [DOI] [PubMed] [Google Scholar]
- 26.Martin, F., and J. F. Kearney. 2002. Marginal-zone B cells. Nature Rev. Immunol. 2:323-335. [DOI] [PubMed] [Google Scholar]
- 27.Melamed, I., G. P. Downey, K. Aktories, and C. M. Roifman. 1991. Microfilament assembly is required for antigen-receptor-mediated activation of human B lymphocytes. J. Immunol. 147:1139-1146. [PubMed] [Google Scholar]
- 28.Melamed, I., and E. W. Gelfand. 1999. Microfilament assembly is involved in B-cell apoptosis. Cell. Immunol. 194:136-142. [DOI] [PubMed] [Google Scholar]
- 29.Melchers, F., E. ten Boekel, T. Seidl, X. C. Kong, T. Yamagami, K. Onishi, T. Shimizu, A. G. Rolink, and J. Andersson. 2000. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol. Rev. 175:33-46. [PubMed] [Google Scholar]
- 30.Minami, A., M. Iseki, K. Kishi, M. Wang, M. Ogura, N. Furukawa, S. Hayashi, M. Yamada, T. Obata, Y. Takeshita, Y. Nakaya, Y. Bando, K. Izumi, S. A. Moodie, F. Kajiura, M. Matsumoto, K. Takatsu, S. Takaki, and Y. Ebina. 2003. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52:2657-2665. [DOI] [PubMed] [Google Scholar]
- 31.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 32.Nobuhisa, I., M. Takizawa, S. Takaki, H. Inoue, K. Okita, M. Ueno, K. Takatsu, and T. Taga. 2003. Regulation of hematopoietic development in the aorta-gonad-mesonephros region mediated by Lnk adaptor protein. Mol. Cell. Biol. 23:8486-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtsuka, S., S. Takaki, M. Iseki, K. Miyoshi, N. Nakagata, Y. Kataoka, N. Yoshida, K. Takatsu, and A. Yoshimura. 2002. SH2-B is required for both male and female reproduction. Mol. Cell. Biol. 22:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver, A. M., F. Martin, G. L. Gartland, R. H. Carter, and J. F. Kearney. 1997. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 27:2366-2374. [DOI] [PubMed] [Google Scholar]
- 35.Osborne, M. A., S. Dalton, and J. P. Kochan. 1995. The yeast tribrid system: genetic detection of trans-phosphorylated ITAM-SH2-interactions. Bio/Technology 13:1474-1478. [DOI] [PubMed] [Google Scholar]
- 36.Pappu, R., A. M. Cheng, B. Li, Q. Gong, C. Chiu, N. Griffin, M. White, B. P. Sleckman, and A. C. Chan. 1999. Requirement for B cell linker protein (BLNK) in B cell development. Science 286:1949-1954. [DOI] [PubMed] [Google Scholar]
- 37.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 38.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F. W. Alt, and R. S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell 94:229-238. [DOI] [PubMed] [Google Scholar]
- 39.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 40.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki, H., Y. Terauchi, M. Fujiwara, S. Aizawa, Y. Yazaki, T. Kadowaki, and S. Koyasu. 1999. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science 283:390-392. [DOI] [PubMed] [Google Scholar]
- 42.Takaki, S., H. Morita, Y. Tezuka, and K. Takatsu. 2002. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J. Exp. Med. 195:151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takaki, S., K. Sauer, B. M. Iritani, S. Chien, Y. Ebihara, K. Tsuji, K. Takatsu, and R. M. Perlmutter. 2000. Control of B cell production by the adaptor protein Lnk: definition of a conserved family of signal-modulating proteins. Immunity 13:599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaki, S., Y. Tezuka, K. Sauer, C. Kubo, S. M. Kwon, E. Armstead, K. Nakao, M. Katsuki, R. M. Perlmutter, and K. Takatsu. 2003. Impaired lymphopoiesis and altered B cell subpopulations in mice overexpressing Lnk adaptor protein. J. Immunol. 170:703-710. [DOI] [PubMed] [Google Scholar]
- 45.Tedford, K., L. Nitschke, I. Girkontaite, A. Charlesworth, G. Chan, V. Sakk, M. Barbacid, and K. D. Fischer. 2001. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2:548-555. [DOI] [PubMed] [Google Scholar]
- 46.Uehara, S., Y. Hitoshi, F. Numata, M. Makino, M. Howard, T. Mizuochi, and K. Takatsu. 1994. An IFN-gamma-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int. Immunol. 6:1937-1947. [DOI] [PubMed] [Google Scholar]
- 47.Wakioka, T., A. Sasaki, K. Mitsui, M. Yokouchi, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing Pleckstrin homology (PH) and Src homology-2 (SH2) domains inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13:760-767. [DOI] [PubMed] [Google Scholar]
- 48.Wang, D., J. Feng, R. Wen, J. C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 49.Yabana, N., and M. Shibuya. 2002. Adaptor protein APS binds the NH2-terminal autoinhibitory domain of guanine nucleotide exchange factor Vav3 and augments its activity. Oncogene 21:7720-7729. [DOI] [PubMed] [Google Scholar]
- 50.Yokouchi, M., R. Suzuki, M. Masuhara, S. Komiya, A. Inoue, and A. Yoshimura. 1997. Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene 15:7-15. [DOI] [PubMed] [Google Scholar]
- 51.Yokouchi, M., T. Wakioka, H. Sakamoto, H. Yasukawa, S. Ohtsuka, A. Sasaki, M. Ohtsubo, M. Valius, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene 18:759-767. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10:323-332. [DOI] [PubMed] [Google Scholar]