Abstract
The Tec protein tyrosine kinase is the founding member of a family that includes Btk, Itk, Bmx, and Txk. Btk is essential for B-cell receptor signaling, because mutations in Btk are responsible for X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (xid) in mice, whereas Itk is involved in T-cell receptor signaling. Tec is expressed in both T and B cells, but its role in antigen receptor signaling is not clear. In this study, we show that Tec protein is expressed at substantially lower levels in primary T and B cells relative to Itk and Btk, respectively. However, Tec is up-regulated upon T-cell activation and in Th1 and Th2 cells. In functional experiments that mimic Tec up-regulation, we find that Tec overexpression in lymphocyte cell lines is sufficient to induce phospholipase Cγ (PLC-γ) phosphorylation and NFAT (nuclear factor of activated T cells) activation. In contrast, overexpression of Btk, Itk, or Bmx does not induce NFAT activation. Tec-induced NFAT activation requires PLC-γ, but not the adapters LAT, SLP-76, and BLNK, which are required for Btk and Itk to couple to PLC-γ. Finally, we show that the unique effector function for Tec correlates with a unique subcellular localization. We hypothesize that Tec functions in activated and effector T lymphocytes to induce the expression of genes regulated by NFAT transcription factors.
Signal transduction through antigen receptors is central to the development and function of T and B lymphocytes. The T-cell receptor (TCR) and B-cell receptor (BCR) signal by recruiting non-receptor protein tyrosine kinases (PTKs) of the Src, Syk, and Tec families. These PTKs phosphorylate adapter proteins, such as LAT, SLP-76, and BLNK, which recruit downstream effectors, including phospholipase Cγ (PLC-γ). Subsequent PLC-γ activation induces calcium mobilization and mitogen-activated protein kinase (MAPK) activation, resulting in the activation of nuclear factor of activated T cells (NFAT) and other transcription factors (reviewed in references 13 and 18).
The Tec family of PTKs is composed of Tec, Btk, Itk, Bmx, and Txk. These PTKs are characterized by an NH2-terminal pleckstrin homology (PH) domain (absent in Txk), a proline-rich region, Src-homology 3 (SH3) and SH2 domains, and a COOH-terminal PTK domain (reviewed in references 17 and 26). An essential role for Tec family PTKs in antigen receptor signaling became clear when mutations in Btk were found to be responsible for the human disease X-linked agammaglobulinemia (XLA) and for the X-linked immunodeficient (xid) mouse. BCR signaling is severely impaired in XLA patients, resulting in a failure of B-cell development and an absence of mature B cells. The xid mouse and the Btk knockout mouse have phenotypes that are similar to that of XLA, but are less severe (reviewed in reference 19). Studies using Btk-deficient cell lines have shown that Btk activation is necessary and sufficient for the phosphorylation and activation of PLC-γ2 (8, 25, 28). Btk is coupled to PLC-γ2 by the BLNK adapter protein (13). In T cells, which do not express Btk, Itk is required for optimal TCR-induced PLC-γ1 activation. The adapters LAT and SLP-76 couple Itk to PLC-γ1 (17, 26).
Tec is expressed in both T and B cells (12), but its role in lymphocytes is not clear. In primary T cells, reduction of Tec expression by using an antisense strategy causes reduced interleukin-2 (IL-2) production in response to TCR stimulation (33). Overexpression of Tec in the Jurkat T-cell line synergizes with phorbol myristate acetate (PMA) to induce NFAT activation, whereas Itk overexpression has no effect (34). These data suggest that Tec is important for optimal TCR signaling and suggest a functional difference between Tec and Itk. However, the Tec knockout mouse was reported to have no obvious defect in lymphocyte function (6), although this may be due to compensation by Itk and Btk. Consistent with this theory, the Tec/Btk double-knockout mouse has a more severe BCR signaling defect than the Btk knockout mouse and lacks mature B cells (6). In addition, Btk-deficient cell lines can be functionally reconstituted with Tec (8, 27). Interpretation of these knockout results is complicated by an absence of data concerning the relative expression levels of Tec, Itk, and Btk in primary T, B, and effector cells.
In this study, we have addressed the role of Tec in T and B cells by comparing its expression level, signaling function, and subcellular localization to other Tec family PTKs. We find that the expression level of Tec in primary T and B cells is low, compared to those of Itk and Btk, but that Tec is up-regulated following T-cell activation and in T helper 1 (Th1) and Th2 cells. In experiments that mimic Tec up-regulation, we show that overexpression of Tec, but not Btk, Itk, or Bmx, can induce NFAT activation in lymphocyte cell lines. Consistent with this unique function, we observe a unique punctate pattern of Tec localization at the cell membrane and immunological synapse. The atypical expression pattern, signaling function, and localization of Tec, compared to other Tec family PTKs, suggest a unique role for Tec that may be particularly important in effector T cells.
MATERIALS AND METHOD
Cells
The Jurkat T-cell line was cultured in RPMI supplemented with 5% fetal bovine serum, penicillin, streptomycin, and glutamine. Jurkat signaling mutant derivatives JCaM1 and JCaM1/Lck (23), P116 and P116/ZAP-70 (C39) (30), JCaM2 and JCaM2/LAT (LAT#3) (7), J14 and J14/SLP-76 (J14-76) (31) were described previously. The DT40 chicken B-cell line was cultured in RPMI supplemented with 10% fetal bovine serum, 1% chicken serum (Sigma, St. Louis, Mo.), penicillin, streptomycin, glutamine, and 50 μM mercaptoethanol. DT40 cell lines rendered deficient for lyn/syk (25), btk (25), grb2/grap (15), blnk (9) and plcγ2 (24) were described previously and were kindly provided by T. Kurosaki (Kansai Medical University, Moriguchi, Japan). The human kidney cell line 293T was cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin, streptomycin, glutamine, and 50 μM mercaptoethanol. The D10 T-cell clone was a gift from M. Krummel (University of California, San Francisco, Calif.). D10 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, nonessential amino acids, penicillin, streptomycin, glutamine, 50 μM mercaptoethanol, and 50-U/ml recombinant human IL-2 (R&D Systems, Minneapolis, Minn.). D10 cells were restimulated every 3 to 4 weeks with irradiated, erythrocyte-depleted splenocytes from B10.BR mice and chicken conalbumin (Sigma). The CH27 mouse B-cell line was maintained in RPMI supplemented with 5% fetal bovine serum, penicillin, streptomycin, and glutamine.
Plasmids
Tec family constructs (mouse Tec, Itk, and Btk and human Bmx), hemagglutinin (HA) tagged at the NH2 terminus, have been described previously (27). For this study, HA-tagged Tec family kinases were subcloned into the pCDEF3 expression vector, which contains the EF-1α promoter. Myr-tagged Tec was generated by PCR, replacing the HA tag with the 17 NH2-terminal amino acids of chicken Src. Tec family constructs, green fluorescent protein (GFP) tagged at the COOH terminus, were generated by PCR and cloning into the pEGFP-C1 vector (BD Biosciences, San Diego, Calif.). HA-PLC-γ1 cDNA was a gift from Q. Ji (First Military Medical University, Guangzhon, China). HA-PLC-γ1 was subcloned into the pEFBOS expression vector, which contains the EF-1α promoter, by D. Yablonski (Technion-Israel Institute of Technology, Haifa, Israel). The following inactivating point mutations were generated by a two-step PCR method (28): Tec R29C (PH domain), Tec P158,161,168,169,170,171A (proline-rich region), Tec W215,216L (SH3 domain), Tec R272A (SH2 domain), and Tec K397E (kinase domain). The NFAT luciferase reporter contains three copies of the distal NFAT site from the IL-2 promoter (21). The AP-1 luciferase reporter contains four copies of the AP-1 site from the metallothionein promoter (21). The NF-κB luciferase reporter contains three copies of the κB site of the immunoglobulin (Ig) κ-chain promoter (1). The RE-AP luciferase reporter contains four copies of the RE-AP site from the IL-2 promoter (22). The pEF6-lacZ expression construct was obtained from Invitrogen (Carlsbad, Calif.).
Antibodies
The polyclonal antisera for Btk and Itk were described previously (27). The anti-Tec rabbit antiserum, antiphosphotyrosine monoclonal antibody (MAb) 4G10, and anti-PLC-γ1 MAb were obtained from Upstate Biotechnology (Charlottsville, Va.). The anti-pY783 rabbit heteroserum was purchased from BioSource, Inc. (Camarillo, Calif.). The anti-actin MAb was obtained from Chemicon (Temecula, Calif.). The anti-HA tag MAb was 16B12 (Covance Research Products, Berkeley, Calif.), and the anti-myc tag MAb was 9B11 (Cell Signaling Technology, Beverley, Mass.). The anti-TCRβ MAb C305, for Jurkat cell stimulations, was described previously (29).
Biochemical analyses
Immunoprecipitations and Western blotting were performed as previously described (10). Western blots were visualized with Western Lightning chemiluminescence reagents (Perkin-Elmer, Boston, Mass.) on a Kodak Image Station (Kodak, Rochester, N.Y.). Quantitation of bands utilized Kodak 1D image analysis software, version 3.5.
Lymphocyte purification
Lymph nodes and spleens from 5- to 6-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were dispersed through a 70-μm-pore-diameter nylon filter to create single-cell suspensions. B and T cells were purified by negative selection with MACS Microbeads and LS columns (Miltenyi Biotec, Auburn, Calif.). CD43 and CD11b Microbeads were used for B-cell purification, and major histocompatibility complex (MHC) class II DX5 and CD11b Microbeads were used for T-cell purification. B and T cells were isolated to 90 to 95% purity as measured by CD19 and CD3 fluorescence-activated cell sorter (FACS) analyses. For T-cell activation, 24-well plates were first coated with 10-μg/ml rabbit anti-hamster IgG (Sigma) followed by 1-μg/ml anti-mouse CD3ɛ MAb 145-2C11 (BD Biosciences). T cells were then added at 106 cells per ml in RPMI supplemented with 10% fetal bovine serum, nonessential amino acids, penicillin, streptomycin, glutamine, 50 μM mercaptoethanol, and 1-μg/ml anti-mouse CD28 MAb 37.51 (BD Biosciences). Cells were harvested after 1, 2, and 3 days for Western blotting.
Th1/Th2 generation
Single-cell suspensions were prepared as described above. CD4+ T cells were partially purified by negative selection with the MACS CD4+ T-cell isolation kit and LS columns (Miltenyi Biotec). Cells were then labeled with phycoerythrin (PE)-conjugated anti-CD4, allophycocyanin-conjugated anti-CD62L, and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 and anti-CD19 (BD Biosciences). Naïve CD4+ CD62Lhi cells were sorted to >98% purity with a Mo-Flo Multi-Laser flow cytometer from Cytomation (Fort Collins, Colo.) and stimulated on plate-bound anti-CD3 and soluble anti-CD28 antibodies as described above. For Th1 generation, medium was supplemented with 5-ng/ml recombinant murine IL-12 (R&D Systems), 20-μg/ml neutralizing anti-IL-4 MAb 11B11 (a gift from R. Locksley, University of California, San Francisco) and 50-U/ml recombinant human IL-2 (R&D Systems). For Th2 generation, medium was supplemented with 50-ng/ml recombinant murine IL-4 (R&D Systems), 10-μg/ml neutralizing anti-IL-12 MAb (BD Biosciences), 50-μg/ml neutralizing anti-IFN-γ MAb XMG1.2 (a gift from R. Locksley, University of California, San Francisco), and 50-U/ml recombinant human IL-2 (R&D Systems). After 3 days on plate-bound anti-CD3 and soluble anti-CD28, Th1 and Th2 cells were replated in fresh medium without stimulating antibodies, but supplemented with the cytokines and neutralizing antibodies described above. Th1 and Th2 cells were harvested on day 6 for Western blotting. A fraction of the cells were restimulated with plate-bound anti-CD3 and soluble anti-CD28 to confirm Th1 and Th2 effector function by subsequent IFN-γ and IL-4 enzyme-linked immunosorbent assays (ELISAs). Th1 supernatants contained IFN-γ but no IL-4, and Th2 supernatants contained IL-4 and some IFN-γ, suggesting that the Th2 cells were not pure and contained some Th1 cells (data not shown).
Transfections
Jurkat, D10, and DT40 cells were transfected in a volume of 0.4 ml of RPMI (without serum) by electroporation with a Gene Pulser Electroporator (Bio-Rad, Hercules, Calif.) set at 250 V, 960 μF (Jurkat and D10) or 350 V, 500 μF (DT40). 293T cells were transfected by the calcium phosphate method with the CalPhos mammalian transfection kit (BD Biosciences).
Luciferase assays
Jurkat and DT40 cells were transfected as described above with the expression construct of interest, in addition to 20 μg of the luciferase reporter construct and 2 μg of pEF6-lacZ to control for transfection efficiency. Sixteen hours after transfection, live cells were counted by Trypan blue exclusion, and samples were divided for luciferase assay, β-galactosidase assay, and Western blotting. Luciferase assays were performed in triplicate with 105 live cells in a total volume of 100 μl. Samples were stimulated for 6 h with a 1:100 dilution of C305 MAb (anti-TCR) concentrated tissue culture supernatant plus 2-μg/ml anti-CD28 MAb (Caltag, Burlingame, Calif.) for NF-κB and RE-AP assays, or with 50-ng/ml PMA plus 1 μM ionomycin (Calbiochem, San Diego, Calif.). The assay was harvested by adding 11 μl of lysis buffer containing 10% Triton X-100, 200 mM NaPO4 (pH 7.8), and 4 mM dithiothreitol (DTT). One hundred microliters of this lysate was added to 100 μl of assay buffer containing 200 mM NaPO4 (pH 7.8), 20 mM MgCl2, and 10 mM ATP. Following the addition of 50 μl of 20 mM luciferin, luciferase activity was measured with a microplate luminometer (Berthold Technologies, Oak Ridge, Tenn.). β-Galactosidase assays were performed with 106 cells using the Galacto-Light chemiluminescent reporter assay, according to the manufacturer's instructions (Tropix, Bedford, Mass.). β-Galactosidase activity was measured in triplicate using a microplate luminometer (Berthold Technologies). All luciferase assay data were normalized to β-galactosidase values. Expression of each construct was confirmed by Western blotting.
Microscopy
For conjugate formation, D10 cells, transfected with GFP-tagged Tec family PTKs, were mixed with equal numbers of CH27 cells, preloaded for 3 to 4 h with the cognate chicken conalbumin peptide at 8 μM. Cells were centrifuged at 1,200 × g for 1 min, and conjugates were allowed to form for 20 min at 37°C. The medium was aspirated, and the cells were gently resuspended in phosphate-buffered saline (PBS)-1% bovine serum albumin (BSA) by pipetting up and down three times with a 1-ml micropipette. Approximately 105 cells were applied to glass slides, coated with poly-l-lysine, and allowed to settle for 10 min. Cells were fixed with 4% paraformaldehyde for 20 min, washed with PBS, permeabilized with 0.1% Triton X-100 for 4 min, and blocked for 30 min with PBS-1% BSA-4% normal goat serum. Cells were stained for 30 min with 4-μg/ml protein kinase Cθ (PKC-θ) rabbit antiserum (Santa Cruz Biotechnology, Santa Cruz, Calif.), washed three times for 5 min each with PBS-0.2% BSA, stained for 30 min with goat anti-rabbit-Cy3 (Jackson Immunoresearch Laboratories, West Grove, Pa.), diluted 1:100, and washed as described above. Jurkat cells transfected with GFP-tagged or HA-tagged Tec family PTKs were similarly applied to slides, fixed, and permeabilized. HA staining was done with Alexa 488-conjugated 16B12 MAb (Covance Research Products), diluted 1:500. Cholera toxin staining was done on nonpermeabilized cells by using 2-μg/ml biotinylated cholera toxin (Sigma) followed by 1-μg/ml Alexa 647-conjugated streptavidin (Molecular Probes, Eugene, Oreg.). Antiphosphotyrosine staining was done with 1-μg/ml antiphosphotyrosine MAb 4G10 (Upstate Biotechnology) followed by goat anti-mouse-Cy5 (Jackson Immunoresearch Laboratories) diluted 1:100. Nuclei were stained with Hoechst 33342 (Molecular Probes). The slides were mounted with coverslips by using polyvinyl mounting medium (Sigma). Slides were examined with an Intelligent Imaging Innovations Marianas system (Denver, Colo.), consisting of a Zeiss Axiovert microscope fitted with a Zeiss Plan-Apochromat, ×63/1.4 NA oil objective and a Cooke Sensicam cooled charge-coupled device (CCD) camera. Approximately 20 fluorescence and differential interference contrast (DIC) images were collected per stack (z axis), at intervals of 0.5 μm, and calibrated to fall within the linear range of the camera. Data stacks of fluorescence images were subjected to constrained iterative deconvolution with Slidebook software, version 4.0.
RESULT
Tec is expressed at low levels, relative to Itk and Btk, in primary mouse T and B cells
Itk and Btk are essential for optimal antigen receptor signaling in T and B cells, respectively. This has been clearly demonstrated in studies of gene-targeted mice and Btk-deficient XLA patients (16, 19). Tec is expressed in both T and B cells (12), but its function is not clear. Moreover, the expression level of Tec, compared to those of Itk and Btk, has not been determined. To address this fundamental issue, we have used identically tagged forms of Tec, Itk, and Btk, together with specific antisera for each, to quantify their relative expression levels in primary mouse T and B cells.
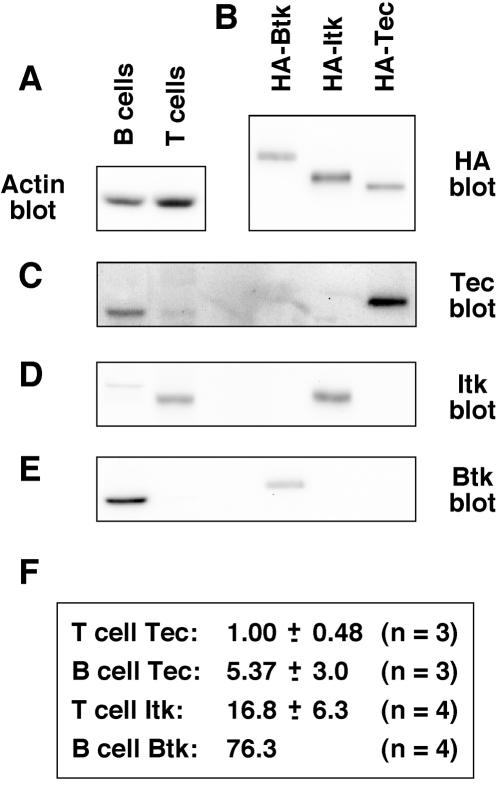
T and B cells were purified from mixed spleen and lymph nodes from 5- to 6-week-old C57BL/6 mice. Purity of T and B cells was between 90 and 95%, as assessed by FACS analyses for CD3 and CD19 (data not shown). T- and B-cell lysates were Western blotted for actin (Fig. 1A), and the bands were quantitated with a Kodak Image Station. Actin levels were assumed to equate to whole-protein levels in the samples. HA-tagged forms of mouse Tec, Itk, and Btk were transiently transfected into 293T cells and immunoprecipitated with anti-HA MAb, and their relative expression levels were quantitated after anti-HA Western blotting (Fig. 1B). The HA-tagged forms of Tec, Itk, and Btk (Fig. 1B) were then compared to the T- and B-cell lysates (Fig. 1A) by Western blotting for Tec (Fig. 1C), Itk (Fig. 1D), and Btk (Fig. 1E). As expected, Tec was expressed in T and B cells (Fig. 1C), Itk was expressed in T cells only (Fig. 1D), and Btk was expressed in B cells only (Fig. 1E). The Tec antiserum identified multiple bands in whole-cell lysates; therefore, the identity of the specific Tec band was confirmed by immunoprecipitation and blotting with a second Tec antiserum (27; data not shown). The faint band detected in B-cell lysate by the Itk antiserum was probably nonspecific, because it was not detected by blotting with a second Itk MAb, 2F12 (a gift from L. Berg, University of Massachusetts Medical School, Worcester, Mass.) (data not shown). Bands were quantitated, and the relative levels of Tec, Itk, and Btk were calculated by correcting for antiactin (Fig. 1A) and anti-HA (Fig. 1B) values. As shown in Fig. 1F, the expression level of Tec was approximately fivefold greater in B cells than in T cells. In addition, Tec expression levels were approximately 17- and 15-fold lower than Itk and Btk expression levels in T and B cells, respectively.
FIG. 1.
Tec expression is relatively low in primary mouse T and B cells, relative to Itk and Btk. (A) B and T lymphocytes were isolated to 90 to 95% purity by MACS from mixed spleen and lymph nodes from 5- to 6-week-old C57BL/6 mice. Cells were lysed, and approximately 5 × 106 cell equivalents were Western blotted for actin as a control for protein loading. (B) HA-tagged forms of Btk, Itk, and Tec were transfected into 293T cells, immunoprecipitated with anti-HA MAb, and Western blotted for HA. (C) Protein samples from panels A and B were Western blotted with Btk antiserum. (D) Protein samples from panels A and B were Western blotted with Itk antiserum. The faint band detected in B-cell lysates is probably nonspecific, since it was not detected by the anti-Itk MAb 2F12 (data not shown). (E) Protein samples from panels A and B were Western blotted with Tec antiserum. (F) Bands were quantitated, and the relative levels of Btk, Itk, and Tec in B and T cells were calculated with the HA-tagged proteins as a common reference. The data represent means and standard deviations from three or four independent experiments. Measurement of Btk levels in B cells was included in each experiment; therefore, this value was fixed arbitrarily.
Tec is up-regulated upon T-cell activation and in effector cells
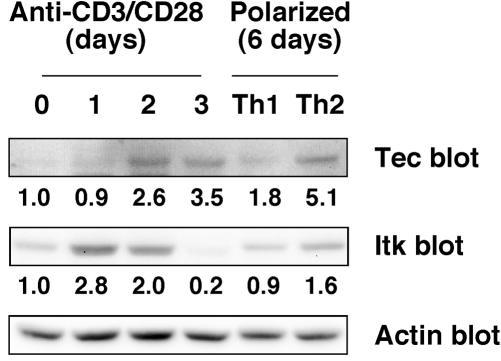
The relatively low levels of Tec in freshly isolated mouse T and B cells suggest that Tec may not play an important role in naïve lymphocytes. Therefore, we measured Tec expression upon activation of primary T and B cells from C57BL/6 mice. No up-regulation of Tec or Btk was detected in B cells stimulated for 1 or 2 days with anti-IgM plus anti-CD40 antibodies, lipopolysaccharide (LPS), or PMA plus ionomycin (data not shown). In contrast, Tec and Itk were up-regulated in T cells stimulated with plate-bound anti-CD3 plus anti-CD28 antibodies, as measured by Western blotting of whole-cell lysates (Fig. 2). Up-regulation of Itk was transient, peaking at 2.8-fold on day 1 after stimulation. Up-regulation of Tec was delayed until day 2, but was sustained to at least day 3 (3.5-fold) after stimulation. Actin blotting was used to control for loading (Fig. 2). To determine whether Tec was also up-regulated in effector Th1 or Th2 subsets, T cells were polarized for 6 days with IL-12 plus anti-IL-4 or IL-4 plus anti-IL-12 and anti-IFN-γ, respectively. Tec was up-regulated in Th1 and Th2 cells and was substantially higher in Th2 cells (up to approximately 5-fold), whereas Itk was only up-regulated in Th2 cells to 1.6-fold (Fig. 2B). These data suggest that Tec may play a role in activated T cells and in effector Th1 and Th2 cells.
FIG. 2.
Tec and Itk are up-regulated in activated T cells and in Th1 and Th2 effector cells. For T-cell activation, C57BL/6 mouse T cells were isolated as described in the legend to Fig. 1A and stimulated with plate-bound anti-CD3 MAb and soluble anti-CD28 MAb. Cells were harvested at the indicated time points, and lysates were Western blotted for Tec, Itk, and actin. For generation of Th1 and Th2 cells, T cells were sorted for CD4+ CD62hi naïve cells to over 98% purity. T cells were stimulated with plate-bound anti-CD3 MAb and soluble anti-CD28 and differentiated under Th1 or Th2 polarizing conditions for 6 days, and then cells were lysed and Western blotted for Tec, Itk, and actin. Quantitation of Tec and Itk bands is shown after correcting for actin. The data are representative of three experiments.
Transient transfection of Tec, but not Itk, Btk, or Bmx, can induce NFAT activation in Jurkat T cells
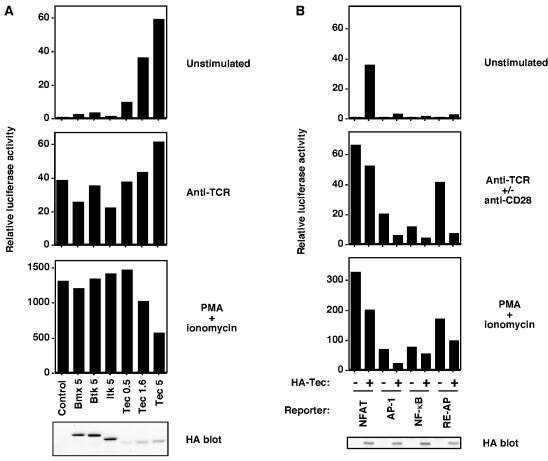
To investigate the potential function of Tec in T cells, up-regulation of Tec was mimicked by transient transfection into the Jurkat T-cell line. Previous Jurkat studies have shown that overexpression of Tec, but not Itk, can synergize with PMA to induce activation of NFAT, IL-2, and IL-4 reporter constructs (33-36). In this study, we attempted to repeat these findings and to determine whether this property of Tec is unique among Tec family PTKs. A luciferase reporter construct, under the control of NFAT sites from the IL-2 promoter, was cotransfected to measure signaling responses in transfected cells. A β-galactosidase construct was cotransfected as a control, and all data were normalized to β-galactosidase readings. Transfection of HA-tagged Tec, in the absence of any stimulation, was sufficient to induce constitutive NFAT activation in a dose-dependent manner (Fig. 3A, upper panel). In contrast, transfection of HA-tagged forms of the other PH domain-containing Tec family kinases, namely Bmx, Btk, and Itk, did not induce NFAT activation (Fig. 3A, upper panel). Western blotting of whole-cell lysates with an anti-HA MAb confirmed expression of each construct, and Tec expression was actually lower than expression of Bmx, Btk, and Itk (Fig. 3A, lower panel). No synergy or additive effect was observed for Tec in combination with anti-TCR stimulation (Fig. 3A). Indeed, TCR levels were substantially down-regulated in Tec-expressing cells (data not shown), probably because of downstream PKC activation, which can induce TCR down-regulation (5). Control stimulation with PMA-ionomycin, which bypasses receptor-proximal signaling by activating RasGRP/PKC and inducing calcium mobilization, respectively, was robust for each transfection (Fig. 3A). However, signaling by PMA-ionomycin was somewhat impaired in Tec-expressing cells (Fig. 3A), probably because chronic signaling by Tec induces negative feedback pathways. The engineering of Tec kinases with NH2-terminal HA tags did not appear to affect function, since tagged and nontagged Tec induced similar levels of NFAT activation (data not shown).
FIG. 3.
Overexpression of Tec, but not Bmx, Btk, or Itk, is sufficient to activate an NFAT reporter in Jurkat T cells. (A) Jurkat T cells were transfected with an NFAT-luciferase reporter construct, a β-galactosidase construct driven by the EF-1α promoter to control for transfection efficiency, and HA-tagged forms of Bmx, Btk, Itk, or Tec, with the quantities transfected indicated in micrograms. Sixteen hours posttransfection, cells were either left unstimulated or were stimulated with anti-TCR MAb C305 or PMA plus ionomycin. Six hours later, cells were lysed and assayed for luciferase and β-galactosidase, and expression of the Tec family constructs was confirmed by anti-HA Western blotting. Luciferase data were normalized for β-galactosidase values. Note the change of scale on the y axis of the PMA-ionomycin graph. The data are representative of at least three experiments. (B) Jurkat T cells were transfected with NFAT, AP-1, NF-κB, or RE-AP luciferase reporter constructs, in the presence or absence of 5 μg of HA-Tec. Luciferase assays were performed and are presented as described for panel A. Anti-CD28 MAb stimulation was included with anti-TCR stimulation for NF-κB and RE-AP assays.
To determine whether constitutive Tec signaling is specific to NFAT, Jurkat T cells were cotransfected with Tec and luciferase reporter constructs for AP-1, NF-κB, and RE-AP, which, in common with NFAT, are important sites within the IL-2 promoter. As before, Tec induced activation of NFAT (Fig. 3B, upper panel). However, activation of AP-1, NF-κB, and RE-AP was relatively weak (Fig. 3B, upper panel). These data are consistent with those from a previous study that used Tec in combination with PMA (35). Control stimulations with anti-TCR (for AP-1), anti-TCR plus anti-CD28 (for NF-κB and RE-AP), and with PMA and ionomycin were sufficient to induce activation of each reporter (Fig. 4, middle panels). The reduced AP-1, NF-kB, and RE-AP responses to TCR stimulation in the presence of Tec are likely due to TCR down-regulation in Tec-expressing cells (data not shown). The similarly reduced PMA-ionomycin responses in the presence of Tec are possibly due to negative feedback pathways induced by chronic Tec signaling. These results suggest that up-regulation of Tec, but not other Tec family members, may induce the expression of genes that are controlled by NFAT sites.
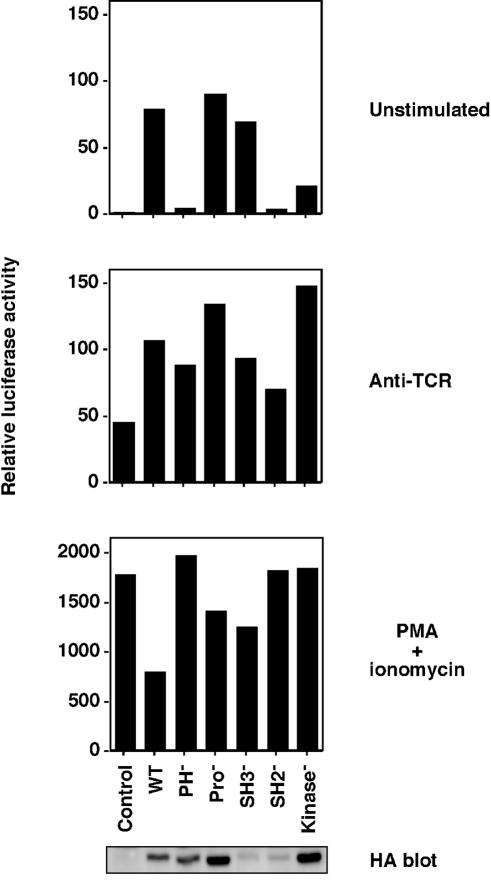
FIG. 4.
Tec-induced NFAT activation requires intact PH and SH2 domains. Jurkat T cells were transfected with an NFAT-luciferase reporter construct and 5 μg of wild-type HA-Tec or constructs with inactivating point mutations of the PH domain, the proline-rich region, or the SH3, SH2, or kinase domain. Luciferase assays were performed and are presented as described in the legend to Fig. 3A.
Tec-induced NFAT activation requires intact PH and SH2 domains
Previous studies have shown that Tec signaling requires an intact PH domain for PIP3 membrane targeting (33) and an intact kinase domain (34-36). To further investigate the mechanism for Tec-induced NFAT activation in our system, inactivating point mutations were made in each domain of Tec. As shown in Fig. 4 (upper panel), NFAT activation required intact PH and SH2 domains of Tec. The inactivity of the SH2 mutant was not due to its relatively low expression level, since it remained inactive at higher expression levels (up to 20 μg of DNA; data not shown). This is consistent with the data of Yang et al. (33) and with the PH and SH2 domain requirements for Btk function in BCR signaling (25). NFAT activation did not require the two proline-containing motifs or SH3 domain of Tec (Fig. 4, upper panel). Indeed, the SH3 point mutant appeared to be hyperactive, since NFAT activation was similar to that of wild-type Tec, but its expression level was lower. This hyperactive phenotype of the SH3 mutant was more striking when wild-type Tec levels were reduced such that the protein levels were equal (data not shown) and is consistent with the proposed negative regulatory role of the Itk SH3 domain (17). The kinase-inactive form of Tec induced NFAT activation at a level that was approximately 25% of wild-type Tec (Fig. 4, upper panel). This effect of kinase-inactive Tec was not restricted to Jurkat T cells, because we had similar results in HUT78 T cells, DT40 B cells, and RBL-2H3 mast cells (data not shown). The mechanism remains unclear, but a partial function for kinase-inactive Btk has also been reported (28). These data suggest that Tec family PTKs have a kinase-independent adapter function. In response to control anti-TCR, or PMA-ionomycin stimulations, similar levels of NFAT activation were observed under all transfection conditions (Fig. 4, lower panels). Taken together, these results suggest that the constitutive signaling mechanism for Tec is similar to that of Btk in BCR signaling.
Lck and PLC-γ are required for Tec-induced NFAT activation, but the LAT, SLP-76, and BLNK adapters are not
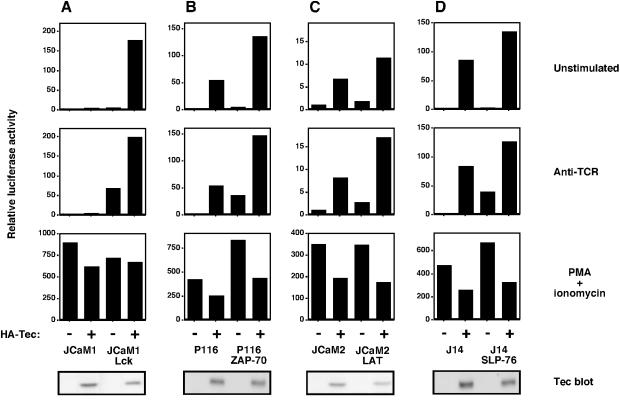
Studies using mutant Jurkat T cells and gene-targeted DT40 B cells have shown that Itk and Btk require Src family kinases for their activation and LAT/SLP-76/BLNK adapters to couple to their substrate PLC-γ (13, 17, 26). To determine whether Tec signals to NFAT by the same mechanism, we transfected Tec into Jurkat and DT40 signaling mutants and measured NFAT induction (Fig. 5 and 6). In Jurkat cells (Fig. 5, upper panels), Tec-induced NFAT required Lck (JCaM1, Fig. 5A), but not ZAP-70 (P116, Fig. 5B), LAT (JCaM2, Fig. 5C), or SLP-76 (J14, Fig. 5D). TCR-induced NFAT did require each of these signaling proteins (Fig. 5, middle panels), and control PMA-ionomycin stimulation was similar in all transfections (Fig. 5, lower panels). Mutant Jurkat lines stably reconstituted with Lck, ZAP-70, LAT, and SLP-76, respectively, were used as controls, and the expression of Tec was confirmed by Western blotting.
FIG. 5.
Tec-induced NFAT activation requires Lck, but not ZAP-70, LAT, or SLP-76. (A) The Lck-deficient Jurkat T-cell line JCaM1 and JCaM1 stably reconstituted with Lck were transfected with an NFAT-luciferase reporter construct in the presence or absence of 5 μg of HA-Tec. Luciferase assays were performed and are presented as described in the legend to Fig. 3A. (B) The ZAP-70-deficient Jurkat T-cell line P116 and P116 stably reconstituted with ZAP-70 were treated as described for panel A. (C) The LAT-deficient Jurkat T-cell line JCaM2 and JCaM2 stably reconstituted with LAT were treated as described for panel A. (D) The SLP-76-deficient Jurkat T-cell line J14 and J14 stably reconstituted with SLP-76 were treated as described for panel A.
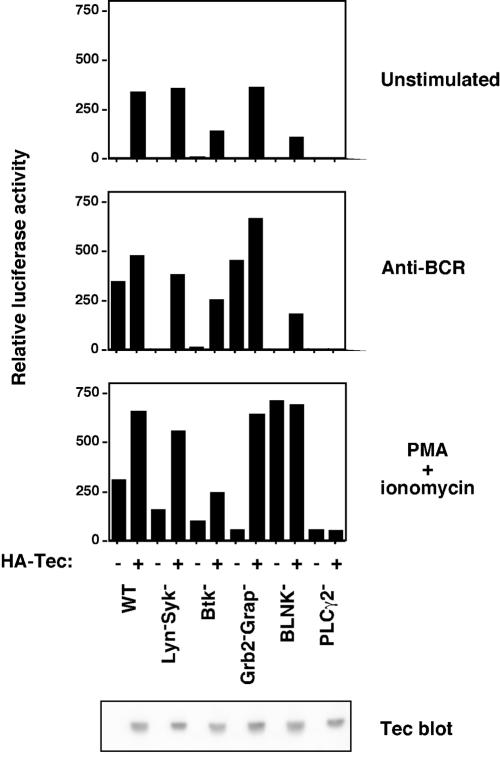
FIG. 6.
Tec-induced NFAT activation requires PLC-γ2, but not Lyn, Syk, Btk, Grb2, Grap, or BLNK. NFAT-luciferase assays were performed as described in the legend to Fig. 5A, using the parental DT40 B-cell line (WT) or lines rendered deficient by homologous recombination for Lyn and Syk, Btk, Grb2 and Grap, BLNK, or PLC-γ2.
In the wild-type DT40 chicken B-cell line, Tec-induced NFAT activation (Fig. 6, upper panel) was comparable to that induced by anti-BCR stimulation (Fig. 6, middle panel) or PMA plus ionomycin (Fig. 6, lower panel). Transfection of Tec into DT40 mutant lines showed that Tec-induced NFAT did not require the PTK Lyn, Syk, or Btk or the adapter Grb2, Grap, or BLNK, but did require PLC-γ2 (Fig. 6, upper panel). Anti-BCR-induced NFAT was impaired in each of these lines, except for the Grb2 Grap doubly-deficient line (Fig. 6, middle panel). PMA-ionomycin control stimulation induced NFAT in all DT40 lines (Fig. 6, lower panel), although the level of induction varied between cell lines (from approximately 70-fold in the Grb2− Grap− and PLC-γ2− cells, to approximately 700-fold in BLNK− cells). Tec expression was confirmed by Western blotting.
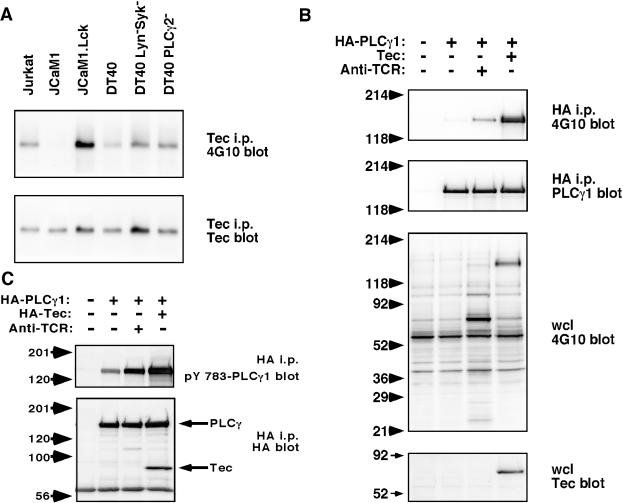
The requirement for Lck in Tec-induced NFAT activation is consistent with the role of Lck in activating Tec family PTKs upon TCR signaling. The requirement for PLC-γ2 in Tec-induced NFAT activation is consistent with the idea that PLC-γ is the major downstream substrate of Tec family PTKs. To address these issues, Tec was transfected into relevant Jurkat and DT40 lines, and its activation status was assessed by Tec immunoprecipitation followed by phosphotyrosine blotting (Fig. 7A), which correlates with Tec kinase activity (34). In parental Jurkat and DT40 lines, Tec was found to be tyrosine phosphorylated, suggesting that Tec is constitutively active. Tec phosphorylation was impaired in JCaM1 cells, which lack Lck, but was restored in JCaM1 cells that stably express Lck (Fig. 7A), suggesting that Lck is upstream of Tec and may provide a tonic signal to allow Tec to be active. Tec phosphorylation was not impaired in DT40 cells that lack Lyn and Syk, consistent with normal NFAT induction by Tec in these cells and suggesting that another PTK in DT40 is able to activate Tec. Phosphorylation by a non-Lyn PTK may actually be facilitated in Lyn-deficient cells, because Lyn is known to have an important negative regulatory role in BCR signaling by phosphorylating immunoreceptor tyrosine-based inhibition motifs (ITIMs), which recruit SHP and SHIP family phosphatases (reviewed in reference 14). Tec phosphorylation was also not impaired in PLC-γ2-deficient DT40 cells (Fig. 7A), consistent with the idea that PLC-γ2 is a downstream target of Tec.
FIG. 7.
Lck is required for Tec tyrosine phosphorylation, and PLC-γ1 is tyrosine phosphorylated upon Tec overexpression. (A) Tec was transfected into Jurkat, JCaM1, Lck-reconstituted JCaM1, DT40, Lyn− Syk− DT40, and PLC-γ2− DT40 cells. Sixteen hours later, cells were lysed, and Tec was immunoprecipitated (i.p.) with Tec antiserum. Samples were Western blotted with antiphosphotyrosine MAb 4G10 or with Tec antiserum. (B) Jurkat cells were transfected with HA-PLC-γ1 in the presence or absence of Tec. Sixteen hours later, cells were harvested and stimulated with anti-TCR MAb C305 where indicated. PLC-γ1 was immunoprecipitated with anti-HA MAb and Western blotted with antiphosphotyrosine MAb 4G10 or with anti-PLC-γ1 MAb. Control Western blotting of whole-cell lysates (wcl) was done with 4G10 or Tec antiserum. (C) Jurkat cells were transfected with HA-PLC-γ1 in the presence or absence of HA-Tec. Sixteen hours later, cells were harvested and stimulated with anti-TCR MAb C305 where indicated. PLC-γ1 and Tec were immunoprecipitated with anti-HA MAb and Western blotted with rabbit anti-pY 783 PLC-γ1 or anti-HA MAb.
If PLC-γ is indeed a target for Tec, PLC-γ1 would be predicted to be tyrosine phosphorylated in Tec-expressing cells. To test this prediction, Tec was transfected into Jurkat cells in the presence or absence of HA-tagged PLC-γ1 (Fig. 7B). Immunoprecipitation of HA-PLC-γ1 followed by antiphosphotyrosine blotting showed that Tec could induce PLC-γ1 phosphorylation that was more robust than with anti-TCR stimulation (Fig. 7B, upper panel). Phosphotyrosine blotting of whole-cell lysates identified a major phosphoprotein of 145 kDa that was weakly induced by TCR stimulation but strongly induced by Tec (Fig. 7B, third panel) and is likely to be HA-PLC-γ1. Moreover, expression of Tec led to phosphorylation of a key tyrosine, Y783, which is known to be associated with PLC-γ1 activation (Fig. 7C), suggesting that Tec expression leads to PLC-γ1 activation. The level of Tec-induced pY783 was even greater than that induced by TCR stimulation.
Taken together, these data suggest a mechanism for Tec-induced NFAT. Lck appears to play an upstream role in providing a tonic stimulus for Tec activation, whereas PLC-γ is likely to be the downstream target for Tec. Interestingly, Tec may couple to PLC-γ by a novel mechanism, since the LAT/SLP-76/BLNK adapters were dispensable for Tec-induced NFAT activation.
Tec, Itk, and Btk exhibit different subcellular localization patterns when transiently expressed in the Jurkat T-cell line
Previous studies have demonstrated that GFP-tagged forms of Tec (33) and Itk (3, 4) can colocalize with the TCR upon TCR cross-linking in Jurkat cells. To determine whether the unique Tec functional effect that we have observed in unstimulated Jurkat cells correlates with a unique subcellular localization, we compared the localizations of GFP-tagged Tec, Itk, and Btk.
Tec, Itk, and Btk constructs, GFP tagged at the COOH terminus, were generated and first tested functionally in the Btk-deficient DT40 cell line. The GFP constructs behaved in a similar manner to NH2-terminally HA-tagged constructs in transient NFAT assays. Tec constructs induced constitutive NFAT activation, whereas Itk and Btk reconstituted BCR-induced NFAT activation (data not shown). Therefore GFP tagging does not appear to impair the function of these Tec family PTKs.
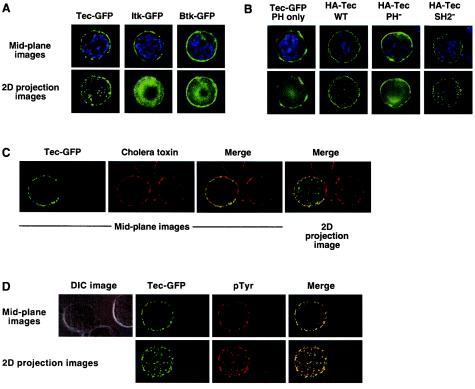
To compare subcellular localizations of Tec, Itk, and Btk in Jurkat cells, the cells were transiently transfected with GFP-tagged constructs, and the cells were imaged by fluorescence microscopy using deconvolution software. Tec and Itk were localized primarily to the cell membrane, whereas Btk was localized to the membrane and cytoplasm (Fig. 8A, mid-plane images). In contrast to Itk, Tec appeared to be clustered and exhibited a punctate localization pattern at the cell surface. Tec clusters were most noticeable when the z-stacks for each image were merged to generate a two-dimensional projection image (Fig. 8A). The unique Tec expression pattern was not a consequence of a different level of expression, since the observed patterns for each construct were consistent across a broad range of GFP intensities (data not shown).
FIG. 8.
Tec, but not Itk or Btk, is localized in clusters at the cell membrane in Jurkat cells. (A) Jurkat cells were transfected with GFP-tagged Tec, Itk, or Btk. Twenty hours posttransfection, cells were fixed on glass slides and imaged by florescence microscopy with deconvolution software. Representative cells are presented with GFP in green and nuclear staining in blue. The mid-plane image represents a single, central z-section. The two-dimensional (2D) projection represents the sum total of approximately 20 z-stacks collapsed to form one image. (B) Jurkat cells were transfected with GFP-tagged Tec PH domain or HA-tagged Tec wild type, PH, or SH2 point mutants. For detection of HA-tagged Tec, cells were fixed and permeabilized and then stained with Alexa 488-conjugated anti-HA MAb (shown in green). Nuclear staining is shown in blue. Images were collected as described for panel A. (C) Jurkat cells were transfected with GFP-tagged Tec. For detection of GEMs, cells were fixed and stained with biotinylated cholera toxin followed by Alexa 647-conjugated streptavidin (shown in red). Images were collected as described for panel A. (D) Jurkat cells were transfected with GFP-tagged Tec. For detection of phosphotyrosine, cells were fixed and permeabilized and then were stained with antiphosphotyrosine MAb 4G10 followed by Cy5-conjugated antimouse antibody (shown in red). Images were collected as described for panel A. The DIC image is shown to indicate the absence of a phosphotyrosine signal in nontransfected cells.
Tec clustering was also observed with an HA-tagged form of Tec, imaged by anti-HA immunoflorescence (Fig. 8B), suggesting that the localization pattern was not an artifact of GFP tagging. Tec clustering was not a downstream consequence of chronic signaling in Tec-expressing cells, since Tec with a mutated SH2 domain does not signal (Fig. 4) and yet retained punctate localization (Fig. 8B). An intact PH domain was required for punctate localization (Fig. 8B), but was not sufficient, since a GFP-tagged Tec PH domain construct did not exhibit punctate localization (Fig. 8B). In addition, the punctate pattern did not represent clustered glycosphingolipid-enriched microdomains (GEMs), since no substantial colocalization was observed with cholera toxin (Fig. 8C). We also observed no concentration of endogenous PLC-γ1 at the Tec clusters (data not shown), although we cannot rule out the possibility that a small percentage of PLC-γ1 is localized. However, Tec did colocalize with antiphosphotyrosine antibody (Fig. 8D). Therefore, Tec patches may represent active signaling complexes.
Tec, Itk, and Btk, when transiently transfected into D10 T cells, can localize to the immunological synapse following stimulation with APCs plus peptide
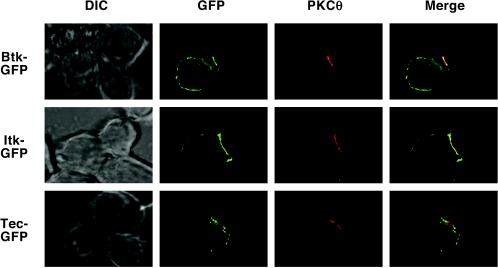
To determine whether punctate Tec localization could be observed in a more physiological system, GFP-tagged Tec family PTKs were transfected into the D10 T-cell clone. In resting cells, Tec, Itk, and Btk were cytoplasmic (data not shown), consistent with low PIP3 levels at the cell membrane. Following stimulation with antigen-presenting cells (APCs) plus cognate peptide, Tec, Itk, and Btk relocalized to the cell membrane and to the T-cell-APC interface (Fig. 9). Tec and Itk localized relatively efficiently to the immunological synapse, as detected by PKC-θ costaining, but Btk was more diffusely localized around the entire cell membrane. Consistent with the Jurkat cell localization data, Tec showed a punctate localization pattern at the T-cell-APC interface (Fig. 9). These data suggest a role for Tec in T-cell signaling in response to APC-peptide stimulation. Furthermore, the unique subcellular localization of Tec may be related to its unique signaling function.
FIG. 9.
Tec, but not Itk or Btk, is localized in clusters at the immunological synapse of the D10 T-cell clone. The D10 T-cell clone was transfected with GFP-tagged Tec, Itk, or Btk. Twenty hours posttransfection, conjugates were formed between D10 cells and CH27 APCs plus cognate peptide, and the cells were fixed on glass slides and permeabilized. To confirm the formation of productive conjugates, cells were stained with PKC-θ rabbit antiserum, followed by Cy5-conjugated anti-rabbit antibody, and imaged by florescence microscopy with deconvolution software. Representative conjugates are presented as mid-plane images with GFP in green and PKC-θ in red.
DISCUSSION
Gene-targeting experiments have shown that Itk and Btk perform essential roles in activating PLC-γ following TCR and BCR signaling, respectively (13, 17, 26). The role of Tec in lymphocytes is not clear, although there is some evidence to suggest a function in antigen receptor signaling (6, 8, 27, 33, 34). However, interpretation of existing data is difficult, because the expression level of Tec in lymphocytes, relative to those of Itk and Btk, is not known. In this study, we have quantitated Tec, Itk, and Btk expression levels in primary mouse T and B cells, using specific antisera combined with HA-tagged constructs as a reference. We have not analyzed the atypical Tec family PTK Rlk (mouse Txk) in this study due to the lack of a good Rlk antibody, although clearly Rlk is important for normal TCR-induced PLC-γ1 activation (20), and it would be useful to determine its relative expression level in T cells. We find that Tec is expressed at approximately 17- to 15-fold-lower levels than Itk and Btk in T and B cells, respectively. In B cells, Tec expression is approximately fivefold higher than in T cells, but Tec is up-regulated in activated T cells and in Th1/Th2 effector cells. These data allow a reevaluation of the phenotypes of Tec family knockout mice. In particular, Itk−/− Rlk−/− mice were defective in TCR signaling but made functional Th2 responses to a schistosome parasite (20). It is possible that low levels of Tec in naïve T cells can partially compensate for the absence of Itk and Rlk. Upon T-cell activation and differentiation, Tec is up-regulated and may now more efficiently compensate, allowing Th2 effector function. In Btk−/− Tec−/− mice, BCR signaling is defective in the absence of any Tec family PTKs, resulting in a complete failure to develop B cells (6). In Btk−/− mice, B cells do develop but are 50% reduced in number and show defective BCR signaling. These data are consistent with weak compensation by the relatively low level of Tec in B cells. Finally, the Tec−/− mice were reported to have no obvious phenotype and no antigen receptor signaling defect (6). This is not surprising given that Tec is expressed at low levels and that Itk and Btk may compensate for the loss of Tec. However, since Tec is up-regulated upon T-cell activation, a phenotype might be revealed upon examination of Th1/Th2 effector responses.
Functional experiments reported by other groups have shown that Tec overexpression in cell lines can activate NFAT, IL-2, IL-4, and c-fos reporter constructs (32-36). In this study, we have extended these findings to show that Tec is unique, within the PH domain-containing members of the Tec family, in its capacity to induce constitutive NFAT activation when overexpressed in lymphocyte lines. It is not clear why Tec has this unique function. We have generated a number of Tec/Itk chimeric proteins to address this question, although they have not provided a definitive answer. For example, Tec retained its capacity to induce NFAT activation when regions of Tec were substituted for with the corresponding regions of Itk. These included the PH domain, the proline/SH3 region, the SH2 domain, and the SH2/kinase region (M. G. Tomlinson and A. Weiss, unpublished data). Thus, the ability of Tec to induce NFAT activation is unlikely to be the result of any single domain that enables Tec to be uniquely localized or enables a unique effector function. An intrinsically more active kinase domain is also not likely, because Tec has in vitro kinase activity that is comparable to that of other family members (data not shown). It is possible that the unique Tec function is related to its relatively low expression level. If we assume that Tec-specific negative regulators are expressed at correspondingly low levels, whereas Itk and Btk negative regulators are expressed at relatively high levels, it may be easier to overcome negative regulation by overexpression of Tec than for other family members. Candidate Tec-specific negative regulators are the lipid phosphatases SHIP1 and SHIP2. These phosphatases are potent inhibitors of Tec-induced NFAT, but another lipid phosphatase, PTEN, has only a weak inhibitory effect (Tomlinson and Weiss, unpublished). Moreover, the SH3 domain of Tec, but not Itk, can interact with SHIP family phosphatases (Tomlinson and Weiss, unpublished). It is currently unclear whether Tec can interact with SHIP phosphatases in vivo.
We have studied the mechanism by which Tec induces NFAT activation by using mutant Jurkat and DT40 cell lines. Consistent with the proposed role of Itk, Btk, and Rlk (Txk) in activating PLC-γ, Tec induction of NFAT activation required PLC-γ2 in DT40 cells, and Tec was sufficient to induce PLC-γ1 phosphorylation in Jurkat cells. Based on previous studies of Itk and Btk, Tec would be predicted to also require LAT/SLP-76 and BLNK adapters to couple to PLC-γ (13, 17, 26). Surprisingly these adapters were not required for Tec-induced NFAT activation. Since we could not detect a direct interaction between Tec and PLC-γ1 in Jurkat cells (data not shown), we hypothesize that Tec utilizes a novel adapter to couple to PLC-γ. The SH2 domain of Tec is the most likely candidate for adapter binding, because the SH2 domains of Itk and Btk couple to SLP-76 and BLNK, respectively, and because the SH2 domain is required for Tec-induced NFAT activation. Interestingly we have found that the SH2 domains of Tec and Itk, expressed as glutathione S-transferase fusion proteins, have overlapping, but also distinct, binding partners. In particular, the Tec SH2 domain does not efficiently interact with SLP-76 and LAT, whereas, as reported by other groups, the Itk SH2 domain does (Tomlinson and Weiss, unpublished). Therefore, we favor a model in which Tec induces NFAT activation by activating PLC-γ, through SH2 domain coupling to an unidentified adapter.
The mechanism by which Tec activates NFAT is not entirely dependent on kinase activity. We found that a kinase-inactive form of Tec consistently induced NFAT activation to a level of approximately 25% that of kinase-active Tec. This effect of kinase-inactive Tec was not restricted to Jurkat cells, since it was also observed in the HUT78 T-cell line, the DT40 B-cell line, and the RBL-2H3 mast cell line (Tomlinson and Weiss, unpublished). The mechanism of action of kinase-inactive Tec is not clear, but is suggestive of an adapter function. A potential adapter function has been proposed for Btk, since kinase-inactive Btk can partially rescue BCR signaling in Btk-deficient DT40 cells (28). Characterization of the signaling capabilities of kinase-inactive forms of Tec family PTKs is of major importance. Tec PTKs are attractive drug discovery candidates for the inhibition of activation of T, B, and mast cells (11). However, any small molecule inhibitor that inhibits kinase activity may be only partially effective if the kinase retains an adapter function.
The unique signaling function of Tec, among Tec family PTKs, suggested that Tec might also localize to a unique subcellular compartment. Using fluorescence microscopy and GFP-tagged forms of Tec, Itk, and Btk, we indeed observed a unique localization pattern for Tec that was punctate at the cell membrane. This was observed both in Jurkat cells, which are known to have elevated PIP3 levels, and at the immunological synapse of a T-cell clone interacting with APC/peptide. The punctate Tec pattern resembles the small, TCR-enriched clusters, which induced calcium fluxes, observed by Bunnell et al. (2) after allowing Jurkat cells to settle onto stimulatory coverslips. Similar to our observations, these clusters are enriched in phosphotyrosine but lack GEMs. The Tec clusters required an intact PH domain, but the PH domain alone was not sufficient. Tec clustering was not a consequence of chronic signaling, because the signaling-deficient Tec SH2 mutant formed clusters. We hypothesize that Tec localizes to the membrane in a PIP3-dependent manner, where it recruits other signaling proteins, which may include Lck and PLC-γ, into clusters that are sufficient to induce NFAT activation.
In summary, we have identified some features of Tec that are unique among Tec family PTKs. We have shown that Tec has a unique subcellular localization and can constitutively activate the NFAT transcription factor in a LAT/SLP-76/BLNK-independent manner. These findings are surprising, because previous studies have suggested that Tec family PTKs are functionally redundant. We propose that Tec function may be important in activated T cells and in Th1/Th2 effector populations, where its expression is up-regulated. In particular, Tec could play an amplification role by increasing the sensitivity of memory or effector cells to subsequent stimulation.
Acknowledgments
We thank Cliff McArthur for Mo-Flo sorting, Lee Reinhardt and other members of Richard Locksley's laboratory for Th1/Th2 reagents, Pietro Andres for cytokine ELISAs, and Tomohiro Kurosaki for providing DT40 mutant cell lines. We also thank Victoria Heath, Joseph Lin, and other members of the Weiss laboratory for helpful advice and comments.
This work was supported in part by the Rosalind Russell Medical Research Center for Arthritis and by a grant (CA72531) from the National Cancer Institute.
REFERENCE
- 1.Arenzana-Seisdedos, F., B. Fernandez, I. Dominguez, J.-M. Jacque, D. Thomas, M. T. Diaz-Meco, J. Moscat, and J. L. Virelizier. 1993. Phosphatidylcholine hydrolysis activates NF-κB and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J. Virol. 67:6596-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunnell, S. C., D. I. Hong, J. R. Kardon, T. Yamazaki, C. J. McGlade, V. A. Barr, and L. E. Samelson. 2002. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158:1263-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ching, K. A., J. A. Grasis, P. Tailor, Y. Kawakami, T. Kawakami, and C. D. Tsoukas. 2000. TCR/CD3-induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. J. Immunol. 165:256-262. [DOI] [PubMed] [Google Scholar]
- 4.Ching, K. A., Y. Kawakami, T. Kawakami, and C. D. Tsoukas. 1999. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. J. Immunol. 163:6006-6013. [PubMed] [Google Scholar]
- 5.Dietrich, J., X. Hou, A. M. Wegener, and C. Geisler. 1994. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 13:2156-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellmeier, W., S. Jung, M. J. Sunshine, F. Hatam, Y. Xu, D. Baltimore, H. Mano, and D. R. Littman. 2000. Severe B cell deficiency in mice lacking the tec kinase family members Tec and Btk. J. Exp. Med. 192:1611-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finco, T. S., T. Kadlecek, W. Zhang, L. E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity 9:617-626. [DOI] [PubMed] [Google Scholar]
- 8.Fluckiger, A. C., Z. Li, R. M. Kato, M. I. Wahl, H. D. Ochs, R. Longnecker, J. P. Kinet, O. N. Witte, A. M. Scharenberg, and D. J. Rawlings. 1998. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 17:1973-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiai, M., M. Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity 10:117-125. [DOI] [PubMed] [Google Scholar]
- 10.Kane, L. P., P. G. Andres, K. C. Howland, A. K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2:37-44. [DOI] [PubMed] [Google Scholar]
- 11.Kanner, S. B., and J. J. Perez-Villar. 2003. Altering T-cell activation by targeting the multidomain tyrosine kinase Itk. Trends Immunol. 24:249-253. [DOI] [PubMed] [Google Scholar]
- 12.Kitanaka, A., H. Mano, M. E. Conley, and D. Campana. 1998. Expression and activation of the nonreceptor tyrosine kinase Tec in human B cells. Blood 91:940-948. [PubMed] [Google Scholar]
- 13.Kurosaki, T. 2002. Regulation of B-cell signal transduction by adaptor proteins. Nat. Rev. Immunol. 2:354-363. [DOI] [PubMed] [Google Scholar]
- 14.Latour, S., and A. Veillette. 2001. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 13:299-306. [DOI] [PubMed] [Google Scholar]
- 15.Liou, J., F. Kiefer, A. Dang, A. Hashimoto, M. H. Cobb, T. Kurosaki, and A. Weiss. 2000. HPK1 is activated by lymphocyte antigen receptors and negatively regulates AP-1. Immunity 12:399-408. [DOI] [PubMed] [Google Scholar]
- 16.Lucas, J. A., A. T. Miller, L. O. Atherly, and L. J. Berg. 2003. The role of Tec family kinases in T cell development and function. Immunol. Rev. 191:119-138. [DOI] [PubMed] [Google Scholar]
- 17.Miller, A. T., and L. J. Berg. 2002. New insights into the regulation and functions of Tec family tyrosine kinases in the immune system. Curr. Opin. Immunol. 14:331-340. [DOI] [PubMed] [Google Scholar]
- 18.Samelson, L. E. 2002. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371-394. [DOI] [PubMed] [Google Scholar]
- 19.Satterthwaite, A. B., Z. Li, and O. N. Witte. 1998. Btk function in B cell development and response. Semin. Immunol. 10:309-316. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer, E. M., G. S. Yap, C. M. Lewis, M. J. Czar, D. W. McVicar, A. W. Cheever, A. Sher, and P. L. Schwartzberg. 2001. Mutation of Tec family kinases alters T helper cell differentiation. Nat. Immunol. 2:1183-1188. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro, V. S., M. N. Mollenauer, W. C. Greene, and A. Weiss. 1996. c-rel regulation of IL-2 gene expression may be mediated through activation of AP-1. J. Exp. Med. 184:1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro, V. S., K. E. Truitt, J. B. Imboden, and A. Weiss. 1997. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol. Cell. Biol. 17:4051-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70:585-593. [DOI] [PubMed] [Google Scholar]
- 24.Takata, M., Y. Homma, and T. Kurosaki. 1995. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J. Exp. Med. 182:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takata, M., and T. Kurosaki. 1996. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma 2. J. Exp. Med. 184:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takesono, A., L. D. Finkelstein, and P. L. Schwartzberg. 2002. Beyond calcium: new signaling pathways for Tec family kinases. J. Cell Sci. 115:3039-3048. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson, M. G., T. Kurosaki, A. E. Berson, G. H. Fujii, J. A. Johnston, and J. B. Bolen. 1999. Reconstitution of Btk signaling by the atypical tec family tyrosine kinases Bmx and Txk. J. Biol. Chem. 274:13577-13585. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson, M. G., D. B. Woods, M. McMahon, M. I. Wahl, O. N. Witte, T. Kurosaki, J. B. Bolen, and J. A. Johnston. 8 June 2001, posting date. A conditional form of Bruton's tyrosine kinase is sufficient to activate multiple downstream signaling pathways via PLC gamma 2 in B cells. BMC Immunol. 2:4. [Online.] http://www.biomedcentral.com. [DOI] [PMC free article] [PubMed]
- 29.Weiss, A., and J. D. Stobo. 1984. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 160:1284-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams, B. L., K. L. Schreiber, W. Zhang, R. L. Wange, L. E. Samelson, P. J. Leibson, and R. T. Abraham. 1998. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 18:1388-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yablonski, D., M. R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-γ1 in an SLP-76-deficient T cell. Science 281:413-416. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita, Y., S. Watanabe, A. Miyazato, K. Ohya, U. Ikeda, K. Shimada, N. Komatsu, K. Hatake, Y. Miura, K. Ozawa, and H. Mano. 1998. Tec and Jak2 kinases cooperate to mediate cytokine-driven activation of c-fos transcription. Blood 91:1496-1507. [PubMed] [Google Scholar]
- 33.Yang, W. C., K. A. Ching, C. D. Tsoukas, and L. J. Berg. 2001. Tec kinase signaling in T cells is regulated by phosphatidylinositol 3-kinase and the Tec pleckstrin homology domain. J. Immunol. 166:387-395. [DOI] [PubMed] [Google Scholar]
- 34.Yang, W. C., M. Ghiotto, B. Barbarat, and D. Olive. 1999. The role of Tec protein-tyrosine kinase in T cell signaling. J. Biol. Chem. 274:607-617. [DOI] [PubMed] [Google Scholar]
- 35.Yang, W. C., M. Ghiotto, R. Castellano, Y. Collette, N. Auphan, J. A. Nunes, and D. Olive. 2000. Role of Tec kinase in nuclear factor of activated T cells signaling. Int. Immunol. 12:1547-1552. [DOI] [PubMed] [Google Scholar]
- 36.Yang, W. C., and D. Olive. 1999. Tec kinase is involved in transcriptional regulation of IL-2 and IL-4 in the CD28 pathway. Eur. J. Immunol. 29:1842-1849. [DOI] [PubMed] [Google Scholar]