Abstract
Lichens are a key component of forest biodiversity. However, a comprehensive study analyzing lichen species richness in relation to several management types, extending over different regions and forest stages and including information on site conditions is missing for temperate European forests. In three German regions (Schwäbische Alb, Hainich-Dün, Schorfheide-Chorin), the so-called Biodiversity Exploratories, we studied lichen species richness in 631 forest plots of 400 m2 comprising different management types (unmanaged, selection cutting, deciduous and coniferous age-class forests resulting from clear cutting or shelterwood logging), various stand ages, and site conditions, typical for large parts of temperate Europe. We analyzed how lichen species richness responds to management and habitat variables (standing biomass, cover of deadwood, cover of rocks). We found strong regional differences with highest lichen species richness in the Schwäbische Alb, probably driven by regional differences in former air pollution, and in precipitation and habitat variables. Overall, unmanaged forests harbored 22% more threatened lichen species than managed age-class forests. In general, total, corticolous, and threatened lichen species richness did not differ among management types of deciduous forests. However, in the Schwäbische-Alb region, deciduous forests had 61% more lichen species than coniferous forests and they had 279% more threatened and 76% more corticolous lichen species. Old deciduous age classes were richer in corticolous lichen species than young ones, while old coniferous age-classes were poorer than young ones. Overall, our findings highlight the importance of stand continuity for conservation. To increase total and threatened lichen species richness we suggest (1) conserving unmanaged forests, (2) promoting silvicultural methods assuring stand continuity, (3) conserving old trees in managed forests, (4) promoting stands of native deciduous tree species instead of coniferous plantations, and (5) increasing the amount of deadwood in forests.
Introduction
The main reasons for the ongoing decline of lichen species richness are habitat degradation caused by human interference such as disturbances by management activities, and air pollution. Together these factors have resulted in large numbers of threatened lichen species [1]–[3]. Because of their sensitivity to land-use and habitat changes lichens are very important environmental indicators [2]. In particular, rare and threatened species should be considered in conservation-oriented forest-management plans [4], [5]. Between the 1950’s and 1980’s, air pollution was high in certain parts of Europe (e.g. [6]). In particular the extent of sulfur dioxide (SO2) deposition, which reduced bark pH and regional lichen species pools [7]–[9], differed strongly among Central European regions. Hence, studying different regions is required to reach general conclusions about forest-management effects on lichen diversity.
Temperate European forests have long management histories, and forests without human influence are restricted to remote or inaccessible areas [10]. Hence, European forests are fragmented with large areas dominated by economically profitable age-class forests. These contain stands with even-aged tree structure, which had either resulted from clear cuts or from shelterwood logging. In the second case all trees of a stand are removed in a series of two cuts: first, approximately 60% of the tree cover is harvested, leaving scattered shelter trees for seedlings and secondly, after establishment of a closed stand cover of young trees, the remaining shelter trees are also removed. Such even-aged forest stands are often managed as plantations of fast growing conifers [11]. As both methods replace the whole stand by a new tree layer in a relatively short time period, stands are non-continuous in both cases. In recent years, silvicultural methods were promoted which attempt to mimic the natural-forest cycles typical for a particular vegetation zone. These methods focus on reproducing natural gap dynamics and regeneration and on promoting original vegetation, such as mixed forests dominated by deciduous tree species [10], [12]. In comparison to conventional clear cuts and shelterwood logging, which result in age-class forests, silvicultural methods such as selection cuttings assure continuous tree cover by harvesting single trees or small groups of trees, thus promoting uneven-aged stands. Selection cuttings should maintain and enhance biodiversity in forests whilst producing timber in an economically efficient way [12].
In Europe the area of protected forest reserves without management has recently been increased, mainly to conserve vulnerable and rare forest ecosystems, and to establish a reserve network [10]. However, because nearly all of these forests were formerly more or less intensively managed. Central European unmanaged forests are not comparable with natural forests in North America, Siberia or some parts of Eastern and Northern Europe which have been largely untouched by man for centuries [12]. In temperate European forests the efficacy of forest protection and of different silvicultural systems for maintaining lichen diversity is still poorly investigated, calling for a comprehensive analysis [13].
Lichens, symbiotic associations between mycobiontic and photobiontic partners, occur on a wide range of substrates in most terrestrial ecosystems of the world, including the bark of trees (corticolous lichens), soil (terricolous lichens), rocks (saxicolous lichens) and deadwood (lignicolous lichens). Nevertheless, many particular lichen species are restricted to a narrow ecological niche with specific requirements concerning substrate (e.g. bark, deadwood, rocks, soil), pH value, and nutrient status. Thus, particular habitats, and even successional stages within habitats, harbor distinctive lichen communities with successional variation in their lichen composition [2].
Case studies in North America and Europe showed higher corticolous and threatened lichen species richness in unmanaged than in managed forests [13]–[15]. In addition, it was suggested that silvicultural systems assuring stand continuity of forests, such as selection cutting or prolonged rotation periods, might maintain and increase lichen species richness and should therefore be favored over conventional forestry methods including clear cuts [16], [17].
Unfortunately, to date there have been no comprehensive, comparative studies from temperate European forests on the response of lichen species richness to management. No studies have included different regions, management types and developmental stages, along with detailed information on site conditions [13]. Furthermore, rather than addressing all lichen species in defined areas of differently managed forests, only corticolous species on individual trees were usually recorded [18], [19]. In addition, studies on the effects of stand age or stand characteristics on lichen species richness are rare outside Fennoscandia, where case studies have been carried out (e.g. [20]–[23]).
We present a comparative study analyzing the response of the species richness of all lichens, of lichens separated by substrate (corticolous, lignicolous, saxicolous), and of threatened lichens to management and habitat variables (standing biomass, cover of deadwood, cover of rocks). This is the most extensive lichen dataset from Central Europe to date.
Our main questions are:
(1) How does lichen species richness respond to forest management?
(2) How does lichen species richness respond to habitat variables?
Materials and Methods
Study system
This study was conducted as part of the Biodiversity Exploratories project (www.biodiversity-exploratories.de) in three German regions: (1) the UNESCO Biosphere area Schwäbische Alb (Swabian Jura), situated in the low mountain ranges of South-western Germany (2) the National Park Hainich and its surrounding areas, situated in the hilly lands of Central Germany, and (3) the UNESCO Biosphere Reserve Schorfheide-Chorin, situated in the young glacial lowlands of North-eastern Germany. The three study regions differ in climate, geology, and topographical situations and harbor land uses as well as species pools typical for large parts of temperate Europe ([24]; Table 1). Past mean annual SO2 depositions had been low in the Schwäbische Alb, high in Schorfheide-Chorin, and very high in Hainich-Dün ([25]; Table 1).
Table 1. Main geographic and habitat characteristics of the three Biodiversity Exploratories.
| Schwäbische Alb | Hainich-Dün | Schorfheide-Chorin | |
| Location | SW Germany | Central Germany | NE Germany |
| Size | ∼422 km2 | ∼1300 km2 | ∼1300 km2 |
| Geology | Calcareous bedrock | Calcareous bedrock | Young glacial landscape |
| Altitude a.s.l. | 460–860 m | 285–550 m | 3–140 m |
| Annual mean temperature | 6.0–7.0 °C | 6.5–8.0 °C | 8.0–8.5 °C |
| Annual mean precipitation | 700–1000 mm | 500–800 mm | 500–600 mm |
| SO2 deposition | |||
| until 1985 | 25–<50 µg/m3 | >150 µg/m3 | 25–<50 µg/m3 |
| 1985 to 1990 | <25 µg/m3 | >150 µg/m3 | 50–<75 µg/m3 |
| 1990 to 1995 | <25 µg/m3 | 25–<50 µg/m3 | 50–<75 µg/m3 |
| since 1995 | <25 µg/m3 | <25 µg/m3 | <25 µg/m3 |
| Number of plots | 152 | 172 | 307 |
| Standing biomass [m3/ha] | |||
| mean (SD) | 336.7 (187.8) | 408.6 (188.9) | 444.8 (187.0) |
| range | 1.2–1017.4 | 3.9–881.5 | 18.3–1001.5 |
| Cover deadwood [%] | |||
| mean (SD) | 3.6 (3.0) | 3.1 (2.8) | 3.8 (3.6) |
| range | 0.5–20.0 | 0.5–15.0 | 0.5–25.0 |
| Cover rocks [%] | |||
| mean (SD) | 1.0 (1.6) | 0.2 (0.5) | 0.3 (0.6) |
| range | 0.0–11.0 | 0.0–4.0 | 0.0–5.5 |
Plot selection
Each region, of at least 20 km by 30 km, contains more than 500 forest plots selected from the intersection points of a 100 m×100 m grid, after discarding plots fully or partially overlapping with settlements, grasslands, agricultural fields, water bodies and plots intersected by roads [24]. From these plots, we randomly selected 631 plots for this study, which cover all management types in each region: 152 in the Schwäbische Alb, 172 in Hainich-Dün, and 307 in Schorfheide-Chorin. Thus, we consider our plot sample as unbiased with regard to studying differences in forest management.
Management data
To assess the management system and stand characteristics of forests, a forest inventory had been conducted on a circular area of 500 m2 (radius 12.62 m) in each plot. Unmanaged forests were mature, deciduous forests dominated mainly by European beech (Fagus sylvatica). Age-class forests were dominated by European beech, Norway spruce (Picea abies) or Scots pine (Pinus sylvestris) and had different developmental stages of even-aged structure due to harvests at 80- to 120-year intervals. Selection forests were uneven-aged deciduous stands dominated by European beech, in which single or small groups of trees were harvested selectively. As stand characteristics, we counted the number of trees (>7 cm diameter at breast height; DBH), measured their DBH, and their height using an ultrasonic tree height meter (Vertex III Forester, Haglöf, Langsele, Sweden). We then calculated standing biomass (m3/ha) using height and DBH of each occurring tree accounting for tree species specific trunk shapes (for details see [26]). Standing biomass can be used as a combined indicator for both tree densities (negative relation with standing biomass; [27]) and stand age (positive relation with standing biomass). We additionally recorded the percentage ground covered by rocks and deadwood respectively as indicators of substrate quantity. Furthermore, we recorded the occurrence of logging trails. Spacing of logging trails turned out to be about 20 m in coniferous and about 40 m in deciduous forests.
Vegetation data
During 2007 and 2008 the first author recorded lichens in all 631 plots. In each case lichens were recorded on 20 m×20 m (in the center of each plot and concentric with the forest inventory circle). All lichen species per plot were identified and lichens were also recorded separately for each of the four substrate categories: bark (corticolous species, up to 2.5 m height on tree trunks and branches of shrubs), rocks (saxicolous species), deadwood (lignicolous species), and soil (terricolous species), resulting in total and substrate-specific richness values. Further, we obtained the number of lichen species classified as critically endangered to vulnerable in the red list of threatened lichens of Germany [1]. As we recorded very few terricolous lichen species we considered them when analyzing total lichen species richness but, in contrast to the other groups, did not analyze them separately.
As an additional measurement of habitat diversity for corticolous lichen species, we recorded the number of tree species (>5 m height) per plot, estimated their percentage cover and summed these cover estimates (cumulative tree cover). Across age-class forests we used the proportion of coniferous tree cover, to separate coniferous (≥70%; N = 115) and deciduous age-class forests (including mixed and pure deciduous stands; N = 379).
Statistical analysis
We analysed the response of lichen species richness to management and habitat variables. Response variables were the species richness of all lichens and the species richness of the separate lichen groups (corticulous, saxiculous and ligniculous). Explanatory variables were management type, total cover of rocks, total cover of deadwood, standing biomass, age of the oldest tree per plot (max. DBH/plot), the number of tree species, and cumulative tree cover. As we were analyzing count data we used GLM models with quasi-Poisson errors to correct for overdispersion. We used subsets of the data for three separate analyses to compare effects of different management types: (1) unmanaged vs. age-class forests (all regions), (2) unmanaged vs. deciduous age-class vs. selection forests (without Schorfheide-Chorin, where no selection forests were available in the dataset), and (3) coniferous age-class vs. deciduous age-class forests (all regions). Furthermore, we included interactions with region, management type and standing biomass. Sequential F-tests were used to test the significance of deviance changes associated factors added progressively to the model (the sequence is shown in Tables 2, 3, 4). Covariables (cover of rocks and cover of deadwood) were fitted before management, meaning that the management effect is corrected for these variables and effects of management on lichens are not due to differences in the amount of deadwood or rocks between management types. As the number of tree species per plot and the occurrence of logging trails had no effect on lichen species richness in our study, we removed these variables from the analyses. We also excluded maximum DBH and cumulative tree cover because these were correlated with standing biomass (for both variables: r = 0.623, p < 0.0001). Data were analyzed using R, Version 2.13.1 [28].
Table 2. GLM results for differences in lichen species richness between 86 unmanaged and 494 age-class forests.
| Species richness | |||||||||||
| All lichens | Threatened lichens | Corticolous lichens | Saxicolous lichens | Lignicolous lichens | |||||||
| Source of variation | df | F | p | F | p | F | p | F | p | F | p |
| Region | 2 | 435.42 | <0.001 | 275.47 | <0.001 | 301.85 | <0.001 | 57.135 | <0.001 | 41.538 | <0.001 |
| Rock cover | 1 | 23.75 | <0.001 | 16.60 | <0.001 | –– | –– | 138.140 | <0.001 | –– | –– |
| Deadwood cover | 1 | 1.68 | 0.196 | 16.87 | <0.001 | –– | –– | –– | –– | 11.704 | 0.001 |
| Management | |||||||||||
| Management (unmanaged vs. age class) | 1 | 0.32 | 0.572 | 20.61 | <0.001 | 0.424 | 0.515 | 23.047 | <0.001 | 5.460 | 0.020 |
| Standing biomass | 1 | 3.43 | 0.065 | 7.11 | 0.008 | 14.970 | <0.001 | 0.005 | 0.944 | 25.587 | <0.001 |
| Management × standing biomass | 1 | 0.06 | 0.812 | 0.25 | 0.615 | 0.160 | 0.690 | 0.697 | 0.404 | 2.265 | 0.133 |
| Regional interactions | |||||||||||
| Region × rock cover | 2 | 0.18 | 0.839 | 3.76 | 0.024 | –– | –– | 13.711 | <0.001 | –– | –– |
| Region × deadwood cover | 2 | 13.00 | <0.001 | 2.85 | 0.059 | –– | –– | –– | –– | 0.036 | 0.965 |
| Region × management | 2 | 1.24 | 0.290 | 4.38 | 0.013 | 2.332 | 0.098 | 10.800 | <0.001 | 0.412 | 0.663 |
| Region × standing biomass | 2 | 1.25 | 0.287 | 5.07 | 0.007 | 1.710 | 0.182 | 2.330 | 0.098 | 4.003 | 0.019 |
| Residual Deviance | ≥564 | 1072.40 | 814.86 | 1045.13 | 605.33 | 840.47 | |||||
Significant differences are indicated by bold p values.
Table 3. GLM results for differences in lichen species richness among 516 deciduous forests (86 unmanaged vs. 379 age-class vs. 51 selection forests).
| Species richness | ||||||||||||
| All lichens | Threatened lichens | Corticolous lichens | Saxicolous lichens | Lignicolous lichens | ||||||||
| Source of variation | df | F | p | F | p | F | p | F | p | F | p | |
| Region | 1 | 717.40 | <0.001 | 479.84 | <0.001 | 495.94 | <0.001 | 133.81 | <0.001 | 26.51 | <0.001 | |
| Rock cover | 1 | 4.85 | 0.028 | 5.70 | 0.018 | –– | –– | 83.47 | <0.001 | –– | –– | |
| Deadwood cover | 1 | 2.54 | 0.112 | 0.65 | 0.423 | –– | –– | –– | –– | 6.33 | 0.012 | |
| Management | ||||||||||||
| Management (Unmanaged vs. age class vs. selection) | 2 | 0.31 | 0.731 | 7.15 | 0.001 | 0.34 | 0.714 | 2.14 | 0.119 | 0.23 | 0.794 | |
| Standing biomass | 1 | 10.19 | 0.002 | 19.74 | <0.001 | 22.12 | <0.001 | 0.14 | 0.712 | 22.72 | <0.001 | |
| Management × standing biomass | 2 | 3.81 | 0.023 | 1.40 | 0.248 | 1.66 | 0.193 | 3.00 | 0.052 | 1.34 | 0.263 | |
| Regional interactions | ||||||||||||
| Region × rock cover | 1 | 0.03 | 0.859 | 4.39 | 0.037 | –– | –– | 14.26 | <0.001 | –– | –– | |
| Region × deadwood cover | 1 | 10.20 | 0.002 | 1.33 | 0.250 | –– | –– | –– | –– | 0.41 | 0.521 | |
| Region × management | 2 | 4.45 | 0.013 | 5.46 | 0.005 | 7.97 | <0.001 | 4.68 | 0.010 | 0.10 | 0.908 | |
| Region × standing biomass | 1 | 0.02 | 0.901 | 3.83 | 0.051 | 0.50 | 0.481 | 0.14 | 0.709 | 0.00 | 0.983 | |
| Residual Deviance | ≥272 | 540.05 | 370.10 | 493.88 | 359.15 | 332.63 | ||||||
Significant differences are indicated by bold p values.
Table 4. GLM results for differences in lichen species richness between 379 deciduous and 115 coniferous age-class forests.
| Species richness | |||||||||||
| All lichens | Threatened lichens | Corticolous lichens | Saxicolous lichens | Lignicolous lichens | |||||||
| Source of variation | df | F | p | F | p | F | p | F | p | F | p |
| Region | 2 | 392.86 | <0.001 | 284.51 | <0.001 | 277.88 | <0.001 | 47.73 | <0.001 | 35.86 | <0.001 |
| Rock cover | 1 | 27.88 | <0.001 | 19.58 | <0.001 | –– | –– | 83.02 | <0.001 | –– | –– |
| Deadwood cover | 1 | 1.44 | 0.231 | 14.39 | <0.001 | –– | –– | –– | –– | 7.28 | 0.007 |
| Management | |||||||||||
| Management (deciduous vs. coniferous age class) | 1 | 16.20 | <0.001 | 63.94 | <0.001 | 39.13 | <0.001 | 5.28 | 0.022 | 26.00 | <0.001 |
| Standing biomass | 1 | 4.34 | 0.038 | 14.15 | <0.001 | 16.81 | <0.001 | 0.29 | 0.592 | 25.82 | <0.001 |
| Management × standing biomass | 1 | 11.62 | 0.001 | 20.67 | <0.001 | 10.12 | 0.002 | 3.11 | 0.078 | 0.68 | 0.411 |
| Regional interactions | |||||||||||
| Region × rock cover | 2 | 0.35 | 0.704 | 3.22 | 0.041 | –– | –– | 6.21 | 0.002 | –– | –– |
| Region × deadwood cover | 2 | 9.30 | <0.001 | 2.26 | 0.105 | –– | –– | –– | –– | 0.20 | 0.817 |
| Region × management | 2 | 6.07 | 0.002 | 0.92 | 0.401 | 8.34 | <0.001 | 3.04 | 0.049 | 1.10 | 0.334 |
| Region × standing biomass | 2 | 0.20 | 0.821 | 2.06 | 0.129 | 0.05 | 0.953 | 1.03 | 0.357 | 7.00 | 0.001 |
| Residual Deviance | ≥478 | 896.93 | 582.89 | 846.33 | 435.47 | 688.15 | |||||
Significant differences are indicated by bold p values.
Results
Overall and regional lichen species richness
We recorded 202 lichen species, including 73 which are threatened in Germany. Of these 202 species, 124 were corticolous, 84 lignicolous, 59 saxicolous and 18 terricolous. Across all 631 plots the species richness of corticolous lichens was positively correlated with the species richness of lignicolous and saxicolous lichens (lignicolous versus corticolous, r = 0.0838, p = 0.0354; corticolous versus saxicolous, r = 0.3091, p < 0.0001).
In the Schwäbische Alb region we recorded 177 species, in Hainich-Dün 59, and in Schorfheide-Chorin 70. Thirty-three of the recorded species were found in all three regions, 52 species were shared by the Schwäbische Alb and Hainich-Dün regions, 47 by the Schwäbische Alb and Schorfheide-Chorin regions, and 36 by the Hainich-Dün and Schorfheide-Chorin regions. One-hundred and eleven species were recorded exclusively in the Schwäbische Alb region, 7 in Hainich-Dün, and 23 in Schorfheide-Chorin.
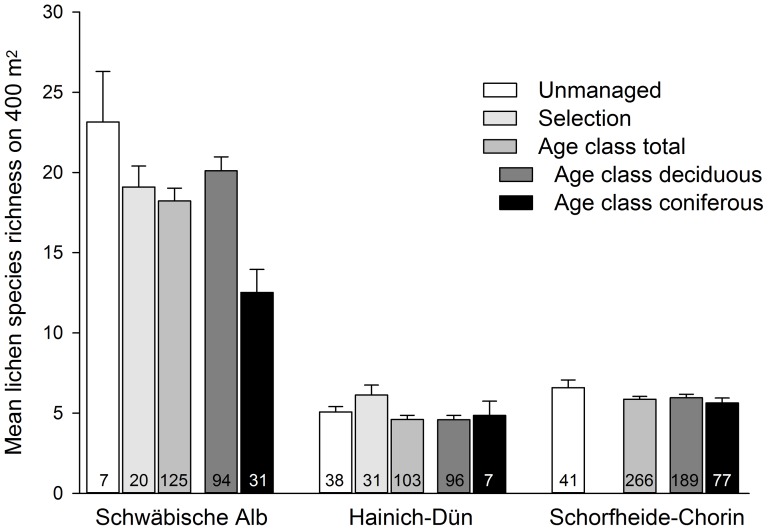
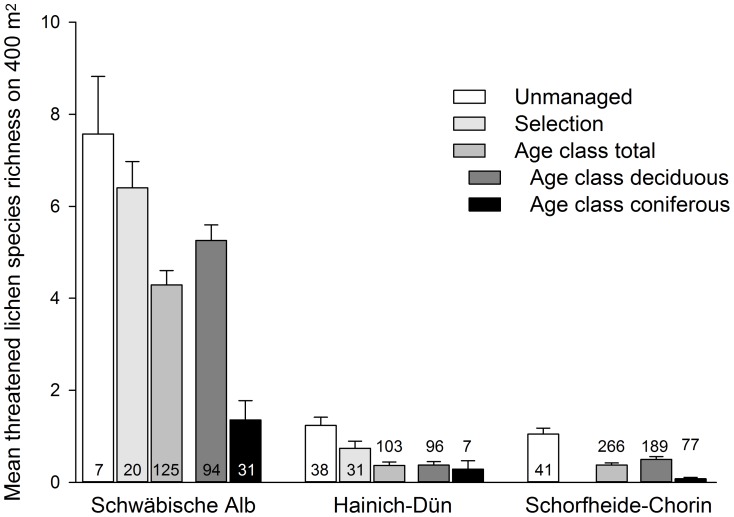
Species richness per plot of all lichens and of threatened lichens was significantly higher in the Schwäbische Alb than in both other regions (Figures 1, 2). Total species richness per plot was on average 18.6 (SD 8.6) in the Schwäbische Alb, 5.0 (2.7) in Hainich-Dün, and 6.0 (3.0) in Schorfheide-Chorin.
Figure 1. Mean lichen species richness (+SE) per plot for each of the forest management types in the three study regions.
Figure 2. Mean species richness of threatened lichens (+SE) per plot for each of the forest management types in the three study regions.
Management effects on lichen species richness
Total and corticolous lichen species richness did not differ between unmanaged and age-class forests. However, unmanaged forests harbored 21.9% more threatened lichen species than managed age-class forests (Tables 2, 5).
Table 5. Mean lichen species richness per 400 m2 (untransformed mean ±SE) in unmanaged and age-class forests, and in deciduous and coniferous age-class forests, in total and for the three study regions.
| N | All lichens | Threatened lichens | Corticolous lichens | Saxicolous lichens | Lignicolous lichens | ||||||
| Total | |||||||||||
| Unmanaged | 86 | 7.3 | (0.6) | 1.7 | (0.2) | 5.4 | (0.4) | 1.3 | (0.3) | 0.6 | (0.1) |
| Age classtotal | 494 | 8.7 | (0.3) | 1.4 | (0.1) | 6.9 | (0.3) | 0.5 | (0.1) | 1.2 | (0.1) |
| Age classdeciduous | 379 | 9.1 | (0.4) | 1.6 | (0.1) | 7.3 | (0.3) | 0.5 | (0.1) | 0.9 | (0.1) |
| Age classconiferous | 115 | 7.4 | (0.5) | 0.4 | (0.1) | 5.4 | (0.4) | 0.2 | (0.1) | 2.0 | (0.2) |
| Schwäbische Alb | |||||||||||
| Unmanaged | 7 | 23.1 | (3.2) | 7.6 | (1.3) | 14.0 | (2.6) | 6.9 | (2.2) | 0.7 | (0.4) |
| Age classtotal | 125 | 18.2 | (0.8) | 4.3 | (0.3) | 13.8 | (0.7) | 1.2 | (0.2) | 1.2 | (0.2) |
| Age classdeciduous | 94 | 20.1 | (0.9) | 5.3 | (0.3) | 15.4 | (0.7) | 1.3 | (0.2) | 1.0 | (0.2) |
| Age classconiferous | 31 | 12.5 | (1.4) | 1.4 | (0.4) | 8.7 | (1.2) | 0.7 | (0.2) | 1.8 | (0.4) |
| Hainich-Dün | |||||||||||
| Unmanaged | 38 | 5.1 | (0.3) | 1.2 | (0.2) | 4.7 | (0.3) | 0.1 | (0.1) | 0.3 | (0.1) |
| Age classtotal | 103 | 4.6 | (0.3) | 0.4 | (0.1) | 3.8 | (0.2) | 0.4 | (0.1) | 0.3 | (0.1) |
| Age classdeciduous | 96 | 4.6 | (0.3) | 0.4 | (0.1) | 3.8 | (0.2) | 0.4 | (0.1) | 0.3 | (0.1) |
| Age classconiferous | 7 | 4.9 | (0.9) | 0.3 | (0.2) | 4.0 | (1.0) | 0.0 | (0.0) | 0.4 | (0.4) |
| Schorfheide-Chorin | |||||||||||
| Unmanaged | 41 | 6.6 | (0.5) | 1.0 | (0.1) | 4.5 | (0.3) | 1.6 | (0.2) | 0.9 | (0.2) |
| Age classtotal | 266 | 5.9 | (0.2) | 0.4 | (0.0) | 4.8 | (0.2) | 0.2 | (0.0) | 1.5 | (0.1) |
| Age classdeciduous | 189 | 6.0 | (0.2) | 0.5 | (0.1) | 5.1 | (0.2) | 0.2 | (0.0) | 1.2 | (0.1) |
| Age classconiferous | 77 | 5.6 | (0.3) | 0.1 | (0.0) | 4.2 | (0.2) | 0.0 | (0.0) | 2.2 | (0.2) |
In general, total and corticolous lichen species richness did not differ among management types of deciduous stands. However, among those stands the species richness of threatened lichens was highest in unmanaged, intermediate in selection and lowest in age-class forests (Tables 3, 6). In general, threatened lichen species richness was higher in deciduous than in coniferous age-class forests. This was also the case for corticolous lichen species richness in the Schwäbische Alb and Schorfheide-Chorin, and for the total lichen species richness only in the Schwäbische Alb. In contrast, the species richness of lignicolous lichens was generally higher in coniferous than in deciduous age-class forests (Tables 4, 5; Figures 1, 2). Thus, promoting stands with site-typical composition of tree species appears important for promoting lichen species richness and threatened lichen species in forests.
Table 6. Mean lichen species richness per 400 m2 (untransformed mean ±SE) in unmanaged and differently managed deciduous forests in total, and for the Schwäbische Alb and Hainich-Dün regions.
| N | All lichens | Threatened lichens | Corticolous lichens | Saxicolous lichens | Lignicolous lichens | ||||||
| Total | |||||||||||
| Unmanaged | 45 | 7.9 | (1.1) | 2.2 | (0.4) | 6.2 | (0.7) | 1.2 | (0.5) | 0.4 | (0.1) |
| Age classdeciduous | 190 | 12.3 | (0.7) | 2.8 | (0.2) | 9.5 | (0.6) | 0.8 | (0.1) | 0.7 | (0.1) |
| Selectiondeciduous | 51 | 11.2 | (1.1) | 3.0 | (0.5) | 8.1 | (0.8) | 1.7 | (0.3) | 0.5 | (0.1) |
| Schwäbische Alb | |||||||||||
| Unmanaged | 7 | 23.1 | (3.2) | 7.6 | (1.3) | 14.0 | (2.6) | 6.9 | (2.2) | 0.7 | (0.4) |
| Age classdeciduous | 94 | 20.1 | (0.9) | 5.3 | (0.3) | 15.4 | (0.7) | 1.3 | (0.2) | 1.0 | (0.2) |
| Selectiondeciduous | 20 | 19.1 | (1.3) | 6.4 | (0.6) | 12.7 | (1.1) | 4.0 | (0.5) | 0.7 | (0.2) |
| Hainich-Dün | |||||||||||
| Unmanaged | 38 | 5.1 | (0.3) | 1.2 | (0.2) | 4.7 | (0.3) | 0.1 | (0.1) | 0.3 | (0.1) |
| Age classdeciduous | 96 | 4.6 | (0.3) | 0.4 | (0.1) | 3.8 | (0.2) | 0.4 | (0.1) | 0.3 | (0.1) |
| Selectiondeciduous | 31 | 6.1 | (0.6) | 0.7 | (0.2) | 5.1 | (0.5) | 0.2 | (0.1) | 0.4 | (0.1) |
In the Schwäbische Alb, species richness of saxicolous lichens was 428% higher in unmanaged than in deciduous age-class forests and also 73% higher than in selection forests (Tables 3, 6).
Standing biomass and lichen species richness
Overall, old forests with large quantities of standing biomass were slightly richer in corticolous lichen species than were young forests with low quantities of standing biomass: we found an increase of 1.2 species with an increase of 500m3 standing biomass per ha (Table 2). Among deciduous stands this relationship was even more pronounced (+2.0 species/500 m3). Furthermore, threatened lichen species richness increased with standing biomass, more strongly for the Schwäbische Alb (+2.6 species/500 m3) than for the Hainich-Dün (+0.6 species/500 m3), as indicated by the significant region - by - standing biomass interaction (Table 3). Overall, we found opposing effects of standing biomass on the richness of corticolous and threatened lichen species between deciduous and coniferous age-class forests (significant standing biomass - by - management interaction; Table 4). High standing biomass was associated with higher richness of corticolous (+2.0 species/500 m3) and of threatened lichen species (+0.7 species/500 m3) in deciduous stands but with lower species richness of corticolous (-1.3 species/500 m3) and of threatened lichen species (-1.2 species/500 m3) in coniferous stands (Table 4). These findings indicate that the conservation of old forests dominated by native broadleaved tree species might enhance species richness of corticolous lichens and promote suitable habitats for threatened lichen species. Interestingly, the richness of lignicolous lichen species decreased (-0.7 species/500 m3) with an increase in standing biomass (Tables 2, 3, 4). This was probably because of higher amounts of deadwood in younger stands due to recent timber harvesting.
Cover by deadwood and rocks and lichen species richness
Overall, deadwood cover decreased with standing biomass (N = 631, r = -0.1762; p < 0.0001). Lignicolous lichen species richness was generally positively related to the cover of deadwood, increasing by 0.7 species per 10% increased deadwood cover (Tables 2, 3, 4). These findings indicate that increasing the amount of deadwood in forests may lead to an increase of lichen species richness.
Overall, species richness of saxicolous lichens increased by 1.2 species per 1% increased rock cover (Tables 2, 3, 4). However, effects varied among regions and management types due to varying cover values (Table 1).
Discussion
Differences in lichen species richness among the regions
Our results showed strong differences in lichen species richness between the Schwäbische Alb and the two other regions, although all forests have similar management methods. These differences are not related to the protection status of the regions or to management activities but rather to the former intensity of atmospheric pollutants, especially SO2, which was responsible for the decline of many lichen species in Germany [1]. Until the strong decrease of SO2 pollution around 1990 in Germany, SO2 deposition was low in the Schwäbische Alb, high in Schorfheide-Chorin and very high in Hainich-Dün ([25]; Table 1). Regionally, this resulted in a shift in lichen species composition to “lichen deserts” with only a few toxitolerant species [7], [8]. Today, levels of SO2 pollution have considerably decreased and many species are re-colonizing these areas [7], [9]. However, the characteristic lichen communities have not yet recovered completely and therefore current lichen community composition in these areas might still be influenced by former air pollution. Furthermore, the higher mean rock cover and mean annual precipitation in the Schwäbische Alb than in the other regions (Table 1), might also contribute to the much higher lichen species richness in the Schwäbische Alb.
Management effects on lichen species richness
In our study, total and corticolous lichen species richness did not differ between unmanaged and age-class forest sites. Bergamini et al. [15] and Paillet et al. [13], who used much smaller datasets from other European countries, found higher total and corticolous lichen species richness in unmanaged compared with managed forests. Our results also contrast with those of Friedel et al. [18], who found higher species richness of corticolous lichens in 45 unmanaged than in 45 managed European beech forests in northeastern Germany, and with the findings of Rudolphi and Gustafsson [29], who compared 19 unmanaged and 19 young stands, originating from clear-cutting, in boreal forests in Sweden. The differences among the studies might be because the investigated unmanaged stands that we surveyed had not yet reached the degeneration phase and still showed signs of former management, such as a fairly even-aged structure and a dense canopy cover (as outlined in [30]). However, overall, unmanaged forests harbored more threatened lichen species than managed age-class forests. This confirms the particular importance of unmanaged forests for the conservation of lichen species with long generation times [3] or with specific habitat requirements that mean they are restricted to old growth forests [21]. Other management-related disturbances such as logging trails had no negative effects on lichen species richness, showing that management involving forestry equipment does not need to reduce the species richness of lichens if suitable habitats are spared.
We found no differences in total and corticolous lichen species richness among deciduous forests. However, we did find highest species richness of threatened lichens in unmanaged forests, with intermediate richness in selection forests, and the lowest richness in age-class forests. This result underlines the importance of silvicultural methods which ensure temporal forest continuity for many lichen species. This is line with several other studies pointing out the importance of stand continuity to preserve lichen species richness and communities with rare or threatened lichen species [13], [22], [31]–[34]. Alternatively, patches of old trees could be retained in forests to conserve lichen species richness in managed stands. Peterson and McCune [35] compared 51 forest stands in Oregon and found that retention patches or old trees were essential for the persistence of lichen communities that depend on old-growth forest conditions.
Our study provides further evidence for the idea that older forests harbor more lichen species and particularly more threatened species [5], [36]–[39]. These positive effects might probably be explained by characteristics of old trees which make them better lichen habitats. These include, a larger bark surface which increases the probability of colonization [40], pronounced bark textures including crevices [22], [40] and rot holes [41], which provide a range of microhabitats, and the fact that many lichen species associated with old-growth conditions can only establish on older trees [42]. The retention of numerous mature to over-mature trees in forests appears important for maintaining a high species richness of epiphytic lichens [43]–[45] and to conserve rare and threatened lichen species in managed forests [21], [29], [46], [47]. These trees can act as population centers for the dispersal of lichen propagules and provide refuges for those species which depend on old trees [3], [48]–[50].
Interestingly, species richness of lignicolous lichens decreased with higher standing biomass. Most likely, this finding was due to the higher amounts of deadwood, following recent timber harvesting, in our younger stands of low standing biomass than in our older stands of high standing biomass. Thus, we suggest that increasing the amount of deadwood in older forests may lead to an increase in lichen species richness, in agreement with the findings of Moning et al. [19] based on 113 plots within the National Park Bavarian Forest (Germany).
Clear cutting generally replaced site-characteristic forests with even-aged and homogeneous plantations often with different tree species and this had a very pronounced effect on lichen species richness and composition [3], [44], [51]. In general, we found more threatened lichen species in deciduous forests than in coniferous forests. In the Schwäbische Alb we also found more corticolous lichen species in deciduous forests. This is in line with the findings of Humphrey et al. [36] who observed higher lichen species richness in native deciduous stands than in conifer plantations in different forest sites in Britain. Furthermore, Neitlich and McCune [52] pointed out the importance of deciduous tree patches interspersed in young coniferous plantations for both lichen species richness and the species richness of specialized lichens with high conservation value. Thus, promoting stands or retaining patches with a site-typical composition of tree species appears important for promoting lichen species richness and threatened lichen species in forests.
Rock cover and lichen species richness
Overall we found positive relations between rock cover and the species richness of saxicolous lichens. Clearly, not just the presence but also the quantity of this substrate matters for lichen species richness. As managed stands occur on more easily accessible, flat sites where rocks are scarce, the higher species richness of saxicolous lichens in unmanaged than in age-class or selection forests might well be related to the lower rock quantity in unmanaged forests, rather than to the absence of management per se.
Deadwood cover and lichen species richness
In our study, the species richness of lignicolous lichens generally increased with increasing cover of deadwood. Similarly, in their deadwood-focused study Caruso et al. [53] reported a positive relationship between deadwood volume and the species richness of lignicolous lichens in 30 stands of planted boreo-nemoral Swedish forests. Thus, similar to Moning et al. [19] and Humphrey et al. [36] we recommend actively enhancing deadwood quantity, diversity of types and decay stages of standing deadwood and deadwood on the ground.
Lichen species richness in tree crowns
We probably have underestimated the overall lichen richness because we could not assess species restricted to tree crowns, as this would have required lengthy tree climbing of thousands of trees. According to this difficulty, we are not aware of any other study that attempted to assess lichen diversity in tree crowns of differently managed forests.
Conclusions
Our findings demonstrate the importance of management systems that ensure stand continuity for lichen conservation. Clearly, the conservation of old forests with high standing biomass is absolutely necessary to maintain a high species richness of lichens and to promote threatened lichen species. To increase total and threatened lichen species richness without overly compromising timber production, our results, combined with those of the other studies discussed above, lead us to the following recommendations for managed forests: (1) to promote silvicultural methods that assure stand continuity, e.g. by selection cutting rather than clear cutting and shelterwood logging, (2) to conserve retention patches with groups of old, mature to over-mature trees in managed forests, (3) to conserve at least single mature to over-mature trees which may serve as sources for colonization, (4) to select, as far as visibly obvious, such retention trees of high lichen abundance and species richness composition and population size rather than selecting them at random, (5) to promote stands of native deciduous tree species instead of coniferous plantations, and (6) to increase the amount of deadwood in forests.
Acknowledgments
We thank S. Gockel, A. Hemp, K. Wells, and S. Pfeiffer for maintaining the plot and project infrastructure, and the late E.K.V. Kalko, K.E. Linsenmair, J. Nieschulze, I. Schöning, F. Buscot, and W.W. Weisser for their role in setting up the Biodiversity Exploratories project. Furthermore, we thank V. Otte and L.B. Sparrius for verification of the identity of some critical species, and E. Allan for revising the English. Field work permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG).
Funding Statement
The DFG (German Research Foundation; http://www.dfg.de/en/index.jsp) funded the study in the framework of the Biodiversity Exploratories SSP 1374 "Infrastructure-Biodiversity-Exploratories" (FI 1246/6-1, FI 1246/9-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wirth V, Schöller H, Scholz P, Feuerer T, Gnüchtel P, et al. (1996) Rote Liste der Flechten (Lichenes) der Bundesrepublik Deutschland. In: Bundesamt für Naturschutz, editor. Schriftenreihe Vegetationskunde 28, Rote Liste gefährdeter Pflanzen Deutschlands. Bundesamt für Naturschutz, pp. 307–368.
- 2. Seaward MRD (2008) Environmental role of lichens. In: Lichen Biology Nash TH III, editor. Cambridge: Cambridge Univ. Press, Ed; 2.: 274–298. [Google Scholar]
- 3. Scheidegger C, Werth S (2009) Conservation strategies for lichens: insights from population biology. Fungal Biology Reviews 23: 55ss66. [Google Scholar]
- 4. Thor G (1995) Red Lists–aspects of their compilation and use in lichen conservation. Mitt Eidgenöss Forsch anst Wald Schnee Landsch 70: 29–39. [Google Scholar]
- 5. Gustafsson L, Appelgren L, Jonsson F, Nordin U, Persson A, et al. (2004) High occurrence of red-listed bryophytes and lichens in mature managed forests in boreal Sweden. Basic Appl Ecol 5: 123–129. [Google Scholar]
- 6. Berge E, Jakobsen HA (1998) A regional scale multi-layer model for the calculation of long-term transport and deposition of air pollution in Europe. Tellus 50: 205–223. [Google Scholar]
- 7.Gilbert OL (1992) Lichen reinvasion with declining air pollution. In: Bates JW,Farmer A, editors. Bryophytes and Lichens in a Changing Environment.Oxford:Clarendon Press, pp. 159–177.
- 8. Kirschbaum U, Windisch U, Vorbeck A, Hanewald K (2006) Mapping lichen diversity in Wetzlar and Giessen as an indicator of air quality–Comparison between the surveys of 1970, 1985, 1995 and 2005. Gefahrenst Reinhalt Luft 66: 272–280. [Google Scholar]
- 9. Purvis OW, Tittley I, Chimonides PDJ, Bamber R, Hayes PA, et al. (2010) Long-term biomonitoring of lichen and bryophyte biodiversity at Burnham Beeches SAC and global environmental change. Syst Biodivers 8: 193–208. [Google Scholar]
- 10. Parviainen J, Bücking W, Vandekerkhove K, Schuck A, Päivinen R (2000) Strict forest reserves in Europe: efforts to enhance biodiversity and research on forests left for free development in Europe (EU-COST-Action E4). Forestry 73: 107–118. [Google Scholar]
- 11.Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen.–6 th edition. Stuttgart:Ulmer.1357 p.
- 12. Parviainen J, Frank G (2003) Protected forests in Europe–approaches harmonising the definitions for international comparison and forest policy making. J Environ Manage 67: 27–36. [DOI] [PubMed] [Google Scholar]
- 13. Paillet Y, Berges L, Hjalten J, Odor P, Avon C, et al. (2010) Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv Biol 24: 101–112. [DOI] [PubMed] [Google Scholar]
- 14. Lesica P, McCune B, Cooper SV, Hong WS (1991) Differences in lichen and bryophyte communities between old-growth and managed second-growth forests in the Swan Valley, Montana. Can J Bot 69: 1745–1755. [Google Scholar]
- 15. Bergamini A, Scheidegger C, Stofer S, Carvalho P, Davey S, et al. (2005) Performance of macrolichens and lichen genera as indicators of lichen species richness and composition. Conserv Biol 19: 1051–1062. [Google Scholar]
- 16. Horstkotte T, Moen J, Lämås T, Helle T (2011) The Legacy of Logging–Estimating Arboreal Lichen Occurrence in a Boreal Multiple-Use Landscape on a Two Century Scale. PLoS ONE 6(12): e28779 doi:10.1371/journal.pone.0028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rolstad J, Gjerde I, Storaunet KO, Rolstad E (2001) Epiphytic lichens in Norwegian coastal spruce forest historic logging and present forest structure. Ecol Appl 11: 421–436. [Google Scholar]
- 18. Friedel A, von Oheimb G, Dengler J, Haerdtle W (2006) Species diversity and species composition of epiphytic bryophytes and lichens–a comparison of managed and unmanaged beech forests in NE Germany. Feddes Repert 117: 172–185. [Google Scholar]
- 19. Moning C, Werth S, Dziock F, Bässler C, Bradtka J, et al. (2009) Lichen diversity in temperate montane forests is influenced by forest structure more than climate. Basic Appl Ecol 258: 745–751. [Google Scholar]
- 20. Lõhmus A, Lõhmus P, Vellak K (2007) Substratum diversity explains landscape-scale co-variation in the species-richness of bryophytes and lichens. Biol Conserv 135: 405–414. [Google Scholar]
- 21. Fritz Ö, Niklasson M, Churski M (2008) Tree age is a key factor for the conservation of epiphytic lichens and bryophytes in beech forests. Appl Veg Sci 12: 93–106. [Google Scholar]
- 22. Fritz Ö, Gustafsson L, Larsson K (2008) Does forest continuity matter in conservation?–A study of epiphytic lichens and bryophytes in beech forests of southern Sweden. Biol Conserv 141: 655–668. [Google Scholar]
- 23. Jüriado I, Liira J, Paal J, Suija A (2009) Tree and stand level variables influencing diversity of lichens on temperate broad-leaved trees in boreo-nemoral floodplain forests. Biodivers Conserv 18: 105–125. [Google Scholar]
- 24. Fischer M, Bossdorf O, Gockel S, Hänsel F, Hemp A, et al. (2010) Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories. Basic Appl Ecol 11: 473–485. [Google Scholar]
- 25.Umweltbundesamt (2005) Daten zur Umwelt–Der Zustand der Umwelt in Deutschland. Berlin: Erich Schmidt Verlag.352 p
- 26. Hessenmöller D, Nieschulze J, von Lüpke N, Schulze E-D (2011) Identification of forest management types from ground-based and remotely sensed variables and the effects of forest management on forest structure and composition. Forstarchiv 82: 171–183. [Google Scholar]
- 27. Luyssaert S, Schulze E-D, Börner A, Knohl A, Hessenmöller D, et al. (2008) Old-growth forests as global carbon sinks. Nature 455: 213–215. [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
- 29. Rudolphi J, Gustafsson L (2011) Forests regenerating after clear-cutting function as habitat for bryophyte and lichen species of conservation concern. PLoS ONE 6(4): e18639 doi:10.1371/journal.pone.0018639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graae BJ, Heskjær VS (1997) A comparison of understorey vegetation between untouched and managed deciduous forest in Denmark. Forest Ecol Manag 96: 111–123. [Google Scholar]
- 31. Nascimbene J, Marini L, Nimis PL (2007) Influence of forest management on epiphytic lichens in a temperate beech forest of northern Italy. Forest Ecol Manag 247: 43–47. [Google Scholar]
- 32. Nascimbene J, Marini L, Nimis PL (2010) Epiphytic lichen diversity in old-growth and managed Picea abies stands in Alpine spruce forests. Forest Ecol Manag 260: 603–609. [Google Scholar]
- 33. Jairus K, Lõhmus A, Lõhmus P (2009) Lichen acclimatization on retention trees: a conservation physiology lesson. J Appl Ecol 46: 930–936. [Google Scholar]
- 34. Marmor L, Tõrra T, Saag L, Randlane T (2011) Effects of forest continuity and tree age on epiphytic lichen biota in coniferous forests in Estonia. Ecol Ind 11: 1270–1276. [Google Scholar]
- 35. Peterson EB, McCune B (2001) Diversity and succession of epiphytic macrolichen communities in low-elevation managed conifer forests in Western Oregon. J Veg Sci 12: 511–524. [Google Scholar]
- 36. Humphrey JW, Davey S, Peace AJ, Ferris R, Harding K (2002) Lichens and bryophyte communities of planted and semi-natural forests in Britain: the influence of site type, stand structure and dead wood. Biol Conserv 107: 165–180. [Google Scholar]
- 37. Pykälä J, Heikkinen RK, Toivonen H, Jääskeläinen K (2006) Importance of Forest Act habitats for epiphytic lichens in Finnish managed forests. Basic Appl Ecol 223: 84–92. [Google Scholar]
- 38. Moning C, Müller J (2009) Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol Ind 9: 922–932. [Google Scholar]
- 39. Svoboda D, Peksa O, Veselá J (2010) Epiphytic lichen diversity in central European oak forests: Assessment of the effects of natural environmental factors and human influences. Environ Pollut 158: 812–819. [DOI] [PubMed] [Google Scholar]
- 40. Ranius T, Johansson P, Berg N, Niklasson M (2008) The influence of forest age and microhabitat quality on the occurrence of crustose lichens associated with old oaks. J Veg Sci 19: 653–662. [Google Scholar]
- 41. Fritz Ö, Heilmann-Clausen J (2010) Rot holes create key microhabitats for epiphytic lichens and bryophytes on beech (Fagus sylvatica). Biol Conserv 143: 1008–1016. [Google Scholar]
- 42. Werth S, Wagner HH, Gugerli F, Holderegger R, Csencsics D, et al. (2006) Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 87: 2037–2046. [DOI] [PubMed] [Google Scholar]
- 43. Lõhmus A, Lõhmus P (2010) Epiphyte communities on the trunks of retention trees stabilize in 5 years after timber harvesting, but remain threatened due to tree loss. Biol Conserv 143: 891–898. [Google Scholar]
- 44.Rose F (1992) Temperate forest management: its effects on bryophyte and lichen floras and habitats. In: Bates JW, Farmer A, editors. Bryophytes and Lichens in a Changing Environment.Oxford:Clarendon Press, pp. 211–233.
- 45. Uliczka H, Angelstam P (2000) Assessing conservation values of forest stands based on specialised lichens and birds. Biol Conserv 95: 343–351. [Google Scholar]
- 46. Edmann M, Eriksson A-M, Villard M-A (2008) Effects of selection cutting on the abundance and fertility of indicator lichens Lobaria pulmonaria and Lobaria quercizans . J Appl Ecol 45: 26–33. [Google Scholar]
- 47. Fedrowitz K, Kuusinen M, Snäll T (2012) Metapopulation dynamics and future persistence of epiphytic cyanolichens in a European boreal forest ecosystem. J Appl Ecol 49: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sillett SC, McCune B, Peck JE, Rambo TR, Ruchty A (2000) Dispersal limitations of epiphytic lichens result in species dependent on old-growth forests. Ecol Appl 10: 789–799. [Google Scholar]
- 49. Muir PS, Rambo TR, Kimmerer RW, Keon DB (2006) Influence of overstory removal on growth of epiphytic mosses and lichens in western Oregon. Ecol Appl 16: 1207–1221. [DOI] [PubMed] [Google Scholar]
- 50. Perhans K, Appelgren L, Jonsson F, Nordin U, Söderström B, et al. (2009) Retention patches as potential refugia for bryophytes and lichens in managed forest landscapes. Biol Conserv 142: 1125–1133. [Google Scholar]
- 51. Wolseley PA, Stofer S, Mitchell R, Truscott AM, Vanbergen A, et al. (2006) Variation of lichen communities with landuse in Aberdeenshire, UK. Lichenologist 38: 307–322. [Google Scholar]
- 52. Neitlich PN, McCune B (1997) Hotspots of epiphytic lichen diversity in two young managed forests. Conserv Biol 11: 172–182. [Google Scholar]
- 53. Caruso A, Rudolphi J, Thor G (2008) Lichen species diversity and substrate amounts in young planted boreal forests: A comparison between slash and stumps of Picea abies. Biol Conserv 141: 47–55. [Google Scholar]