Progression through critical events in cell division relies to a large extent on the destruction of key effectors of the cell cycle. An example can be seen in the regulation of the major cell cycle effector, the cyclin-dependent kinases (CDKs). CDKs depend upon the binding of their activators, the cyclins, to drive specific events at particular stages in the cell division cycle. As different cyclins are synthesized at different phases of the cell cycle, the CDK, when associated with cyclins, essentially exhibits distinct activities in each phase of the cell cycle. The destruction of cell cycle stage-specific cyclins therefore plays an important role in limiting the activities of the CDKs at the end of each stage so that the CDKs can associate with yet other cyclins to drive progression of the cell cycle (reviewed in reference 45).
Cyclins were initially discovered as proteins whose levels oscillated in a highly specific manner in sea urchin Arbacia punctulata embryos undergoing successive rounds of cell division (12). Initial hints that the cyclical nature of the cyclins is important for the regulation of the cell cycle came from the observation that destruction of mitotic cyclins, leading to a decrease in mitotic kinase activities, is required for the exit from mitosis (47). Further analyses led to the understanding that ubiquitination is key to cyclin destruction (19, 28). An E3 ubiquitin-ligase known as the anaphase-promoting complex (APC) was subsequently purified from extracts of the clam Spisula solidissima (29, 64) and African clawed frog Xenopus laevis (33) and shown to be responsible for the ubiquitination of mitotic cyclins.
In the process of ubiquitination, the E3 ligase adds a chain of ubiquitin, a 76-residue polypeptide, to proteins destined for destruction. The proteins marked with a ubiquitin chain are recognized as substrates by the 26S proteosome and duly destroyed (reviewed in references 26 and 70). Studies in the budding yeast Saccharomyces cerevisiae revealed that mutants of the APC subunits such as cdc16 and cdc23 were defective for anaphase progression (31), indicating that the APC is required for mediating destruction of regulators for metaphase to anaphase transition in addition to the ubiquitination of mitotic cyclins. To date, the evolutionarily conserved APC (reviewed in references 25 and 54) has been shown to consist of at least 11 subunits (Table 1), and its activity is cell cycle regulated such that it is active in mitosis (reviewed in reference 50). Activation of the APC activity depends upon the association of WD-40 repeat proteins known as Cdc20/Fizzy and its homologue, Cdh1/Fizzy-related (reviewed in references 25, 50, 54 and 78). These activators have been found to be highly conserved across species (reviewed in references 25 and 54).
TABLE 1.
Orthologues of APC subunits in C. elegans
| Known APC subunitb | APC orthologue in C. elegans (reference[s]) | Function(s) in C. elegansa |
|---|---|---|
| Cdc16 (TPR motif) | emb-27/pod-6 (17, 56) | Progression through meiosis, a-p polarity |
| Cdc23 (TPR motif) | mat-3/pod-4 (9, 20, 56) | Progression through meiosis, mitosis, a-p polarity, affects development (e.g., male tail) |
| Cdc27 (TPR motif) | mat-1/pod-5 (20, 56, 61) | Progression through meiosis, a-p polarity, affects development (e.g., vulva and male tail) |
| Apc1 (Rpn1/2 homology) | mat-2/pod-3 (9, 20, 49) | Progression through meiosis, a-p polarity, affects development of vulva and male tail |
| Apc2 (Cullin homology) | K06H7.6 (9) | Progression through meiosis |
| Apc4 (WD40 repeats) | emb-30 (17) | Progression through meiosis, mitosis, affects development (e.g., vulva and male tail) |
| Apc5 (TPR motif) | M163.4 (9) | Mitosis |
| Apc-10 | F15H10.3 (9) | Mitosis |
| Apc11 | F35G12.9 (9) | Progression through meiosis |
| Cdc26 | ||
| Doc-1 | ||
| Ama-1 | ||
| Cdc20 (WD40 repeats) | fzy-1 (36, 56) | Progression through meiosis, mitosis, a-p polarity |
| Cdh1 (WD40 repeats) | fzr-1 (14) | Mitosis, cyclin destruction, affects germ line proliferation |
a-p, anterior-posterior.
TPR, tetratricopeptide repeat.
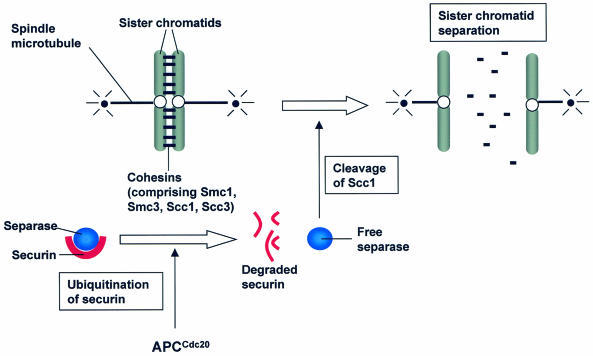
While the functions of each individual APC subunit remain unclear, a comprehensive picture of the roles of the APC in mitosis has emerged through several important studies in budding yeast. The APCCdc20 and APCHct1 complexes have essentially been shown to be required for two main transitions in mitosis, metaphase-to-anaphase transition and exit from mitosis. In metaphase, sister chromatids are held together by a cohesin complex presently known to consist of at least four subunits, Smc1, Smc3, Scc1/Mcd1/Rad21, and Scc3 (reviewed in reference 48) (Fig. 1). In order that sister chromatids separate, Scc1 has to be cleaved by separase, a cysteine protease belonging to the CD clan (68, 69). Separase, however, is normally held inactive during metaphase through its association with the anaphase inhibitor securin. APCCdc20 functions to promote sister chromatid separation by ubiquitination of securin and in so doing causes its destruction (reviewed in reference 48) (Fig. 1). The liberated separase is then free to cleave Scc1, leading to loss of sister chromatid cohesion, thereby allowing chromatids to be pulled apart by the spindle microtubules (Fig. 1). Cdc20 is the target of the spindle assembly checkpoint, which is activated in the presence of spindle defects. The spindle assembly checkpoint components such as Mad1, Mad2, Mad3, Bub1, Bub3, and Mps1 are involved in a pathway that serves to sequester Cdc20, thereby preventing the onset of anaphase, when spindle microtubules are disrupted (reviewed in reference 77).
FIG. 1.
Diagrammatic representation of sister chromatid separation. Sister chromatids are held together by cohesin complexes (short black bars) during metaphase. APCCdc20 ubiquitination of securin (red) leads to liberation of separase (blue). Separase cleaves Scc1, one of the subunits of the cohesin complex, and sister chromatids are pulled apart by spindle microtubules (long black bars).
Homologues of the APC subunits, securin, separase, and cohesin subunits and even the spindle assembly checkpoint components have been found in different organisms (reviewed in reference 48), a strong indication that the pathway leading to sister chromatid separation is perhaps rather well conserved. APCCdc20 also triggers the destruction of S and M phase cyclins to initiate exit from mitosis so that cells can enter a new round of cell division. The initial lowering of the mitotic kinase activities by APCCdc20 (3, 30, 73, 76) allows APCHct1 to be activated by the mitotic exit network (MEN), comprising components such as Tem1 (GTPase), Cdc15 (kinase), Dbf2 (kinase), and Cdc14 (phosphatase) (reviewed in reference 46). The MEN pathway is the target for another type of spindle checkpoint, the spindle position checkpoint. This checkpoint depends upon Bub2 and monitors the orientation and position of the spindle apparatus. In the event that the spindle apparatus is misoriented, Bub2 acts through the MEN pathway to delay mitotic exit via Hct1 until the spindle apparatus is properly oriented (reviewed in reference 40).
It has also been shown in budding yeast that homologous chromosome separation during meiosis takes place only in the presence of a functional APC. As in sister chromatid segregation, the APC promotes the ubiquitination of securin in meiosis I and II (59). Degradation of securin by the 26S proteosome frees separase (4, 69), which cleaves Rec8, a meiosis-specific cohesin subunit (37) that shows homology to Scc1 (see above). During meiosis I, the Rec8 molecules localized to the longitudinal arms are destroyed by separase, leading to homologous chromosome disjunction, while Rec8 in the proximity of the centromeres is protected from proteolysis until meiosis II (4, 37). The direct involvement of separase in meiosis II has yet to be confirmed. The sequential destruction in meiosis of Rec8 homologues also occurs in other organisms, such as Schizosaccharomyces pombe (74), Mus musculus (39), and Arabidopsis thaliana (5).
Insofar as our understanding of APC function in cell division is concerned, the budding yeast has been instrumental in providing the initial insights. With the identification of homologues of the APC subunits and activators and the other components of chromosome separation and cohesion pathways (reviewed in reference 48), it would be interesting to establish the extent of conservation in the functions of the APC in different organisms. Furthermore, the question now arises as to whether the APC, which is essential in cell division, also contributes to other processes in multicellular organisms. Indeed, recent reviews on the APC have alluded to the possibility that the APC performs functions in cellular processes other than mitosis and meiosis (25, 54). Support for additional roles of the APC comes from studies showing that Hct1/Cdh1 and APC subunits can be detected in postmitotic murine neuronal cells (18) and that multiple copies of Hct1/Cdh1 genes exist in chickens (72). Interestingly, however, work by various groups on the APC in Caenorhabditis elegans in the past few years has not only implicated APC subunits in meiosis and mitosis; more significantly, the data indicate that the functions of the APC in meiotic and mitotic divisions have important consequences in the development of the worm (Table 1). This would suggest that at least in C. elegans, additional roles of the APC, such as in development, are a result of its contribution to proper cell division.
Although C. elegans has not been the major organism of choice for studying the APC, it has nonetheless lent itself as a useful system for a better understanding of APC function for several reasons. First, as the APC subunits, Cdc20/Fizzy and Hct1/Fizzy-related have been found in C. elegans (Table 1), comparisons can clearly be made to establish the similarities and differences in APC function across different species. Second, an advantage of using C. elegans is the availability of traditional genetic techniques which make the generation of APC mutant worms for functional studies relatively easy. Also, as a reverse genetics approach, the newly established double-stranded RNA interference (dsRNAi) method provides a powerful and convenient tool for targeting genes in knockdown experiments in C. elegans (15). Furthermore, the well-characterized behavior of the homologous chromosomes (five pairs of autosomes and one pair of sex chromosomes in the hermaphrodite) in worm oocytes has been useful for examining meiotic progression, while the single-celled embryo has characteristics which allow cytological observations of mitotic division processes such as spindles, nucleus, and centrosomes by Nomarski optics. Finally, with the complete documentation of the entire cell lineage (65) and an anatomy that has been well studied (reviewed in reference 38), C. elegans can potentially serve as an important model for investigating the role of the APC in development. This review takes a look at what is currently known about the contributions of APC in cell division as well as how the functions of the APC in cell division impinge upon the development of C. elegans.
APC IN MEIOSI
The evidence from budding yeast that the APC is essential for progression through meiosis is clear. Nonetheless, despite the conservation of APC subunits and cohesin components in different organisms (reviewed in reference 48), whether there is indeed a widespread requirement for the APC in chromosome segregation during meiosis is far from certain. In fact, two studies have shown that the APC is dispensable for meiosis in Xenopus laevis (53, 66), casting some doubt on the relevance of extrapolating the data from budding yeast to higher organisms. In this regard, C. elegans has provided evidence showing support for the data obtained with budding yeast that the APC is indeed essential for meiotic progression.
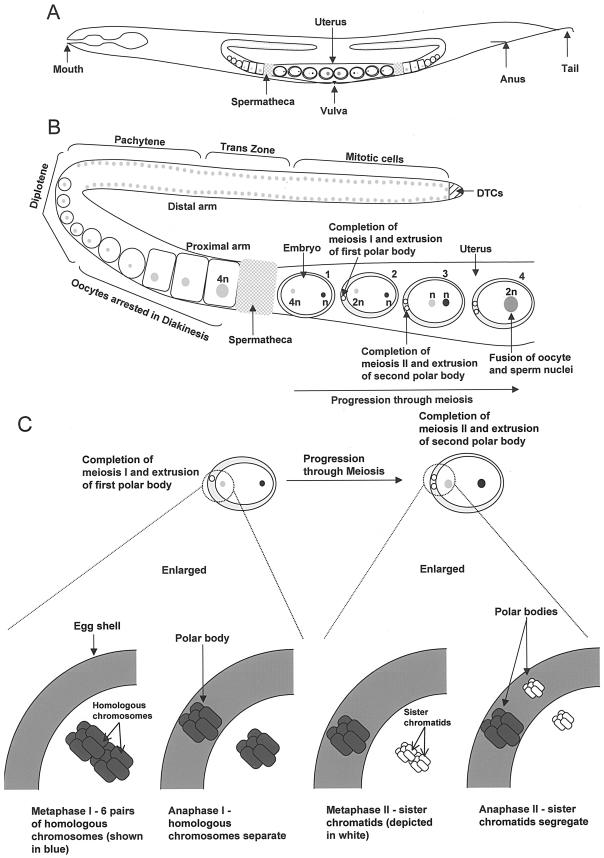
The hermaphrodite reproductive system in C. elegans has been a useful tool for the examination of both mitotic and meiotic cell divisions. The hermaphrodite reproductive system is a U-shaped structure, with one arm of the U extending towards the anterior of the worm and the other towards the posterior (Fig. 2A). Each arm serves as an ovary which ends off with a spermatheca joined to a uterus (Fig. 2A). A vulval opening in the middle of the uterus allows fertilized oocytes to be laid. At the distal end of each arm, germ cells divide mitotically but transit from mitotic to meiotic divisions as they move towards the proximal end near the spermatheca (Fig. 2B). The mitotic proliferation of the germ cells is under the influence of the distal tip cells (Fig. 2B and C), which provide signals via molecules such as GLP-1 (for germ-line proliferation-defective; a transmembrane protein homologous to Notch), LAG-1 (a highly conserved transcription factor acting downstream of GLP-1), and LAG-2 (a transmembrane Delta-like protein which acts as the signaling ligand for GLP-1). Once the cells move to a distance greater than 20 cell diameters away from the distal tip cells, cells exit mitosis and enter meiosis in the transition zone (Fig. 2B, trans zone) as they develop into oocytes. However, the oocytes do not complete meiosis but remain arrested in diakinesis of meiosis I as they move into the proximal arm.
FIG.2.
(A) Adult hermaphrodite showing mouth, anus, and tail with U-shaped gonad. The spermatheca, uterus containing embryos, and vulva are also highlighted (more details in text). (B) Magnified view of one-half of the U-shaped gonad from the adult hermaphrodite with oocyte chromosomes in newly fertilized embryos undergoing meiosis in the uterus (adapted from reference 57). Embryo 1, fertilization of oocyte (grey circle, 4n) by the sperm; embryo 2, completion of meiosis I by oocyte chromosomes (grey circle, 2n) and extrusion of the first polar body; embryo 3, completion of meiosis II and extrusion of the second polar body; embryo 4, fusion of oocyte and sperm nuclei (see the text for more description). (C) Chromosome segregation during meiosis in the C. elegans oocyte (57). Six pairs of homologous chromosomes undergo meiosis I upon fertilization, followed by sister chromatid separation during meiosis II (see the text for details).
Meiosis in the oocyte proceeds to completion only upon fertilization by a sperm (reviewed in reference 60). Once fertilized, the oocyte chromosomes first undergo meiosis I, during which homologous chromosomes or bivalents separate into two sets of univalents, with the one closer to the surface of the embryos extruded as a polar body (Fig. 2B and C). During meiosis II, sister chromatid segregation occurs, with the extrusion of a second polar body (1). The chromosomes in C. elegans during meiosis can be easily observed under standard 4′,6′-diamidino-2-phenylindole (DAPI) staining and fluorescent microscopic examination of the oocytes. Also, the generation of a histone H2B-green fluorescent protein transgenic worm strain by Judith Austin's laboratory has enabled time-lapse imaging in live oocytes of chromosome segregation that can help reveal chromosome dynamics during meiosis and mitosis.
The role of APC in C. elegans meiosis was recently revealed in studies of mutations of two loci, emb-30 and emb-27, previously identified to be essential for embryogenesis (6). The emb-30(g53ts) mutant was initially found to produce one-celled embryos carrying multiple nuclei, while the emb-27(g48) mutant produced one-cell embryos defective in polar body formation when the mutants were grown at the restrictive temperature (6). Detailed analyses showed that homologous chromosomes of emb-30(tn337ts) (17) and emb-27(g48) (20) mutants failed to separate, an indication that the embryonic lethality was due to defects in meiosis. Sequence similarities found between EMB-30 and the human Apc4 and fission yeast Lid1 APC subunit (17), and EMB-27 with Cdc16 (20) therefore implicate the APC in progression through meiosis.
The APC subunits appear to be involved in a general function of homologous chromosome separation, as early events prior to anaphase I such as eggshell deposition, chromosome condensation, and localization of sperm and oocyte chromosomes near the embryo cortex were unaffected by the mutations in both emb-30 and emb-27 mutants (17, 20). Furthermore, the APC is also required in meiosis during spermatogenesis. In general, the main features of spermatogenesis are relatively similar in hermaphrodites and males. Basically, germ cells first enter meiosis to form spermatocytes, which then undergo meiosis to generate spermatids (reviewed in reference 27). Loss of emb-30 or emb-27 function led to a failure to properly segregate chromosomes in the mutant sperm during spermatogenesis (17, 20) though interestingly, cell division continued, resulting in the production of spermatids devoid of chromosomal material (58). These anucleate spermatids surprisingly retained their ability to crawl towards and fertilize the oocytes. The oocytes, once fertilized, became activated and proceeded to complete meiosis. Other events, such as proper eggshell formation, migration of the oocyte pronucleus, and establishment of the resulting embryo, appeared unaffected by mutations in the APC subunits. Therefore, unlike oocytes, which remained arrested in meiosis I, the spermatids were able to continue with the differentiation program to form somewhat functional spermatozoa in the absence of APC activity. Nonetheless, the data from the sperm provide additional indications that the APC is responsible only for promoting proper chromosome segregation and not for spermatid differentiation or for early events in embryogenesis.
The requirement for APC activity in meiosis was further established through studies of another class of mutants isolated recently, known as metaphase-to-anaphase (mat) transition-defective mutants (20) (Table 1). Inactivation of mat genes such as mat-1 (CDC27), mat-2 (APC1), and mat-3 (CDC23) in temperature-sensitive mutants or by dsRNAi led to an arrest in meiosis I in the embryos (9, 20) (Table 1). Other APC subunits, identified through sequence homologies to APC2 and APC11 (K06H7.6 and F35G12.9, respectively) (Table 1), are also essential in meiosis I (9). However, growth of several temperature-sensitive mutant alleles of mat-1 at semirestrictive temperatures led to the observation of different phenotypes; a subset of the mat-1 mutants arrested in the one-celled, meiosis I stage, a second subset of mutants underwent the first meiotic division to give rise to one polar body, and a third group of mutants completed meiosis I and II, resulting in the extrusion of two polar bodies, though the meiosis was by no means normal (61). These observations show that mat-1 contributes to both meiosis I and II, though it appears that different levels of APC activities are required for meiosis I and II. The meiotic defects due to the partial inactivation of the mat-1 mutants also contribute to developmental abnormalities, for instance, failure to establish a proper anterior-posterior axis and laying down of an impermeable, three-layer eggshell (see below).
Defects in homologous chromosome separation in C. elegans can also be seen in worms where the APC activator Cdc20/Fizzy was disrupted. C. elegans fzy-1 was isolated in a suppressor screen by Kitagawa and coworkers (36) by using the gk2 mutant allele of mdf-1, a homologue of the MAD1 spindle assembly checkpoint component (35). Localization of FZY-1 to chromosomes in antibody staining analysis of worm embryos lends support to the idea that FZY-1 functions in metaphase-to-anaphase transition during mitosis and meiosis (36). In addition, Kitagawa and coworkers (36) proposed that a securin-separase pair exists in C. elegans which plays an important role in chromosome separation similar to that in the budding yeast. The authors isolated the securin orthologue, or ify-1 (for interactor of fizzy-1) in a yeast two-hybrid screen with FZY-1 as the bait. Hermaphrodites in which ify-1 function was abolished by dsRNAi produced single-celled embryos with abnormal chromosome morphology due to defective chromosome segregation in meiosis I (36). dsRNAi experiments revealed that IFY-1 may also be required in processes such as centrosome duplication, spindle organization, and cytokinesis (36). Nonetheless, the identification of a destruction box motif in IFY-1 and the stabilization of IFY-1 protein level in an temperature-sensitive emb-30 mutant is consistent with the idea that the APC, together with FZY-1, is responsible for ubiquitination of IFY-1, perhaps for setting free SEP-1 (36), the orthologue of separase.
The notion that IFY-1 and SEP-1 act as securin and separase, respectively, is supported by the physical interaction between IFY-1 and SEP-1 in yeast two-hybrid and glutathione S-transferase assays (36) as well as the meiosis I chromosome segregation phenotype observed in a sep-1 mutant grown at the restrictive temperature (63). The meiosis-specific cohesin REC-8 has also recently been identified in C. elegans and found to localize to chromosomal axes in oocytes at diakinesis (51). Like that of S. cerevisiae, C. elegans REC-8 is partially destroyed in meiosis I and rec-8 depletion in the oocytes caused aberrant separation of chromosomes, resulting in 24 separate chromatids and chromosome fragments (51). This is a strong indication that REC-8 normally serves as a cohesin subunit in keeping chromosomes together.
Other cohesin subunits in C. elegans have also been found through sequence homologies to the families of Smc1, Smc3, and Scc3 subunits from the genome database (44). Furthermore, work by Chan and colleagues established that REC-8 colocalizes with SMC-1, SMC-3, and SCC-3 to meiotic chromosomes (7). Intriguingly, TIM-1, believed to be the paralogue of the Drosophila clock protein TIMELESS, has been coimmunoprecipitated in a cohesin complex comprising SMC-1, SMC-3, SCC-1, and SCC-3 (7). dsRNAi data show that tim-1 is essential for chromosome segregation, as temperature-sensitive tim-1 worms were defective in chromosome separation in meiosis and mitosis. Immunofluorescence studies revealed that non-SMC subunits such as REC-8 and SCC-1 failed to localize to chromosomes in worms where tim-1 was knocked down by dsRNAi, a strong hint that TIM-1 is important for targeting of non-SMC cohesin subunits to the chromosomes.
Taken together, these findings show that, in contrast to meiosis in Xenopus laevis, chromosome separation in C. elegans meiosis is mediated by an APC-dependent pathway similar to that found in S. cerevisiae. Despite this evidence supporting the idea that APC is required for meiosis, it could still be argued that the data from S. cerevisiae and C. elegans are not as useful for understanding meiosis in vertebrates and that the finding in Xenopus laevis (53, 66) may better reflect the situation in higher organisms. However, work from mouse oocytes reveals that the progression through meiosis also depends upon the APC and securin (67), further demonstrating the functional conservation of the APC in meiosis. Terret and coworkers (67) suggested that the apparent lack of requirement of the APC in Xenopus meiosis could be due either to technical difficulties in inactivating the APC in Xenopus oocytes or to the possibility that Xenopus laevis has evolved a unique mechanism of chromosome separation in meiosis.
It should be noted that the complete picture of meiosis in C. elegans is not as straightforward as in budding yeast. Reports showing that the inactivation of ify-1 failed to rescue the mat phenotype in an emb-30 mutant background (36) and that depletion of rec-8 was unable to rescue the apc-11 phenotype (9) point to the possibility that factors other than securin/IFY-1 have to be degraded by the APC for proper meiosis I progression and that REC-8 is probably not the sole substrate of separase in C. elegans. The finding that TIM-1 (7) is also essential for both meiosis and mitosis through its role in localizing cohesin subunits adds to the number of important players required for chromosome segregation in the worm.
APC IN MITOSI
The embryonically lethal phenotype of APC mutations due to defects in meiosis could potentially obscure observations as to whether the APC functions during mitosis in C. elegans. Indeed, of the APC subunits examined, only the inactivation of apc-5 and apc-10 by dsRNA did not result in embryonic lethality at the meiotic one-cell stage; rather, the embryos died at the multicellular stage (9). These data indicate that apc-5 and apc-10 contribute to postmeiotic events, perhaps in mitosis. The observation that mothers in which either apc-5 or apc-10 was disrupted by dsRNAi eventually became sterile due to a failure of germ line maintenance (9) points to the established role of the APC in mitosis (reviewed in references 25, 54, and 78), more specifically, in germ line mitosis. One possible explanation for the absence of a meiotic defect in the apc-5 and apc-10 dsRNAi experiments could be that not all APC subunits are required strictly for meiosis (see above) and that some of the subunits may be needed for proper mitotic divisions and/or specific processes during embryogenesis. Alternatively, it could well be that the dsRNAi failed to completely knock down the gene functions of apc-5 and apc-10. The partial APC activity allowed the embryos to progress through meiosis even though the reduced activity was insufficient for subsequent processes.
Other lines of evidence supporting the contribution of APC to postmeiotic events can be seen in the emb-30 class I and mat-1(ax144) and mat-1(ax212) mutant larvae (61). By allowing these emb-30 class I mutants to grow at the permissive temperature to bypass the meiotic defect due to emb-30 mutation, the embryos were able to develop into adult worms, albeit with developmental defects such as an everted vulva (17). Double mutants carrying either the mat-1(ax212) or mat-1(ax227) allele in combination with other APC mutant alleles were able to develop to the adult stage, but these gave birth to dead embryos at the permissive temperature, although the single mutants were fully fertile (61). These data demonstrate that by compromising APC activity in worms carrying a combination of two mutant alleles of the APC, defects can occur even at the permissive temperature, perhaps due to the APC's contribution to somatic cell division during embryonic and postembryonic development (see below).
The indication that the APC is indeed required for mitosis in somatic tissues came from detailed analysis of mat-1(ax144) and mat-1(ax212) mutant embryos allowed to develop at the permissive temperature into male larvae. Phospho-histone staining revealed an increase in the posterior ends of the male larvae when they were shifted to the restrictive temperature (61), a sign that the cells failed to progress through mitosis. The mitotic progression phenotype in the APC mutants correlates with the well-accepted role of the APC for the ubiquitination of the anaphase inhibitor securin (reviewed in reference 48). However, the early function in meiosis of the C. elegans securin, ify-1, and separase, sep-1 (see above), could have precluded the identification of their role in mitosis, though it is not unreasonable to expect that they should also contribute to sister chromatid segregation, given that the conservation in the components in the APC and the cohesin complex appears to extend across different organisms (reviewed in reference 48).
That an emb-30 mutation can partially rescue the abnormal chromosome separation in an mdf-1 background where chromosome segregation occurs in the presence of a defective spindle assembly checkpoint (17) further indicates that the APC is needed for proper chromosome separation. Analysis of smc-1, smc-3, and scc-3 in C. elegans by dsRNAi revealed that these cohesin subunits are required for proper chromosome segregation during mitosis in embryos (46). More recent work in Chan's group with immunoprecipitation assays found COH-2 in the SMC-1, SMC-3, and SCC3 cohesin complex, providing strong evidence that COH-2 is the functional orthologue of Scc1 (7). Also, tim-1 was immunoprecipitated in the cohesin complex and appears to be essential for mitotic chromosome segregation in both germ line and somatic cells. Furthermore, Kitagawa and Rose found that mdf2, the C. elegans orthologue of the MAD2 spindle assembly checkpoint component in budding yeast, was able to rescue the benomyl-sensitive phenotype in a mad2 deletion strain of budding yeast (35). This work points to the conservation of the sister chromatid segregation pathway, as Mad2 has been shown to act through sequestration of Cdc20 from the APC, inhibiting chromosome segregation upon spindle damage.
APC IN DEVELOPMENT
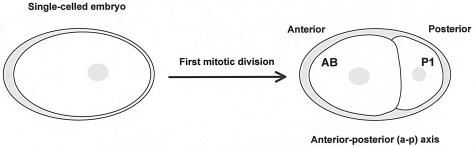
Mitotic cell divisions are important during the development of multicellular organisms for the generation of the correct cell numbers or cell types important for the proper formation of the organisms. Aberrant cell divisions occurring during embryonic or postembryonic development of the worm can result in developmental defects. In the first mitotic division of the one-celled embryo, P0, two unequal daughter cells, a larger anterior AB and a smaller posterior P1 (Fig. 3), are generated, and each subsequently contributes to cells forming different tissue types (65). This asymmetric division depends on the anterior-posterior axis, established through the interactions among the sperm pronucleus/centrosome complex, the associated microtubules and the actin-rich cortex upon fertilization of the oocyte. The sperm pronucleus/centrosome complex triggers cytoplasmic flow and cortical rearrangements that cause the polarized localization of specific cellular determinants such as the PAR proteins. These PAR proteins play an important role in the displacement of the mitotic spindle to ensure an asymmetric division (reviewed in references 41 and 52).
FIG. 3.
First mitotic division in the embryo establishes the anterior-posterior axis. The first cell division is asymmetric, resulting in a larger anterior AB cell and a smaller posterior P1 cell.
A screen for polarity- and osmosis-defective (pod) mutants identified a role for the APC in anterior-posterior axis formation during division of the P0 (56) (Table 1). Complementation analyses of pod and mat mutants showed that the pod mutations constitute different alleles of the mat locus (Table 1). The finding that the mat alleles led to meiosis I arrest (see above) while pod alleles bypassed the meiotic defect suggests that the mat alleles correspond to more severe mutations leading to a greater reduction of APC function (20). Detailed analysis of the pod-3(or319) mutant by Rappleye and coworkers (56) indicated that a partial disruption of the APC-1 subunit orthologue led to the inability of the sperm pronuclei to associate with the cortex, which is key to the establishment of the anterior-posterior axis (71). As a consequence, PAR-3 granules, normally localized to the anterior of the embryo (11), were observed throughout the cortex of the embryo and PAR-2 proteins were displaced to the cytoplasm in the pod embryos instead of their usual location in the posterior cortex. In addition, other cytoplasmic determinants such as the germ cell granules, which normally localize to the posterior of the P0 cells in wild-type worms, accumulated in the middle of the one-celled embryos in the different pod mutants. Shakes and coworkers (61) suggested that the contribution of the APC in establishing proper polarity as seen in the pod mutants could be indirect, as they observed polarity defects due to mat-1 inactivation only in the presence of gross meiotic defects in embryos where only one polar body was formed. This would mean that the progression through an abnormal meiosis could indirectly precipitate the polarity defect.
The proper establishment of the anterior-posterior axis also depends on ubiquitin and the APC activator CDC20/FZY-1, as disruption of either ubiquitin gene ubq-2 or cdc20/fzy-1 by dsRNAi abolished asymmetric division of the P0 embryo (56). Rappleye and coworkers (56) proposed that APCCDC20/FZY-1 contributes to anterior-posterior polarity through the activation of separase in the P0 cell, as the disruption of sep-1 also led to a defect in anterior-posterior polarity establishment. APC-activated separase triggers the association of the sperm centrosome with the posterior cortex, causing the displacement of the PAR-3-containing complex to the anterior cortex. PAR-2 on the other hand is able to localize to the posterior cortex, thereby setting up an asymmetric distribution of cellular determinants in the one-cell P0. A polarized localization of these known cytoplasmic determinants in the wild-type P0 is required for generating a differential pulling force exerted by the mitotic spindles such that asymmetric division occurs (23). Interestingly, the absence of a proper anterior-posterior axis resulted also in the altered timing and misorientation of spindles during the second cell division (56).
The homologue of FZY-1, FZR-1 (for Fizzy-related, a homologue of Cdh1), apparently has no involvement in polarity determination (14). fzr-1 was isolated in a synthetic lethal screen in a lin-35/Rb background (14). lin-35 fzr-1 double mutants were mostly nonviable, though several survived and exhibited hyperproliferation of the seam cells, vulval tissue, gut cells, and tumorous phenotype in the gonadal arm. The fzr-1 single mutant displayed milder defects in hyperproliferation of tissues, although complete inactivation of fzr-1 in RNAi led to a drastic reduction in fertility. The overexpression of cyclins A and E by Fay and Han in the fzr-1 mutant background resulted in an increase in mitotic divisions in the germ cells at the distal arm (14). This led to multiple-armed gonads and has been described as the Shiva (after the Hindu god Shiva, who is usually depicted with four arms) phenotype. This is most likely due to the reduced ability to degrade G1 cyclins such as cyclin E and A. Hence, FZR-1 is required for the proper regulation of cell division during the development of the worm, most probably due to its role in controlling cyclin levels.
The function of the APC subunits in mitotic cell divisions has consequences in the development of the worm during postembryonic development for both germ and somatic cells. For example, emb-30(tn337ts) mutant embryos laid at the permissive temperature but allowed to develop at the restrictive temperature were defective in germ line proliferation, with many of the mitotic germ cells arrested in metaphase (17). Additional emb-30 mutant alleles isolated, such as those in the emb-30(class I) mutants, further point to the requirement of the APC in vulva development, somatic gonad development, and male tail morphogenesis (17). These defects were not due to differentiation defects, as analysis of vulval precursor cells showed that they either delayed or blocked mitosis. Everted vulva and male tail morphogenesis defects were also observed in mat-2 mutants (9) and mat-1 mutants (61), suggesting that certain mutant alleles led to reduced levels of APC activity, which allowed meiosis I progression but not postembryonic development. Male tail abnormalities such as missing rays and complete absence of rays were also found in mat-1(ax144) and mat-1(ax212) mutant male larvae (61). These sensory rays, used during mating for sensing of hermaphrodites, are derived from blast cells through division and differentiation (reviewed in reference 10). As the APC is implicated in mitotic divisions in the worm, the absence of such rays in the mat-1(ax144) and mat-1(ax212) mutant male larvae could be attributed to the lack of or improper cell division.
FUTURE PERSPECTIVE
The coordination of cell division, cell death, and differentiation is critical for the development of a multicellular organism. The development of a multicellular organism can be affected by the cell division cycle in one of several ways. For example, the number of division cycles, which correlates with the number of progeny cells resulting from a progenitor cell, is an important factor in organogenesis for proper shape or size and hence functioning of tissues or organs. For instance, in C. elegans a defect in lin-23, which functions as part of the stem cell factor ubiquitin-ligase complex involved in the degradation of G1 cyclins, led to unrestrained cell proliferation and hyperplasia of all cell types (34). In another C. elegans mutant carrying a truncated cyclin E homolog, cye-1, underproliferation of postembryonic blast lineages was observed (14). The cell cycle stage apparently also affects the differentiation program of the developing organisms. In the vulval development of C. elegans, the vulval progenitor cells at G1 or pre-S phase in the cell cycle can adopt the primary vulval precursor cell fate, whereas only cells in post-S phase can be induced to the secondary vulval precursor cell fate (2). It is therefore noteworthy that the studies on the C. elegans APC described above have provided further links between the machinery involved in cell divisions and proper development of the worm.
A better understanding of how the APC is regulated during cell division could help shed some light on how cell division is coordinated with developmental processes. To this end, C. elegans continues to serve as a useful tool, as genetic screens can be performed relatively easily for isolating regulators and/or substrates of the APC. Screens for mutations affecting cell division, such as the one carried out by Gonczy et al. (21), are useful, as additional genes involved in cell division can be identified. In that particular screen, mutations in 34 loci on chromosome III which affected cell division in the single-cell-stage embryo were obtained. The fact that the spindles, centrosomes, and nuclei of the mutant embryos can be easily visualized and documented by time-lapse differential interference contrast microscopy demonstrates the strength of using the C. elegans one-celled embryo for studying cell division. Using this technique, the authors were able to categorize the mutants into distinct mutant phenotypes, such as defects in spindle assembly, anaphase spindle positioning, chromosome segregation, and cytokinesis, among others, for further studies.
Genetic interactions between genes identified from such screens and genes encoding APC can be tested by standard genetic crosses and relevant candidates can be further characterized in biochemical assays. It would be interesting if distinct pathways leading to APC activation were found for meiotic and mitotic cell division due to differential regulation of the APC. Mutant analyses could possibly lead to the discovery of as yet unidentified subunits of the APC which may assemble different APCs for meiosis and mitosis. A subset of cell division mutants could also allow the isolation of substrates of the APC.
Conversely, a more directed approach to screening for genes involved in cell division is provided by reverse genetics with dsRNAi. Large-scale screens by various groups have been used to target specific clusters of genes, such as those expressed in the worm ovary (55) and genes on chromosome I (16, 79) and chromosome III (22), as well as a genome-wide dsRNAi endeavor by Maeda and colleagues (42) and Kamath and coworkers (32). Consistent with the data showing that the APC is essential for meiosis in C. elegans, dsRNAi of the genes on chromosome III revealed that of the 11 genes required for progression through meiosis, 10 encode components of the proteosome, APC subunits, or ubiquitin (22). The availability of the dsRNAi data on the Wormbase (http://www.wormbase.org) will no doubt speed up the hunt for genes involved in cell division and development. One could essentially perform searches at Wormbase for all possible worm genes implicated in a specific cellular or developmental process based on phenotypes. Genes identified through such searches can then be used to determine functional interactions with the APC.
Studying the developmental context in which the APC functions could pose a bigger challenge for C. elegans, given that the abolishment of APC activity more often than not leads to an early embryonic defect. However, as in the studies by Furuta and coworkers (17) and Shakes et al. (61), when conditional mutants such as temperature-sensitive APC mutants are available, it could still be possible to investigate the regulation of the APC during the development of the worm. By culturing the temperature-sensitive mutants at the permissive temperature to circumvent the embryonically lethal phenotypes, one can shift the mutants to the restrictive temperature and examine developmental defects once the embryos have passed embryonic development. Such temperature shift regimens or perhaps the use of conditional knockouts based on dsRNAi (e.g., heat-inducible promoters) will permit the dissection of various cell division processes involving the APC during postembryonic development.
A screen performed by O'Connell and coworkers (49) for either a temperature-sensitive embryonically lethal (Emb) or a postembryonic phenotype known as sterile and uncoordinated (Stu) led to the identification of novel genes which are involved in cell division. Detailed examination revealed that some of the Stu mutants had defects in meiosis or mitosis, demonstrating that a developmental defect such as Stu is a good predictor for postembryonic cell division defects. Conversely, the mutations leading to the Stu phenotype also caused problems during embryonic cell divisions, yet again indicating the close link between the processes of cell division and development. While these mutants could be used for testing for genetic interactions with mutants of the APC subunits, the general strategy employed by O'Connell and colleagues (49) to obtain conditional mutants can be employed for isolating more players involved in cell division and development.
An established function of the APC which has not been well explored in C. elegans is the destruction of mitotic cyclins for the regulation of exit from mitosis (reviewed in reference 46). Although fzr-1 has been shown to be required for cyclin destruction (13) and components required for mitotic exit such as the MEN have been identified through sequence homologies (24), not much is known about how conserved the pathway leading to mitotic exit is in C. elegans. In addition, while the MEN components have been implicated in cytokinesis (reviewed in reference 43), the APC has only recently been shown to be required for cytokinesis in the sea urchin and sand dollar (62), adding yet another contribution to its regulation of the cell division cycle. C. elegans will no doubt provide a useful system for further work on the APC and the control of exit from mitosis and cytokinesis. How the processes of exit from mitosis and cytokinesis are regulated during the development of the worm also awaits further investigation.
As it stands, studies with C. elegans remain relevant for understanding APC functions. It is known that there are tight controls that maintain the balance between cell division and developmental processes in a multicellular organism (reviewed in reference 8). Understanding what the mechanisms which ensure the proper execution of cell division and development are will be important, as the decoupling of these processes can have dire consequences, such as oncogenesis (reviewed in reference 8). Given the conservation in the APC function in C. elegans and the range of laboratory manipulations afforded by the worm, studies in the worm can yet provide meaningful insights into the coordination of cell division and development in higher organisms.
Acknowledgments
I thank Goh Phuay Yee and Tham Foong Yee for critical comments on the manuscript and the two anonymous reviewers for their very constructive and insightful comments.
Y.F.M. is supported by the Faculty of Medicine start-up fund (grant number R-183-000-104-112) and Provost start-up fund (grant number R-183-000-605-101), National University of Singapore.
REFERENCE
- 1.Albertson, D. G., and J. N. Thomson. 1993. Segregation of holocentric chromosomes at meiosis in the nematode Caenorhabditis elegans. Chromosome Res. 1:15-26. [DOI] [PubMed] [Google Scholar]
- 2.Ambros, V. 1999. Cell cycle-dependent sequencing of cell fate decisions in Caenorhabditis elegans vulva precursor cells. Development 126:1947-1956. [DOI] [PubMed] [Google Scholar]
- 3.Baumer, M., G. H. Braus, and S. Irniger. 2000. Two different modes of cyclin clb2 proteolysis during mitosis in Saccharomyces cerevisiae. FEBS Lett. 468:142-148. [DOI] [PubMed] [Google Scholar]
- 4.Buonomo, S. B., R. K. Clyne, J. Fuchs, J. Loidl, F. Uhlmann, and K. Nasmyth. 2000. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103:387-398. [DOI] [PubMed] [Google Scholar]
- 5.Cai, X., F. Dong, R. E. Edelmann, and C. A. Makaroff. 1999. The Arabidopsis SYN1 cohesin protein is required for sister chromatid arm cohesion and homologous chromosome pairing. J. Cell Sci. 116:2999-3007. [DOI] [PubMed] [Google Scholar]
- 6.Cassada, R., E. Isnenghi, M. Culotti, and G. von Ehrenstein. 1981. Genetic analysis of temperature-sensitive embryogenesis mutants in Caenorhabditis elegans. Dev. Biol. 84:193-205. [DOI] [PubMed] [Google Scholar]
- 7.Chan, R. C., A. Chan, M. Jeon, T. F. Wu, D. Pasqualone, A. E. Rougvie, and B. J. Meyer. 2003. Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature 424:1002-1009. [DOI] [PubMed] [Google Scholar]
- 8.Corn, P. G., and W. S. El-Deiry. 2000. Derangement of growth and differentiation control in oncogenesis. Bioessays 24:83-90. [DOI] [PubMed] [Google Scholar]
- 9.Davis, E. S., L. Wille, B. A. Chestnut, P. L. Sadler, D. C. Shakes, and A. Golden. 2002. Multiple subunits of the Caenorhabditis elegans anaphase-promoting complex are required for chromosome segregation during meiosis I. Genetics 160:805-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emmons, S. W., and P. W. Sternberg. 1997. Male development and mating behavior, p. 295-334. In D. L. Riddle et al. (ed.), C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 11.Etemad-Moghadam, B., S. Guo, and K. J. Kemphues. 1995. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83:743-752. [DOI] [PubMed] [Google Scholar]
- 12.Evans, T., E. T. Rosenthal, J. Youngblom, D. Distel, and T. Hunt. 1983. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33:389-396. [DOI] [PubMed] [Google Scholar]
- 13.Fay, D. S., S. Keenan, and M. Han. 2002. fzr-1 and lin-35/Rb function redundantly to control cell proliferation in C. elegans as revealed by a nonbiased synthetic screen. Genes Dev. 16:503-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fay, D. S., and M. Han. 2000. Mutations in cye-1, a Caenorhabditis elegans cyclin E homolog, reveal coordination between cell cycle control and vulval development. Development 127:4049-4060. [DOI] [PubMed] [Google Scholar]
- 15.Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver, C. C. Mello. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806-811. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, A. G., R. S. Kamath, P. Zipperlen, M. Martinez-Campos, M. Sohrmann, and J. Ahringer. 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408:325-330. [DOI] [PubMed] [Google Scholar]
- 17.Furuta, T., S. Tuck, J. Kirchner, B. Koch, R. Auty, R. Kitagawa, A. M. Rose, and D. Greenstein. 2000. EMB-30: an APC4 homologue required for metaphase-to-anaphase transitions during meiosis and mitosis in Caenorhabditis elegans. Mol. Biol. Cell 11:1401-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gieffers, C., B. H. Peters, E. R. Kramer, C. G. Dotti, and J. M. Peters. 1999. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl. Acad. Sci. USA 96:11317-11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glotzer, M., M. W. Murray, and M. W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349:132-138. [DOI] [PubMed] [Google Scholar]
- 20.Golden, A., P. L. Sadler, M. R. Wallenfang, J. M. Schumacher, D. R. Hamill, G. Bates, B. Bowerman, G. Seydoux, and D. C. Shakes. 2000. Metaphase to anaphase (mat) transition-defective mutants in Caenorhabditis elegans. J. Cell Biol. 151:1469-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonczy, P., H. Schnabel, T. Kaletta, A. D. Amores, T. Hyman, and R. Schnabel. 1999. Dissection of cell division processes in the one cell stage Caenorhabditis elegans embryo by mutational analysis. J. Cell Biol. 144:927-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonczy, P., C. Echeverri, K. Oegema, A. Coulson, S. J. Jones, R. R. Copley, J. Duperon, J. Oegema, M. Brehm, E. Cassin, E. Hannak, M. Kirkham, S. Pichler, K. Flohrs, A. Goessen, S. Leidel, A. M. Alleaume, C. Martin, N. Ozlu, P. Bork, and A. A. Hyman. 2000. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature 408:331-336. [DOI] [PubMed] [Google Scholar]
- 23.Grill, S. W., P. Gonczy, E. H. Stelzer, and A. A. Hyman. 2001. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409:630-633. [DOI] [PubMed] [Google Scholar]
- 24.Gruneberg, U., M. Glotzer, A. Gartner, and E. A. Nigg. 2002. The CeCDC-14 phosphatase is required for cytokinesis in the Caenorhabditis elegans embryo. J. Cell Biol. 158:901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper, J. W., J. L. Burton, and M. J. Solomon. 2002. The anaphase-promoting complex: it's not just for mitosis anymore. Genes Dev. 16:2179-2206. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann-Petersen, R., M. Seeger, and C. Gordon. 2003. Transferring substrates to the 26S proteasome. Trends Biochem. Sci. 28:26-31. [DOI] [PubMed] [Google Scholar]
- 27.Hernault, S. W. L. 1997. Spermatogenesis, p. 271-294. In D. L. Riddle et al. (ed.), C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Hershko, A., D. Ganoth, J. Pehrson, R. E. Palazzo, and L. H. Cohen. 1991. Methylated ubiquitin inhibits cyclin degradation in clam embryo extracts. J. Biol. Chem. 266:16376-16379. [PubMed] [Google Scholar]
- 29.Hershko, A., D. Ganoth, V. Sudakin, A. Dahan, L. H. Cohen, F. C. Luca, J. V. Ruderman, and E. Eytan. 1994. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J. Biol. Chem. 269:4940-4946. [PubMed] [Google Scholar]
- 30.Huang, J. N., I. Park, E. Ellingson, L. E. Littlepage, and D. Pellman. 2001. Activity of the APC(Cdh1) form of the anaphase-promoting complex persists until S phase and prevents the premature expression of Cdc20p. J. Cell Biol. 154:85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irniger, S., S. Piatti, C. Michaelis, and K. Nasmyth. 1995. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81:269-278. [DOI] [PubMed] [Google Scholar]
- 32.Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin, M. Gotta, A. Kanapin, N. Le Bot, S. Moreno, M. Sohrmann, D. P. Welchman, P. Zipperlen, and J. Ahringer. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231-237. [DOI] [PubMed] [Google Scholar]
- 33.King, R. W., J. M. Peters, S. Tugendreich, M. Rolfe, P. Hieter, and M. W. Kirschner. 1995. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81:279-288. [DOI] [PubMed] [Google Scholar]
- 34.Kipreos, E. T., S. P. Gohel, and E. M. Hedgecock. 2000. The C. elegans F-box/WD-repeat protein LIN-23 functions to limit cell division during development. Development 127:5071-5082. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa, R., and A. M. Rose. 1999. Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nat. Cell Biol. 1:514-521. [DOI] [PubMed] [Google Scholar]
- 36.Kitagawa, R., E. Law, L. Tang, and A. M. Rose. 2002. The Cdc20 homolog, FZY-1, and its interacting protein, IFY-1, are required for proper chromosome segregation in Caenorhabditis elegans. Curr. Biol. 12:2118-2123. [DOI] [PubMed] [Google Scholar]
- 37.Klein, F., P. Mahr, M. Galova, S. B. Buonomo, C. Michaelis, K. Nairz, and K. Nasmyth. 1999. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98:91-103. [DOI] [PubMed] [Google Scholar]
- 38.Lambie, E. J. 2002. Cell proliferation and growth in C. elegans. Bioessays 24:38-53. [DOI] [PubMed] [Google Scholar]
- 39.Lee, J., T. Iwai, T. Yokota, and M. Yamashita. 2003. Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J. Cell Sci. 116:2781-2790. [DOI] [PubMed] [Google Scholar]
- 40.Lew, D. J., and D. J. Burke. 2003. The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37:251-282. [DOI] [PubMed] [Google Scholar]
- 41.Lyczak, R., J. E. Gomes, and B. Bowerman. 2002. Heads or tails: cell polarity and axis formation in the early Caenorhabditis elegans embryo. Dev. Cell 3:157-166. [DOI] [PubMed] [Google Scholar]
- 42.Maeda, I., Y. Kohara, M. Yamamoto, and A. Sugimoto. 2001. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 11:171-176. [DOI] [PubMed] [Google Scholar]
- 43.McCollum, D., and K. L. Gould. 2001. Timing is everything: regulation of mitotic exit and cytokinesis by the mitotic exit network and SIN. Trends Cell Biol. 11:89-95. [DOI] [PubMed] [Google Scholar]
- 44.Mito, Y., A. Sugimoto, and M. Yamamoto. 2003. Distinct developmental function of two Caenorhabditis elegans homologs of the cohesin subunit Scc1/Rad21. Mol. Biol. Cell 14:2399-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan, D. O. 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13:261-291. [DOI] [PubMed] [Google Scholar]
- 46.Morgan, D. O. 1999. Regulation of the APC and the exit from mitosis. Nat. Cell Biol. 1:E47-53. [DOI] [PubMed] [Google Scholar]
- 47.Murray, A. W., M. J. Solomon, and M. W. Kirschner. 1989. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339:280-286. [DOI] [PubMed] [Google Scholar]
- 48.Nasmyth, K. 2001. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35:673-745. [DOI] [PubMed] [Google Scholar]
- 49.O'Connell, K. F., C. M. Leys, and J. G. White. 1998. A genetic screen for temperature-sensitive cell-division mutants of Caenorhabditis elegans. Genetics 149:1303-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page, A. M., and P. Hieter. 1999. The anaphase-promoting complex: new subunits and regulators. Annu. Rev. Biochem. 68:583-609. [DOI] [PubMed] [Google Scholar]
- 51.Pasierbek, P., M. Jantsch, M. Melcher, A. Schleiffer, D. Schweizer, and J. Loidl. 2001. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 15:1349-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellettieri, J., and G. Seydoux. 2002. Anterior-posterior polarity in C. elegans and Drosophila—PARallels and differences. Science 298:1946-1950. [DOI] [PubMed] [Google Scholar]
- 53.Peter, M., A. Castro, T. Lorca, C. Le Peuch, L. Magnaghi-Jaulin, M. Doree, and J. C. Labbe. 2000. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol. 3:83-87. [DOI] [PubMed] [Google Scholar]
- 54.Peters, J. M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9:931-943. [DOI] [PubMed] [Google Scholar]
- 55.Piano, F., A. J. Schetter, M. Mangone, L. Stein, and K. J. Kemphues. 2000. RNAi analysis of genes expressed in the ovary of Caenorhabditis elegans. Curr. Biol. 10:1619-1622. [DOI] [PubMed] [Google Scholar]
- 56.Rappleye, C. A., A. Tagawa, R. Lyczak, B. Bowerman, and R. V. Aroian. 2002. The anaphase-promoting complex and separin are required for embryonic anterior-posterior axis formation. Dev. Cell 2:195-206. [DOI] [PubMed] [Google Scholar]
- 57.Rogers, E., J. D. Bishop, J. A. Waddle, J. M. Schumacher, and R. Lin. 2002. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 157:219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadler, P. L., and D. C. Shakes. 2000. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development 127:355-366. [DOI] [PubMed] [Google Scholar]
- 59.Salah, S. M., and K. Nasmyth. 2000. Destruction of the securin Pds1p occurs at the onset of anaphase during both meiotic divisions in yeast. Chromosoma 109:27-34. [DOI] [PubMed] [Google Scholar]
- 60.Schedl, T. 1997. Developmental genetics of the germline, p. 241-271. In D. L. Riddle et al. (ed.), C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 61.Shakes, D. C., P. L. Sadler, J. M. Schumacher, M. Abdolrasulnia, and A. Golden. 2003. Developmental defects observed in hypomorphic anaphase-promoting complex mutants are linked to cell cycle abnormalities. Development 130:1605-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shuster, C. B., and D. R. Burgess. 2002. Transitions regulating the timing of cytokinesis in embryonic cells. Curr. Biol. 12:854-858. [DOI] [PubMed] [Google Scholar]
- 63.Siomos, M. F., A. Badrinath, P. Pasierbek, D. Livingstone, J. White, M. Glotzer, and K. Nasmyth. 2001. Separase is required for chromosome segregation during meiosis I in Caenorhabditis elegans. Curr. Biol. 11:1825-1835. [DOI] [PubMed] [Google Scholar]
- 64.Sudakin, V., D. Ganoth, A. Dahan, H. Heller, J. Hershko, F. C. Luca, J. V. Ruderman, and A. Hershko. 1995. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6:185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sulston, J. E., E. Schierenberg, J. G. White, and J. N. Thomson. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100:64-119. [DOI] [PubMed] [Google Scholar]
- 66.Taieb, F. E., S. D. Gross, A. L. Lewellyn, and J. L. Maller. 2001. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from meiosis I to II in Xenopus oocytes. Curr. Biol. 11:508-513. [DOI] [PubMed] [Google Scholar]
- 67.Terret, M. E., K. Wassmann, I. Waizenegger, B. Maro, J. M. Peters, and M. H. Verlhac. 2003. The meiosis I-to-meiosis II transition in mouse oocytes requires separase activity. Curr. Biol. 13:1797-1802. [DOI] [PubMed] [Google Scholar]
- 68.Uhlmann, F., F. Lottspeich, and K. Nasmyth. 1999. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400:37-42. [DOI] [PubMed] [Google Scholar]
- 69.Uhlmann, F., D. Wernic, M. A. Poupart, E. V. Koonin, and K. Nasmyth. 2000. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103:375-386. [DOI] [PubMed] [Google Scholar]
- 70.Voges, D., P. Zwickl, and W. Baumeister. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68:1015-1068. [DOI] [PubMed] [Google Scholar]
- 71.Wallenfang, M. R., and G. Seydoux. 2000. Polarization of the anterior-posterior axis of C. elegans is a microtubule-directed process. Nature 408:89-92. [DOI] [PubMed] [Google Scholar]
- 72.Wan, Y., and M. W. Kirschner. 2001. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc. Natl. Acad. Sci. USA 98:13066-13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wasch, R., and F. R. Cross. 2001. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418:556-562. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe, Y., and P. Nurse. 1999. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400:461-464. [DOI] [PubMed] [Google Scholar]
- 75.Weinstein, J., F. W. Jacobsen, C. J. Hsu, T. Wu, and L. G. Baum. 1994. A novel mammalian protein, p55CDC, present in dividing cells is associated with protein kinase activity and has homology to the Saccharomyces cerevisiae cell division cycle proteins Cdc20 and Cdc4. Mol. Cell. Biol. 14:3350-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeong, F. M., H. H. Lim, C. G. Padmashree, and U. Surana. 2000. Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol. Cell 5:501-511. [DOI] [PubMed] [Google Scholar]
- 77.Yu, H. 2002. Regulation of APC-Cdc20 by the spindle checkpoint. Curr. Opin. Cell Biol. 14:706-714. [DOI] [PubMed] [Google Scholar]
- 78.Zachariae, W., and K. Nasmyth. 1999. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13:2039-2058. [DOI] [PubMed] [Google Scholar]
- 79.Zipperlen, P., A. G. Fraser, R. S. Kamath, M. Martinez-Campos, and J. Ahringer. 2001. Roles for 147 embryonic lethal genes on C. elegans chromosome I identified by RNA interference and video microscopy. EMBO J. 20:3984-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]