Abstract
The c-Abl nonreceptor tyrosine kinase is activated by growth factor signals such as the platelet-derived growth factor (PDGF) and functions downstream of the PDGF-β receptor (PDGFR) to mediate biological processes such as membrane ruffling, mitogenesis, and chemotaxis. Here, we show that the related kinase Arg is activated downstream of PDGFRs in a manner dependent on Src family kinases and phospholipase C γ1 (PLC-γ1)-mediated phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis, as we showed previously for c-Abl. PIP2, a highly abundant phosphoinositide known to regulate cytoskeletal and membrane proteins, inhibits the tyrosine kinase activities of both Arg and c-Abl in vitro and in cells. We now demonstrate that c-Abl and Arg form inducible complexes with and are phosphorylated by the PDGFR tyrosine kinase in vitro and in vivo. Moreover, c-Abl and Arg, in turn, phosphorylate the PDGFR. We show that c-Abl and Arg exhibit nonredundant functions downstream of the activated PDGFR. Reintroduction of c-Abl into Arg-Abl double-null fibroblasts rescues the ability of PLC-γ1 to increase PDGF-mediated chemotaxis, while reexpression of Arg fails to rescue the chemotaxis defect. These data show that, although both kinases are activated and form complexes with proteins in the PDGFR signaling pathway, only c-Abl functions downstream of PLC-γ1 to mediate chemotaxis.
The mammalian Abelson (Abl) family of nonreceptor tyrosine kinases is comprised of c-Abl and Arg (Abl-related gene), which are encoded by the Abl1 and Abl2 genes, respectively (30). The kinase activities of c-Abl and Arg are tightly regulated in cells by intra- and intermolecular interactions (14, 30). Mutagenesis and structural studies have shown that c-Abl is maintained in an autoinhibited state by intramolecular interactions involving the c-Abl SH3 domain and a proline residue located in the linker region connecting the SH2 and tyrosine kinase (SH1) domains, as well as by additional interactions that pack the SH3 and SH2 domains against the kinase domain (14, 30). Sequences amino terminal to the SH3 domain are also involved in stabilizing the autoinhibited conformation of c-Abl through interactions with the SH3, SH2, and SH1 domains (13, 14, 27). Intermolecular interactions are also involved in maintaining the autoinhibited conformation of c-Abl in cells as overexpression or enzymatic purifications result in c-Abl activation (3, 32), and several proteins have been shown to bind and inhibit the c-Abl kinase (30). Activation of c-Abl may occur through binding or phosphorylation events that disrupt the intramolecular inhibitory interactions (3, 13, 27, 45).
Deregulation of the c-Abl and Arg tyrosine kinases can occur as a result of chromosomal translocation events that produce chimeric proteins associated with the development of human leukemias (31, 39). Abl1 is translocated to the Bcr and Tel genes, resulting in the expression of activated Bcr-Abl and Tel-Abl oncogenic tyrosine kinases, respectively (31). Bcr-Abl is implicated in chronic myelogenous leukemia (CML), while Tel-Abl has been identified in a subset of acute lymphoblastic and myeloid leukemias. Similarly, Abl2 is translocated to the Tel gene, producing the activated Tel-Arg tyrosine kinase associated with a subset of acute myeloid leukemias (31, 39).
Recent studies revealed that, similarly to c-Abl, the Bcr-Abl oncoprotein associated with CML is negatively regulated by intramolecular interactions and that the phosphorylation state of Bcr-Abl affects its ability to bind to pharmacological inhibitors of the Abl kinase such as STI-571 (Gleevec) (43). STI-571 is a 2-phenylaminopyrimidine derivative that binds to the ATP site of the Abl kinase domain and preferentially interacts with the inactive conformation of the kinase (40). STI-571 has proven to be effective in the treatment of patients with the early, chronic phase of CML. However, most patients treated during the aggressive, blast crisis phase of CML evolve drug resistance to STI-571 (7, 38). Patients that are STI-571 resistant have been shown to harbor mutations that are predicted to destabilize the inactive conformation of the Abl kinase (1, 42). These findings underscore the need for novel Abl kinase inhibitors to treat patients who become resistant to drugs like STI-571 that bind to the ATP site of the kinase.
Recently, we uncovered an unexpected mechanism for the regulation of the c-Abl tyrosine kinase by phosphatidylinositol 4,5-bisphosphate (PIP2) (33). We showed that activation of endogenous c-Abl by platelet-derived growth factor (PDGF) requires functional phospholipase C-γ1 (PLC-γ1) activity and its recruitment to the PDGF receptor (PDGFR) complex and that decreasing cellular PIP2 levels by either PLC-γ1-mediated hydrolysis or dephosphorylation by a PIP2-specific inositol polyphosphate 5-phosphatase (INP54) produces a marked increase of endogenous c-Abl tyrosine kinase activity (33). We showed that PIP2 inhibits the c-Abl kinase activity in vitro (33). These findings suggest that PIP2 binding may be a general mechanism for the regulation of Abl family kinases and that new pharmacological agents could be developed to inhibit deregulated Abl tyrosine kinases based on the inhibitory effect of PIP2 on the Abl kinase activity.
Definition of the mechanisms that regulate the activities of the endogenous Abl family kinases is critical to the understanding of a number of cellular processes that have been reported to be affected by loss- or gain-of-function of the Abl kinases. The c-Abl and Arg kinases have redundant roles during mouse development, as homozygous knockout mice lacking both c-Abl and Arg die during embryonic development but the single-knockout mice are viable (23). Notably, the c-Abl and Arg single-knockout mice exhibit distinct phenotypes (23, 41, 46). The differences may be due to differential levels of expression of c-Abl and Arg in various tissues or to unique, nonredundant functions of the two proteins. In this regard, there are differences in the structures and localization of these two tyrosine kinases. Arg and c-Abl are 90% identical in their amino-terminal SH3, SH2, and SH1 domains but are only 29% identical in their carboxy-terminal domains that comprise approximately half of the proteins. While both c-Abl and Arg share actin binding domains in their carboxy terminus, Arg lacks the nuclear localization signals and DNA binding domains present in the c-Abl carboxy terminus (30). Moreover, while c-Abl localizes to the nucleus, plasma membrane, and actin cytoskeleton, Arg does not accumulate in the nucleus. Arg is primarily present in the cytoplasm and colocalizes with F-actin-rich structures (30, 48). Together, these findings suggest that c-Abl and Arg may have overlapping as well as distinct cellular functions.
The c-Abl tyrosine kinase has been reported to be activated by various stimuli in addition to growth factors. These include integrin engagement (24), DNA damage (22, 47), and oxidative stress (44). In contrast, little is known regarding the signals that result in the activation of the endogenous Arg tyrosine kinase. Similar to c-Abl, Arg was reported to be activated by reactive oxygen species and to affect the apoptotic response to oxidative stress in cultured cells (4). However, the molecular mechanism that underlies Arg activation by oxidative stress remains to be defined. More recently, purified Arg was shown to be activated by autophosphorylation and by phosphorylation by a Src family kinase in vitro (45).
Here we show that endogenous Arg is activated by growth factor stimulation and that Arg kinase activity is regulated by PIP2 in vitro and in cells. Moreover, both Arg and c-Abl form a complex with the PDGFR and undergo reciprocal phosphorylation with the receptor tyrosine kinase. Unexpectedly, we found that, although both Arg and c-Abl are activated by PDGF growth factors via a similar mechanism, they have distinct cellular functions downstream of PDGF.
MATERIALS AND METHOD
Materials
U73122 and its inactive analog U73343 were purchased from BioMol (Plymouth Meeting, Pa.). Antibodies directed against c-Abl (K12, Santa Cruz Biotechnology; AB-3, Oncogene Sciences; 8E9, PharMingen), antiphosphotyrosine (4G10, Upstate Biotechnology Inc.; PY99, Santa Cruz Biotechnology), and glutathione S-transferase (GST; Santa Cruz Biotechnology) were purchased commercially. Antibodies directed against the extracellular domain of PDGFR-α (80.8) (6), the intracellular domain of PDGFR-β (30A) (6), PLC-γ1 (19), and c-Abl (Pex4) (34) were described previously. Antibody to Arg was produced by immunizing rabbits with a peptide specific for a unique C-terminal region (9). Phospholipids were purchased from Avanti Polar Lipids, and PDGF-AA and PDGF-BB were obtained from Upstate Biotechnology Inc.
DNA constructs
c-Abl, PLC-γ1, and INP54 constructs were described previously (33, 34). Murine Arg cDNA was previously described (45, 48). Arg-PP was generated by mutating two proline residues in the interlinker region connecting the SH2 and SH1 domains to glutamate, as described for Abl (2). The cDNAs encoding wild-type, activated, and kinase-inactive forms of Arg were cloned into the bicistronic vector pK1, which contains puromycin in the second cistron, as well as into pcDNA1-AMP (Invitrogen Life Technologies).
Cell lines
Ph cells, which lack endogenous PDGFR-α, and Ph cells expressing wild-type and mutant chimeric or PDGF-α receptors were described previously (6, 37). Arg-Abl double-null mouse embryonic fibroblasts (MEFs) were obtained and reconstituted as previously described (23, 33).
Fusion proteins
GST-Crk and GST fusion proteins containing fragments of c-Abl were previously described (34). GST-Arg SH2 fusion protein containing residues 162 to 259 of murine Arg and GST-Arg SH2-SH3, GST-Arg 694-930, and GST-Arg 1034-1182 were as previously described (48).
Transfection, retroviral infection, and Sf9 infection
293T cells were transfected with calcium chloride, fibroblasts were infected with retroviruses, and Sf9 cells were infected as previously described (34). Baculoviruses for GST-Abl-KR (25), GST-Abl-SH2-SH1-KR (34), and murine PDGFR-β (15) were described previously. Wild-type and kinase-inactive Arg were cloned into HT-a and His-tagged baculovirus vectors, and the corresponding baculoviruses were produced as described by the manufacturer (Invitrogen Life Technologies).
Immunoprecipitation, Western blotting-far-Western blotting, and GST pull-down and kinase assays
Cells were lysed in a Triton lysis buffer (used for kinase assays, GST pull-downs, and coimmunoprecipitations) or radioimmunoprecipitation assay buffer (used for immunoprecipitation and phosphotyrosine analyses). Kinase assays were performed as previously described (34). Kinase assays utilizing liposomes containing phospholipids were described previously (33). For far-Western blots, whole-cell lysates or immunoprecipitates were separated by polyacrylamide gel electrophoresis followed by protein transfer to nitrocellulose filters and blotting. Filters were blocked in 50 mM Tris (pH 7.4)-150 mM NaCl (Tris-NaCl) containing 5% bovine serum albumin, followed by incubation with GST fusion proteins (1.5 μg/ml) in Tris-NaCl containing 0.1% Triton X-100, 10% glycerol, and 2% bovine serum albumin. GST binding was visualized by incubation with antibody to GST, followed by horseradish peroxidase-conjugated secondary antibody.
Transwell migration assays
Transwell migration assays were performed as previously described (33), by using Abl-Arg double-null fibroblasts infected with retroviruses encoding vectors (Migr1 and Mig-CD4), c-Abl (Mig-CD4-c-Abl-wt), Arg (PK1-Arg-wt), and PLC-γ1 (Migr1-PLC-γ1). Migr1 is a bicistronic vector containing green fluorescent protein (GFP) in the second cistron. The under surface of the transwell membrane was coated with collagen, and cells were placed in the upper chamber and allowed to migrate in the absence or presence of PDGF-BB. Transfected, GFP-positive cells were counted on a fluorescence microscope.
RESULT
Abl family kinases are activated by PDGFR-α and PDGFR-β
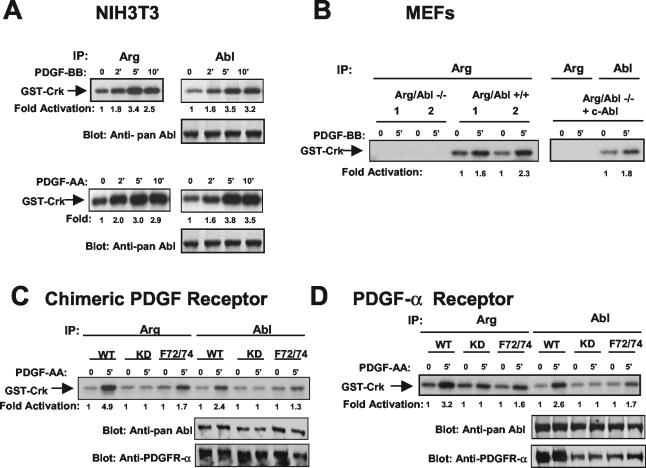
To determine whether Arg is activated by PDGF, we stimulated NIH 3T3 cells with PDGF-BB and assayed Arg kinase activity by an in vitro kinase assay using GST-Crk as a substrate. The Arg kinase was activated by PDGF-BB with the same kinetics as we previously observed for c-Abl (Fig. 1A, top). PDGF-BB induces dimerization and activation of both PDGFR-α and PDGFR-β receptors (12). To determine whether the Abl family kinases are activated downstream of PDGFR-α, NIH 3T3 cells were stimulated with PDGF-AA, which activates only PDGFR-α. The c-Abl and Arg kinases were activated with similar kinetics by PDGF-AA stimulation (Fig. 1A, bottom). To test whether Arg is activated by PDGFR-β signaling events, we employed Ph cells, a murine fibroblast cell line with a deletion of the endogenous PDGFR-α that was engineered to express a chimeric receptor containing the extracellular domain of PDGFR-α and the intracellular domain of PDGFR-β. This chimeric receptor is activated by PDGF-AA specifically but induces PDGFR-β signaling events (6). Arg kinase activity was increased fivefold following stimulation of the PDGFR-β pathway (Fig. 1C). These results show that specific stimulation of PDGFR-α or PDGFR-β results in activation of the endogenous c-Abl and Arg tyrosine kinases.
FIG. 1.
Abl family tyrosine kinase activity is elevated following activation of PDGFR-α and PDGFR-β. (A) NIH 3T3 cells were starved and stimulated with PDGF-BB or PDGF-AA. Endogenous c-Abl and Arg were immunoprecipitated and incubated in an in vitro kinase assay with GST-Crk as a substrate. Lysates were blotted with antibody that recognizes both c-Abl and Arg (pan-Abl; 8E9). (B) MEFs lacking both c-Abl and Arg, fibroblasts from wild-type littermates (two independent sets, 1 and 2), and Arg-Abl null fibroblasts reconstituted with c-Abl by retroviral infection were starved and stimulated with PDGF-BB. Arg and Abl kinase activities were assessed as described above. (C) Ph cells expressing wild-type (WT), kinase-defective (KD), or F72/74 chimeric PDGFRs were starved and stimulated with PDGF-AA. Arg and Abl kinase activities and protein expression were assessed as described above. Lysates also were blotted with antibody to the extracellular domain of PDGFR-α (80.8), which recognizes chimeric receptors. (D) Ph cells expressing wild-type (WT), kinase-defective (KD), or F72/72 PDGFR-α were treated as described for panel C. Arg and Abl kinase activities and protein expression were determined as described above. Lysates also were blotted with antibody that recognizes the PDGFR-α extracellular domain (80.8), which recognizes PDGFR-α. The results are representative of three independent experiments.
To confirm that the Arg antibody specifically immunoprecipitates Arg and does not cross-react with c-Abl or another kinase, Arg was immunoprecipitated from MEFs derived from Arg-Abl null mice, wild-type MEFs, or Arg-Abl null MEFs that were reconstituted with c-Abl (33). The immunoprecipitates were incubated in an in vitro kinase assay. Arg kinase activity was observed only in wild-type fibroblasts and was not observed in Arg-Abl null fibroblasts or the same fibroblasts reconstituted with c-Abl (Fig. 1B), thereby showing that the Arg antibody specifically immunoprecipitates Arg.
We previously showed that activation of c-Abl by PDGF is partially dependent on the prior activation of the Src family of nonreceptor tyrosine kinases (33, 34). To determine whether Arg activation by PDGF also requires Src kinase activity, we employed Ph cells expressing chimeric PDGFRs containing phenylalanine substitutions at two tyrosine residues known to bind to Src in the intracellular domain of PDGFR-β. After PDGF stimulation, the activities of the endogenous Arg and c-Abl kinases were analyzed and compared to that observed in Ph cells expressing a wild-type form of the chimeric receptor. Activation of both Arg and c-Abl by PDGF was reduced but not abolished in cells expressing a receptor that lacked the Src binding sites (F72/74) (Fig. 1C). To determine whether activation of c-Abl and Arg by PDGFR-α is also dependent on the Src kinases, we performed the same assay with Ph cells expressing wild-type or mutant PDGFR-α receptors. Mutation of the Src binding sites also decreased activation of c-Abl and Arg by the PDGFR-α receptor (Fig. 1D). These results show that activation of c-Abl and Arg by both PDGFR-α and PDGFR-β requires activation of the Src family kinases. Previously, we demonstrated that endogenous c-Abl kinase activity is highly elevated in cells expressing oncogenic forms of Src kinases (34). Likewise, here we found that Arg activity was increased over 10-fold in the presence of activated forms of Src (data not shown). Endogenous c-Abl and Arg are tyrosine phosphorylated in the presence of activated Src family kinases (8, 11; data not shown). Additionally, Src family kinases directly phosphorylate and activate both Arg and c-Abl (34, 45). Taken together, these results show that c-Abl and Arg are activated by both PDGFR-α and PDGFR-β receptors in a manner that is partially dependent on the prior activation of the Src family kinases.
Arg activation by PDGF occurs via a PLC-γ1-mediated mechanism
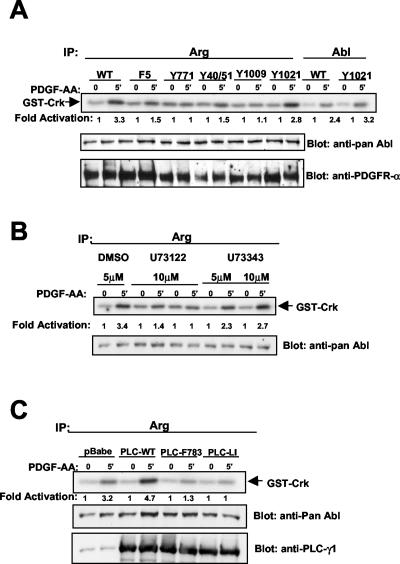
We previously demonstrated that c-Abl activation by PDGF requires not only Src activity but also the activity of PLC-γ1 (33). Src activity, although necessary, was not sufficient for the activation of c-Abl by PDGF (33). To determine whether Arg activation by PDGF occurs via a similar mechanism, we examined whether PLC-γ1 activity was required for Arg activation. The activation of Arg by PDGF was substantially decreased in cells expressing a chimeric PDGFR (F5) lacking the binding sites for phosphoinositide 3-kinase, Ras-Gap, SHP-2, and PLC-γ1 but retaining the Src binding sites (Fig. 2A). Activation of Arg was rescued by restoring the binding site for PLC-γ1, Y1021 (Fig. 2A). These results show that PLC-γ1 activity is required for Arg activation by PDGF. To confirm these data, we utilized a pharmacological PLC-γ1 inhibitor (Fig. 2B) and PLC-γ1 dominant-negative mutants (Fig. 2C). Pretreatment of cells with a PLC-γ1 inhibitor (U73122) inhibited Arg activation by PDGF (Fig. 2B). No inhibition of Arg activity was observed in cells treated with vehicle (dimethyl sulfoxide) or the inactive analog (U73343) (Fig. 2B). Expression of two PLC-γ1 dominant-negative mutant proteins (PLC-F783 and PLC-LI) (33) also inhibited Arg activation following PDGF treatment (Fig. 2C). In particular, the lipase-inactive PLC-LI mutant completely inhibited Arg activation by PDGF (Fig. 2C).
FIG. 2.
Activation of Arg by PDGF requires PLC-γ1 activity. (A) Ph cells expressing wild-type (WT) or mutant chimeric PDGFRs were starved and stimulated with PDGF-AA, and Arg and Abl kinase activities were determined. Lysates were blotted as described for Fig. 1. (B) Ph cells expressing wild-type chimeric PDGFRs were treated for 10 min prior to PDGF-AA stimulation with dimethyl sulfoxide (DMSO), U73122 (PLC-γ1 inhibitor), or U73343 (inactive analog). Arg kinase activity was assessed as described above. (C) NIH 3T3 cells were retrovirally infected with vector (pBabepuro), wild-type PLC-γ1 (WT), or two dominant-negative forms of PLC-γ1 (PLC-F783 and PLC-LI). Arg kinase activity was determined following starvation and stimulation of the infected cells. Lysates were blotted with pan-Abl antibody and antibody to PLC-γ1. The results are representative of three independent experiments.
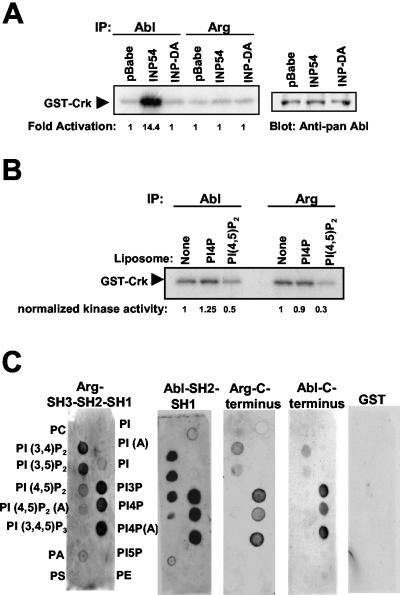
Since PLC-γ1 activity is required for activation of both c-Abl (33) and Arg, we examined whether decreasing the cellular levels of PIP2, the PLC-γ1 substrate, by use of an alternative approach would affect Arg kinase activity. We previously showed that expression of the yeast (INP54, which specifically dephosphorylates PIP2 to produce phosphatidylinositol 4-phosphate (PI4P), potently activates the endogenous c-Abl kinase (Fig. 3A) (33). This finding further supports the notion that decreasing the levels of PIP2 activates c-Abl kinase activity. In contrast, expression of an inactive form of the phosphatase (INP-DA) had no effect on c-Abl kinase activity (Fig. 3A) (33). These data suggest that PIP2 either directly or indirectly inhibits c-Abl kinase activity. To determine whether Arg is also regulated in a PIP2-dependent manner, we tested whether expression of INP54 could activate the endogenous Arg kinase. Unlike c-Abl, Arg was not activated by expression of the active 5-phosphatase (Fig. 3A). These results suggest either that Arg is not regulated by PIP2 or that Arg does not localize to the same subcellular compartment as does the pool of c-Abl that is activated by INP54. To determine whether PIP2 can directly inhibit Arg kinase activity, Arg immunoprecipitates were incubated with liposomes containing PIP2. PIP2 inhibited Arg kinase activity by 75%, while PI4P had no effect (Fig. 3B). PIP2-containing liposomes inhibited c-Abl to a similar level as did Arg (Fig. 3B). PIP2 and other lipids were capable of binding directly to Arg (Fig. 3C). Fragments of Arg and c-Abl containing the kinase domains (SH1) bound to a subset of lipids including PIP2 (Fig. 3C, left). C-terminal fragments of c-Abl and Arg bound to some of the lipids but did not bind PIP2 (Fig. 3C, right). Notably the c-Abl SH2 and Arg SH2 domains did not bind lipids (data not shown), but proteins containing the catalytic SH1 domains fused to their SH2 domains bound strongly to lipids including PIP2. This finding suggests that the kinase domain is the domain that interacts with PIP2. Together, these data show that Arg activation requires PLC-γ1 activity and that PIP2 binds Arg and inhibits its activity. Thus, Arg appears to be regulated by PDGF in the same manner as c-Abl. However, expression of INP54, a PIP2-specific phosphatase, did not increase Arg kinase activity as was observed for c-Abl. The differential effect of INP54 might be explained by localization differences between c-Abl and Arg. Unlike c-Abl, Arg is not expressed in the nucleus (30). The PIP2 phosphatase, which is not targeted to the plasma membrane, might shuttle in and out of the nucleus to dephosphorylate nuclear pools of PIP2. A large pool of c-Abl resides in the nucleus (30), and this may be the pool that is activated by INP54.
FIG. 3.
Arg is inhibited by PIP2 in vitro but is not activated by INP54 expression. (A) NIH 3T3 cells were retrovirally infected with either vector (pBabepuro), INP54, or an inactivated version of the phosphatase (INP-DA). Endogenous c-Abl or Arg was immunoprecipitated and subjected to in vitro kinase assay. (B) c-Abl and Arg were expressed in 293T cells, immunoprecipitated, and subjected to an immune complex kinase assay in the absence or presence of liposomes containing PI4P or PIP2. (C) Nitrocellulose filters containing the indicated phospholipids (Echelon) were incubated with GST alone or with GST fusion proteins of the indicated regions of Arg or c-Abl, followed by antibody to GST and secondary antibody and by chemiluminescence detection.
Abl family tyrosine kinases form a complex with PDGFRs
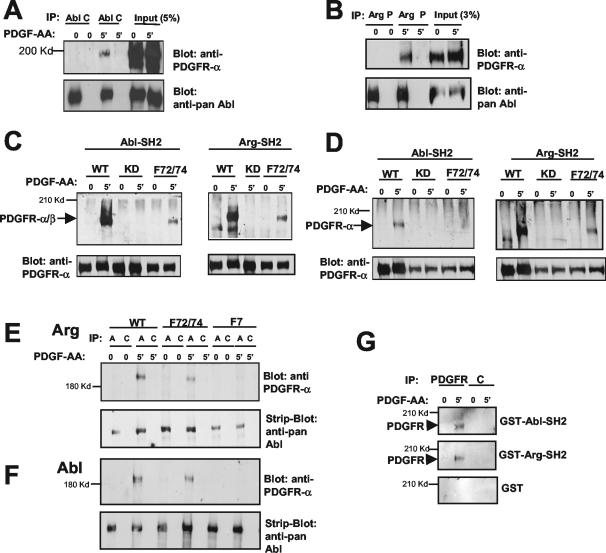
We showed previously that c-Abl binds to PLC-γ1 and that this binding is enhanced after PDGF stimulation (33). Thus, we wished to determine whether Arg and c-Abl could form a complex with the PDGFRs at the plasma membrane in response to PDGF stimulation. We found that both c-Abl and Arg coimmunoprecipitated with wild-type chimeric PDGFRs after PDGF stimulation (Fig. 4A and B). To identify the domains of c-Abl and Arg that interact with the PDGFR, we utilized various fragments of c-Abl and Arg fused to GST in a GST pull-down assay (data not shown; Fig. 4C and D). We found that only the c-Abl-SH2 and Arg-SH2 domains mediated the interaction and bound to wild-type forms of both the chimeric PDGFR-α/β and the PDGFR-α (Fig. 4C and D and data not shown). The c-Abl and Arg SH2 domains did not interact with receptors that lacked kinase activity (Fig. 4C and D). Thus, the c-Abl and Arg SH2 domains bound to phosphorylated tyrosine residues in the intracellular domains of both PDGFR-α and PDGFR-β. Interestingly, mutation of the Src binding sites (F72/74) decreased the interaction between the c-Abl and Arg SH2 domains and the PDGFRs (Fig. 4C and D). Likewise, mutation of the Src binding sites reduced, but did not abolish, the binding of full-length Arg and c-Abl with PDGFR-β (Fig. 4E and F). The wild-type and mutant forms of the PDGFRs were expressed to similar levels in Ph cells (Fig. 4C and D and data not shown). Mutation of the Src binding sites together with the binding sites of phosphatidylinositol 3-kinase, SHP-2, Ras-GAP, and PLC-γ1 (F7) completely eliminated the binding of c-Abl and Arg to the PDGFR-β (Fig. 4E and F). Together, these data show that c-Abl and Arg are recruited to the PDGFR following PDGF stimulation and suggest that Abl family kinases can bind directly or indirectly to Y572/574 and potentially to an additional site(s) on the receptor.
FIG. 4.
Abl family kinases form a complex with PDGFRs. (A, B, E, and F) c-Abl and Arg levels in Ph cells expressing similar levels of chimeric PDGFRs were increased by retroviral infection. c-Abl (A and F) and Arg (B and E) were immunoprecipitated with anti-Abl or anti-Arg antibodies (lanes A in panel E). Irrelevant control antibody (C) (c-Abl) or preimmune serum (P) (Arg) was used as a control. Immunoprecipitates were blotted with antibody to the extracellular domain of PDGFR-α (80.8), which recognizes the chimeric receptor (upper panels). The blots were stripped and reprobed with antibody that recognizes c-Abl and Arg (pan-Abl; 8E9) (lower panels). (C and D) Abl and Arg SH2 domains fused to GST were used to pull down chimeric PDGFR (C) or PDGFR-α (D) from Ph cells expressing wild-type (WT), kinase-dead (KD), or F72/74 receptors (upper panels). Western blot analysis of whole-cell lysates showed similar levels of wild-type and mutant forms of the PDGFRs (lower panels). (G) Nitrocellulose filters containing PDGFR or control (C) immunoprecipitates from cells expressing a chimeric PDGFR were probed with GST, GST-Abl-SH2, or GST-Arg-SH2 protein, followed by Western blot analysis with antibody to the GST tag.
To determine whether c-Abl and Arg could bind directly to the PDGFR without potential bridging proteins, we performed a far-Western analysis on immunoprecipitates from cells expressing a wild-type chimeric PDGFR. Following immunoprecipitation of the PDGFR, the immunoprecipitates were analyzed by gel electrophoresis, and the proteins were transferred to nitrocellulose filters and probed with GST-Abl-SH2, GST-Arg-SH2, or GST alone, followed by Western blot analysis using antibody to GST. GST-Abl-SH2 and GST-Arg-SH2 reacted specifically with immunoprecipitated PDGFR (Fig. 4G), while GST alone did not. These results show that the interaction between the Abl family kinases and PDGFRs is direct and does not require additional proteins to act as a bridge.
Abl family tyrosine kinases are phosphorylated by PDGFRs
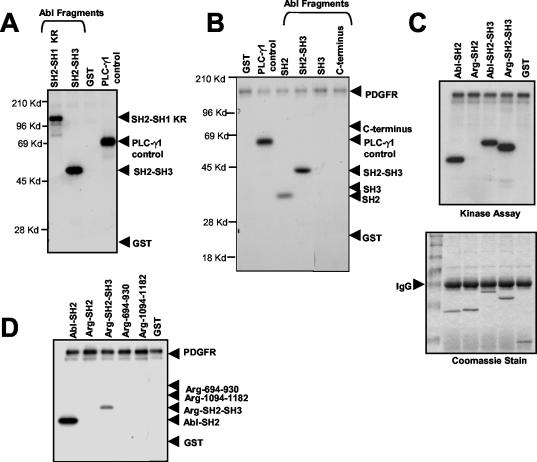
A subset of proteins that bind to activated PDGFRs are tyrosine phosphorylated by the receptors (17). To determine whether this is the case for the Abl family kinases, we immunoprecipitated active chimeric PDGFR from stimulated Ph cells or active PDGFR-β produced following baculovirus infection of insect Sf9 cells and incubated the immunoprecipitates in an in vitro kinase assay with fragments of c-Abl or Arg fused to GST. PDGFR-β phosphorylated all fragments of c-Abl that contained an SH2 domain, including SH2-SH1-KR, SH2-SH3, and SH2 alone (Fig. 5A and B). In contrast, while the Arg SH2 domain alone was not phosphorylated by PDGFR-β, an Arg SH2-SH3 fusion protein was phosphorylated by the receptor kinase (Fig. 5C). The Arg SH2 domain construct contained the same homologous sequence as that present in the c-Abl-SH2 construct, as well as an additional SH2-SH1 linker sequence that was not present in the c-Abl-SH2 construct. These results suggest that the PDGFR may phosphorylate c-Abl and Arg at different sites. The c-Abl protein is most likely phosphorylated in the SH2 domain, while Arg phosphorylation may occur on the SH3 domain. C-terminal sequences from c-Abl (Fig. 5B) and Arg (Fig. 5D) (694 to 930 and 1094 to 1182) were not phosphorylated by PDGFR-β in vitro.
FIG. 5.
PDGFR-β phosphorylates Abl kinases in vitro. (A) Chimeric PDGFR was immunoprecipitated (30A antibody) from starved-stimulated Ph cells and incubated in an in vitro kinase assay with fragments of Abl fused to GST, GST alone, or the positive control PLC-γ1. (B to D) Murine PDGFR-β expressed in Sf9 cells following baculovirus infection was immunoprecipitated (30A antibody) and incubated with fragments of Abl or Arg fused to GST. GST alone and PLC-γ1 served as negative and positive controls, respectively. Substrate amounts were equivalent as determined by Coomassie blue staining (C, lower panel, and data not shown). IgG, immunoglobulin G.
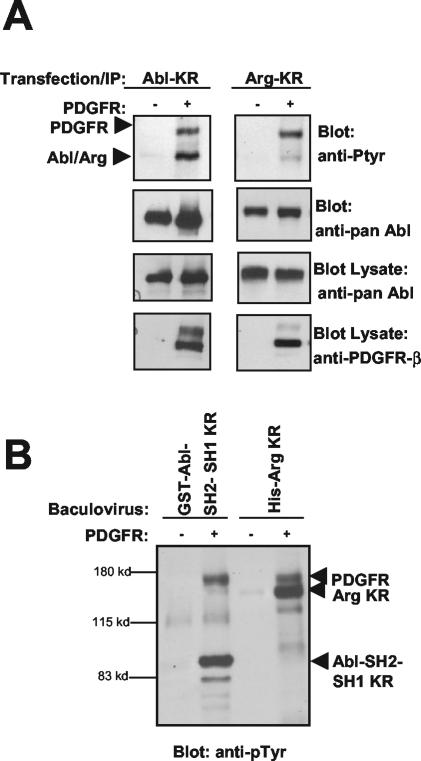
To determine whether c-Abl and Arg are phosphorylated by PDGFR-β in vivo, we coexpressed PDGFR-β with full-length, kinase-inactive forms of c-Abl and Arg. As shown in Fig. 6A, kinase-inactive c-Abl and Arg were tyrosine phosphorylated and coimmunoprecipitated with overexpressed PDGFR-β. Additionally, we coexpressed PDGFR-β with full-length, kinase-inactive c-Abl (GST-Abl-KR); a fragment of Abl containing the SH2 domain and a catalytically inactive kinase domain (GST-Abl-SH2-SH1-KR); and His-tagged, full-length, kinase-inactive Arg (His-Arg-KR) in Sf9 insect cells. The GST-Abl-KR protein exhibited the same molecular weight on the gel as did the PDGFR, and therefore it was difficult to ascertain whether it was phosphorylated by PDGFR-β (data not shown). Coexpression of PDGFR with GST-Abl-SH2-SH1-KR or with His-Arg-KR clearly demonstrated phosphorylation on the c-Abl fragment and the full-length Arg following precipitation and phosphotyrosine blotting (Fig. 6B). These data show that c-Abl and Arg are phosphorylated by the PDGFR in vitro and in vivo.
FIG. 6.
PDGFR-β phosphorylates Abl family kinases in cells. (A) Kinase-inactive c-Abl and Arg were expressed in the absence (−) or presence (+) of the human PDGFR-β in 293T cells. c-Abl and Arg were expressed from different promoters (c-Abl-Migr1 and Arg-pcDNA) in order to obtain equivalent c-Abl and Arg protein expression. The kinase-inactive c-Abl and Arg were immunoprecipitated and probed with antibodies to phosphotyrosine (top). Note that the kinase-defective Abl family kinases are tyrosine phosphorylated only in the presence of active PDGFR-β. The blot was stripped and reprobed with antibody that recognizes both c-Abl and Arg (second panel). Lysates were blotted with antibody that recognizes both c-Abl and Arg (pan-Abl) and with antibody that recognizes PDGFR-β (30A). (B) Baculovirus encoding a kinase-inactive fragment of c-Abl fused to GST (GST-Abl-SH2-SH1-KR) and full-length His-tagged kinase-inactive Arg (His-Arg-KR) was coexpressed with murine PDGFR-β in insect Sf9 cells. The c-Abl and Arg proteins were precipitated with glutathione Sepharose or nickel agarose, respectively, and blotted with antibody to phosphotyrosine.
Abl family kinases phosphorylate PDGFR-β
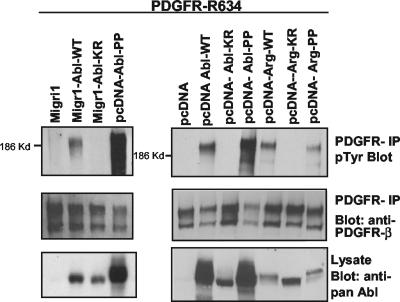
Src family kinases bind to activated PDGFR-β, are phosphorylated by the receptor, and subsequently phosphorylate the receptor, producing a phosphotyrosine binding site for other molecules (12). Therefore, we examined whether the Abl kinases were competent to phosphorylate PDGFR-β. We coexpressed a kinase-inactive form of the PDGFR-β (R634) together with wild-type or constitutively active forms of c-Abl (Abl-PP) (2) and Arg (Arg-PP). Arg-PP was created by mutating two proline residues in the interlinker region to glutamate, analogous to the same mutation previously described for c-Abl (2). Expression of wild-type and constitutively activated forms of c-Abl and Arg induced phosphorylation of the PDGFR, while kinase-inactive forms (KR) of c-Abl and Arg did not (Fig. 7). Although the expression level of wild-type Arg was lower than that for c-Abl, Arg induced similar levels of phosphorylation on the PDGFR (Fig. 7). When expressed at similar levels, c-Abl-PP (constitutively active version) phosphorylated the PDGFR to slightly higher levels than did the wild-type c-Abl. Thus, expression of active forms of c-Abl and Arg induces tyrosine phosphorylation of PDGFR-β.
FIG. 7.
Abl kinases phosphorylate PDGFR-β. Wild-type (WT), kinase-inactive (KR), or constitutively active (PP) forms of c-Abl and Arg were coexpressed with a kinase-inactive version of PDGFR-β (R634) in 293T cells. Migr1 and pcDNA vectors were used in order to obtain low and high levels of expression, respectively. Wild-type and kinase-inactive Abl were expressed in Migr1 (left panels), because in this vector expression of the two proteins is equivalent. Expression of wild-type Abl and Abl-PP from the pcDNA vector resulted in equivalent protein levels (bottom right panel), therefore allowing for direct comparison of PDGFR tyrosine phosphorylation by these two Abl forms (top right panel). The PDGFR was immunoprecipitated with antibody to the intracellular domain of PDGFR-β (30A) and probed with antibody to phosphotyrosine. The blots were stripped and reprobed for PDGFR-β (30A).
PLC-γ-mediated chemotaxis requires c-Abl and not Arg
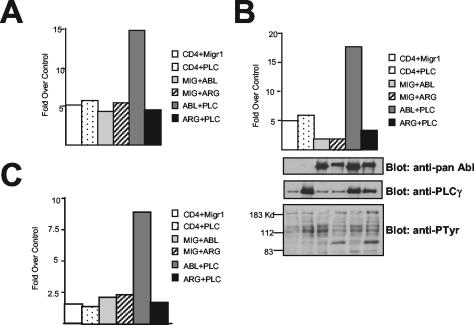
In porcine aortic endothelial cells, PLC-γ1 overexpression increases chemotaxis toward PDGF-BB (36). We previously showed that c-Abl functions downstream of PLC-γ1 during chemotaxis, as expression of kinase-inactive c-Abl blocks PLC-γ1-induced chemotaxis toward PDGF-BB in porcine aortic endothelial cells (33). Therefore, we examined whether c-Abl has a similar function in fibroblasts and whether Arg could function in a redundant fashion with c-Abl during PLC-γ1-mediated PDGF chemotaxis. In MEFs lacking c-Abl and Arg, PLC-γ1 was unable to increase chemotaxis toward PDGF-BB (Fig. 8). Unexpectedly, this migration defect was rescued by reexpression of wild-type c-Abl but not wild-type Arg. Reexpression of c-Abl and Arg proteins in the null cells to levels similar to those present in wild-type cells did not result in the activation of either c-Abl or Arg, as phosphotyrosine levels in the cells reexpressing c-Abl and Arg were similar to levels observed in cells expressing only PLC-γ1 (Fig. 8B, bottom). Therefore, our results show that, although c-Abl and Arg appear to be regulated in a similar fashion by PLC-γ1 and PIP2, c-Abl and Arg functions are not redundant with regards to PLC-γ1-induced chemotaxis toward PDGF, as only c-Abl, not Arg, is capable of rescuing the chemotaxis defect observed in the cells lacking c-Abl-Arg expression.
FIG. 8.
PLC-γ1-mediated chemotaxis requires c-Abl but not Arg. Abl-Arg double-null fibroblasts were retrovirally infected with virus encoding vector (CD4 and Mig), PLC-γ1, c-Abl, Arg, or PLC-γ1 together with c-Abl and Arg. Infected cells were used in a transwell chemotaxis assay. The MigR1 (Mig) vector is a bicistronic vector containing GFP in the second cistron. Migration in the presence of chemoattractant (PDGF-BB) was compared to migration in the absence of PDGF-BB as described previously (33). Transfected, GFP-positive cells which had migrated to the undersurface of the collagen-coated membrane were counted using a fluorescence microscope. (B) An aliquot of the same cells was lysed and blotted for c-Abl and Arg (pan-Abl), PLC-γ1, and phosphotyrosine. Panels A to C represent three independent experiments.
DISCUSSION
Little is known to date regarding the cellular signals that activate the Arg nonreceptor tyrosine kinase. Here we show that Arg is activated by PDGF and that the mechanism of activation involves the activity of two enzymes, Src and PLC-γ1, which are recruited to the PDGFR after growth factor stimulation. We demonstrate that, similarly to c-Abl, Arg is inhibited by PIP2 in vitro and in cells and that PIP2 binds directly to fragments of Arg and c-Abl containing the kinase (SH1) domain, but not to the SH3, SH2, or carboxy-terminal domains of the Abl family kinases. We hypothesize that the binding of PIP2 to the SH1 domains of c-Abl and Arg results in the inhibition of their tyrosine kinase activities either directly or indirectly by recruitment of another molecule (33). Decrease of PIP2 levels by PLC-γ1-mediated hydrolysis and the phosphorylation of Arg and c-Abl by Src kinases produce maximal activation of the Abl family kinases.
Both c-Abl and Arg have two isoforms, 1a and 1b, that result from alternative splicing of the first exon (30). A C14-myristoyl fatty acid residue is covalently attached to the N terminus of the c-Abl-1b protein, while c-Abl-1a is not myristoylated. Recently, a structure of the c-Abl kinase domain, which was crystallized in the presence of myristic acid or a myristoylated peptide, indicated that the myristate group binds in a deep hydrophobic pocket within the C-terminal lobe of the kinase domain (27). Another study showed that the presence of the myristoyl group is required for negative regulation of c-Abl following expression in HEK 293 cells (13). These results suggest that intramolecular interactions between the myristic acid residue and the C-terminal lobe of the kinase domain stabilize the inactive conformation of the c-Abl kinase. However, these findings do not explain why the activity of c-Abl is increased following purification (3) or following high-level overexpression (32). Additionally, the c-Abl-1a isoform is tightly regulated despite lacking a myristate residue, and a c-Abl-1b isoform with a deletion of the myristoylated amino-terminal first 45 amino acids is under negative regulatory control when assayed for in vivo kinase activity and oncogenic potential (10). Together, these data suggest that a soluble inhibitor may be required to maintain the c-Abl and Arg kinases in an inactive state inside the cell.
Our data demonstrate that both c-Abl and Arg are regulated by PIP2 in vivo and in vitro. PIP2 is a relatively abundant lipid. In resting cells the cellular levels of PIP2 are reported to be in the range of 50 μM and are up to 5 mM at the inner leaflet of the plasma membrane (18). These concentrations are in the appropriate range for effective inhibition of c-Abl and Arg kinases in the cell. We propose that binding of PIP2 to the catalytic domains of c-Abl and Arg may cause a similar conformational switch as that reported for myristate, given the presence of similar fatty acid side chains in these lipids. However, our finding that PIP2 exerts a specific inhibitory effect on the Arg and c-Abl kinase activities that is not induced by other lipids that bind the Abl kinases, such as PI4P, suggests that structural determinants present only in PIP2 are required for its profound inhibitory effect on Abl family kinases.
High levels of PIP2 are found not just in the plasma membrane but also in the cell nucleus (20, 29, 49). Moreover, PIP2 has been shown to modulate the function of a number of proteins involved in the regulation of the actin cytoskeleton such as profilin, vinculin, gelsolin, the Arp 2/3 complex, and the Wiskott-Aldrich syndrome protein (21, 35, 50). Thus, we propose that PIP2 is well positioned to regulate the 1a and 1b isoforms of the Abl family kinases in multiple subcellular compartments. This hypothesis is supported by our finding that, while both c-Abl and Arg were activated by PLC-γ1-mediated hydrolysis of PIP2 in vivo and were inhibited by PIP2 in vitro, only c-Abl was activated following expression of the PIP2-specific phosphatase INP54. INP54 was not membrane targeted and therefore would be able to traffic in and out of the nucleus to dephosphorylate nuclear pools of PIP2 (20). Because c-Abl but not Arg accumulates in the nucleus, it is expected that dephosphorylation of nuclear PIP2 pools would affect specifically c-Abl but not Arg kinase activity.
The finding that both c-Abl and Arg kinases are negatively regulated by PIP2 in vivo and in vitro opens the door for the development of novel therapeutic approaches for the treatment of cancers induced or promoted by deregulation of c-Abl and Arg kinase activities. Both c-Abl and Arg kinases are constitutively activated in human leukemias as a consequence of chromosomal translocation events that give rise to fusion proteins such as Bcr-Abl, Tel-Abl, and Tel-Arg (31, 39). Furthermore, Arg has been reported to be expressed at higher levels in primary carcinoma and metastatic colorectal cancer tumors than in adjacent normal tissue or adenomas and therefore may have a role in metastatic progression (5). Thus, inhibition of Abl family kinases in leukemias and other cancers by novel compounds that target the PIP2 binding site may be used alone or together with other drugs that target the ATP binding site of the kinase. This approach may be particularly relevant for those leukemias that become resistant to treatment by conventional Abl kinase inhibitors such as STI-571 (1).
In this study we show that, in addition to binding to PLC-γ1, Abl family kinases also bind directly to intracellular phosphotyrosine residues on PDGFRs via their SH2 domain. These data show that the Abl family kinases are part of a signaling complex involving PDGFRs as well as PLC-γ1. Furthermore, PDGFRs and Abl kinases undergo reciprocal phosphorylation. PDGFRs phosphorylate a subset of molecules that bind phosphotyrosine residues in the intracellular domain of the receptors. Phosphorylation of these substrates may result in their activation (e.g., Src family kinases and PLC-γ1) (16). Further experimentation will be required to determine whether phosphorylation of Abl proteins by the PDGFR regulates their activities or, alternatively, produces phosphotyrosine residues that become binding sites for other signaling molecules containing SH2 domains. If the latter is the case, phosphorylation of c-Abl and Arg by the PDGFR may activate specific signaling pathways by recruiting proteins to the phosphorylated tyrosine residues on the Abl family kinases. Although c-Abl and Arg both are phosphorylated by the PDGFR, the phosphorylation may occur in distinct domains of c-Abl and Arg. This finding suggests that c-Abl and Arg may differentially regulate signaling pathways following PDGF stimulation. In this regard, we found that c-Abl, but not Arg, is involved in PLC-γ1-mediated chemotaxis toward PDGF (Fig. 8). Although both proteins are activated by PDGF and appear to be regulated in a similar manner, the two nonreceptor tyrosine kinases differentially affect chemotaxis pathways downstream of the activated PDGFR, demonstrating that c-Abl and Arg kinases are not functionally redundant in the same cell type in response to this chemotactic stimulus.
In addition to phosphorylating the Abl family kinases, PDGFR-β is in turn phosphorylated by the Abl tyrosine kinases themselves. There is precedence for this type of reciprocal phosphorylation, as the Src kinases bind the PDGFR, are phosphorylated and activated by the PDGFR, and subsequently phosphorylate the receptor, creating a new phosphotyrosine binding site (12). Phosphorylation of the PDGFR by Src has modulatory effects on mitogenesis and chemotaxis (12). Phosphorylation of the PDGFR by Abl kinases may alter the catalytic activity of the receptor, thereby leading to regulation of its activity. We previously showed that phosphorylation of PLC-γ1 by c-Abl downregulates PLC-γ1 activity (33). It is possible that phosphorylation of the PDGFR by Abl family kinases could also downregulate PDGFR activity, leading to downregulation of PDGF signaling. Alternatively, phosphorylation of the receptor by Abl family kinases may create unique phosphotyrosine binding sites for the recruitment of specific signaling molecules, thereby affecting the activation of downstream pathways. While the Abl kinases and the PDGFR are capable of undergoing reciprocal tyrosine phosphorylation in vitro and induce phosphorylation of one another in cells, we cannot rule out the possibility that intervening tyrosine kinases are involved in the in vivo phosphorylation events. Indeed, phosphorylation of Abl kinases by the ligand-activated PDGFR is mediated in part by Src family kinases (11, 34).
Recruitment of Abl family kinases may be a common event induced by activation of several receptor tyrosine kinases. Abl family kinases have been reported to bind to the Eph and MuSK tyrosine kinases, and in both cases the Abl kinases undergo reciprocal phosphorylation with the activated receptor kinases (9, 51). We showed that Abl kinases are required downstream of the MuSK receptor for postsynaptic clustering of acetylcholine receptors at the neuromuscular junction (9). This discovery, together with the finding that c-Abl and Arg localize to the pre- and postsynaptic compartments of hippocampal synapses and that the Abl family kinases modulate short-term synaptic plasticity via a presynaptic mechanism (26), provides another potential link between Abl kinases and PIP2, as this phosphoinositide has been demonstrated to play critical roles in the regulation of synaptic function through its ability to affect vesicle targeting, exocytosis, endocytosis, and ion channel modulation (28).
Our findings show that Abl family kinases are downstream targets of PDGFR-α and PDGFR-β signaling. Significantly, we find that, although both c-Abl and Arg kinases are phosphorylated and activated by the PDGFR, their functions are not redundant, as only c-Abl has a functional role downstream of the PDGFR during PLC-γ1-dependent chemotaxis.
Acknowledgments
This work was supported by NIH grants CA70940 and GM62375 to A.M.P.; NIH training grant CA09111-25 to R.P.; USPHS grant NS39475, NARSAD, Leukemia and Lymphoma Society of America, and Edward Mallinckrodt, Jr. Foundation to A.J.K.; and NIH grant GM48339 to A.K.
We thank Bruce Mayer (University of Connecticut Health Center, School of Medicine, Farmington) for his generous gift of the GST-c-Abl-KR baculovirus. We thank Betsy Burton and Patricia Zipfel for critically reading the manuscript, for advice, and for helping to prepare the manuscript.
REFERENCE
- 1.Azam, M., R. R. Latek, and G. Q. Daley. 2003. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 112:831-843. [DOI] [PubMed] [Google Scholar]
- 2.Barila, D., and G. Superti-Furga. 1998. An intramolecular SH3-domain interaction regulates c-Abl activity. Nat. Genet. 18:280-282. [DOI] [PubMed] [Google Scholar]
- 3.Brasher, B. B., and R. A. Van Etten. 2000. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 275:35631-35637. [DOI] [PubMed] [Google Scholar]
- 4.Cao, C., X. Ren, S. Kharbanda, A. Koleske, K. V. S. Prasad, and D. Kufe. 2001. The ARG tyrosine kinase interacts with Siva-1 in the apoptotic response to oxidative stress. J. Biol. Chem. 276:11465-11468. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W. S., H. J. Kung, W. K. Yang, and W. C. Lin. 1999. Comparative tyrosine kinase profile in colorectal cancers: enhanced Arg expression in carcinoma as compared with adenoma and normal mucosa. Int. J. Cancer 83:579-584. [DOI] [PubMed] [Google Scholar]
- 6.DeMali, K. A., and A. Kazlauskas. 1998. Activation of Src family members is not required for the platelet-derived growth factor β receptor to initiate mitogenesis. Mol. Cell. Biol. 18:2014-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druker, B. J., M. Talpaz, D. J. Resta, B. Peng, E. Buchdunger, J. M. Ford, N. B. Lydon, H. Kantarjian, R. Capdeville, S. Ohno-Jones, and C. L. Sawyers. 2001. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344:1031-1037. [DOI] [PubMed] [Google Scholar]
- 8.Echarri, A., and A. M. Pendergast. 2001. Activated c-Abl is degraded by the ubiquitin-dependent proteasome pathway. Curr. Biol. 11:1759-1765. [DOI] [PubMed] [Google Scholar]
- 9.Finn, A. J., G. Feng, and A. M. Pendergast. 2003. Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat. Neurosci. 6:717-723. [DOI] [PubMed] [Google Scholar]
- 10.Franz, W. M., P. Berger, and J. Y. Wang. 1989. Deletion of an N-terminal regulatory domain of the c-abl tyrosine kinase activates its oncogenic potential. EMBO J. 8:137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furstoss, O., K. Dorey, V. Simon, D. Barila, G. Superti-Furga, and S. Roche. 2002. c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. EMBO J. 21:514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, K., M. Johnell, A. Siegbahn, C. Rorsman, U. Engstrom, C. Wernstedt, C. H. Heldin, and L. Ronnstrand. 1996. Mutation of a Src phosphorylation site in the PDGF receptor leads to increased PDGF-stimulated chemotaxis but decreased mitogenesis. EMBO J. 15:5299-5313. [PMC free article] [PubMed] [Google Scholar]
- 13.Hantschel, O., B. Nagar, S. Guettler, J. Kretzschmar, K. Dorey, and G. Superti-Furga. 2003. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell 112:845-857. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, S. C. 2003. Variation on a Src-like theme. Cell 112:737-740. [DOI] [PubMed] [Google Scholar]
- 15.Heidaran, M. A., J. F. Beeler, J. C. Yu, T. Ishibashi, W. J. LaRochelle, J. H. Pierce, and S. A. Aaronson. 1993. Differences in substrate specificities of alpha and beta platelet-derived growth factor (PDGF) receptors. Correlation with their ability to mediate PDGF transforming functions. J. Biol. Chem. 268:9287-9295. [PubMed] [Google Scholar]
- 16.Heldin, C.-H. 1997. Simultaneous induction of stimulatory and inhibitory signals by PDGF. FEBS Lett. 410:17-21. [DOI] [PubMed] [Google Scholar]
- 17.Heldin, C. H., and B. Westermark. 1990. Signal transduction by the receptors for platelet-derived growth factor. J. Cell Sci. 96:193-196. [DOI] [PubMed] [Google Scholar]
- 18.Insall, R. H., and O. D. Weiner. 2001. PIP3, PIP2 and cell movement—similar messages, different meanings? Dev. Cell 7:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvin, B. J., B. L. Williams, A. E. Nilson, H. O. Maynor, and R. T. Abraham. 2000. Pleiotropic contributions of phospholipase C-γ1 (PLC-γ1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-γ1-deficient Jurkat T-cell line. Mol. Cell. Biol. 20:9149-9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irvine, R. F. 2003. Nuclear lipid signaling. Nat. Rev. Mol. Cell. Biol. 4:1-12. [DOI] [PubMed] [Google Scholar]
- 21.Jamney, P. A. 1994. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu. Rev. Physiol. 56:169-191. [DOI] [PubMed] [Google Scholar]
- 22.Kharbanda, S., R. Ren, P. Pandey, T. D. Shafman, S. M. Feller, R. R. Welchselbaum, and D. W. Kufe. 1995. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376:785-788. [DOI] [PubMed] [Google Scholar]
- 23.Koleske, A. J., A. M. Gifford, M. L. Scott, M. Nee, R. T. Bronson, K. A. Miczek, and D. Baltimore. 1998. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21:1259-1272. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, J. M., R. Baskaran, S. Taagepera, M. A. Schwartz, and J. Y. J. Wang. 1996. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc. Natl. Acad. Sci. USA 93:15174-15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer, B., and D. Baltimore. 1994. Mutagenic analysis of the roles of SH2 and SH3 domains in regulation of the Abl tyrosine kinase. Mol. Cell. Biol. 14:2883-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moresco, E. M. Y., A. J. B. Scheetz, W. G. Bornmann, A. J. Koleske, and R. M. Fitzsimonds. 2003. Abl family nonreceptor tyrosine kinases modulate short-term synaptic plasticity. J. Neurophysiol. 89:1678-1687. [DOI] [PubMed] [Google Scholar]
- 27.Nagar, B., O. Hantschel, M. Young, K. Scheffzek, D. Veach, W. Bornmann, B. Clarkson, G. Superti-Furga, and J. Kuriyan. 2003. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112:859-871. [DOI] [PubMed] [Google Scholar]
- 28.Osborne, S. L., F. A. Meunier, and G. Schiavo. 2001. Phosphoinositides as key regulators of synaptic function. Neuron 32:9-12. [DOI] [PubMed] [Google Scholar]
- 29.Osborne, S. L., C. L. Thomas, S. Gschmeissner, and G. Schiavo. 2001. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J. Cell Sci. 114:2501-2511. [DOI] [PubMed] [Google Scholar]
- 30.Pendergast, A. M. 2002. The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 85:51-100. [DOI] [PubMed] [Google Scholar]
- 31.Pendergast, A. M. 2001. BCR-ABL protein domain, function, and signaling, p. 19-39. In A. M. Carella, G. Q. Daley, C. J. Eaves, J. M. Goldman, and R. Hehlmann (ed.), Chronic myeloid leukaemia: biology and treatment. Martin Dunitz, Ltd., London, United Kingdom.
- 32.Pendergast, A. M., A. J. Muller, M. H. Havlik, R. Clark, F. McCormick, and O. N. Witte. 1991. Evidence for regulation of the human Abl tyrosine kinase by a cellular inhibitor. Proc. Natl. Acad. Sci. USA 88:5927-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plattner, R., B. J. Irvin, S. Guo, K. Blackburn, A. Kazlauskas, R. T. Abraham, J. D. York, and A. M. Pendergast. 2003. A new link between the c-Abl tyrosine kinase and phosphoinositide signaling via PLC-γ1. Nat. Cell Biol. 5:309-319. [DOI] [PubMed] [Google Scholar]
- 34.Plattner, R., L. Kadlec, K. A. DeMali, A. Kazlauskas, and A. M. Pendergast. 1999. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 13:2400-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qualmann, B., M. M. Kessels, and R. B. Kelly. 2000. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150:F111-F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronnstrand, L., A. Siegbahn, C. Rorsman, M. Johnell, K. Hansen, and C. H. Heldin. 1999. Overactivation of phospholipase C-γ1 renders platelet-derived growth factor β-receptor expressing cells independent of the phosphatidylinositol 3-kinase pathway for chemotaxis. J. Biol. Chem. 274:22089-22094. [DOI] [PubMed] [Google Scholar]
- 37.Rosenkranz, S., K. A. DeMali, J. A. Gelderloos, C. Bazenet, and A. Kazlauskas. 1999. Identification of the receptor-associated signaling enzymes that are required for platelet-derived growth factor-AA-dependent chemotaxis and DNA synthesis. J. Biol. Chem. 274:28335-28343. [DOI] [PubMed] [Google Scholar]
- 38.Sawyers, C. L., A. Hochhaus, E. Feldman, J. M. Goldman, C. B. Miller, O. G. Ottmann, C. A. Schiffer, M. Talpaz, F. Guilhot, M. W. Deininger, et al. 2002. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99:3530-3539. [DOI] [PubMed] [Google Scholar]
- 39.Scheijen, B., and J. D. Griffin. 2002. Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease. Oncogene 21:3314-3333. [DOI] [PubMed] [Google Scholar]
- 40.Schindler, T., W. Bornmann, P. Pellicena, W. T. Miller, B. Clarkson, and J. Kuriyan. 2000. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science 289:1938-1942. [DOI] [PubMed] [Google Scholar]
- 41.Schwartzberg, P. L., A. M. Stall, J. D. Hardin, K. S. Bowdish, T. Humaran, S. Boast, M. L. Harbison, E. J. Robertson, and S. P. Goff. 1991. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65:1165-1175. [DOI] [PubMed] [Google Scholar]
- 42.Shah, N. P., J. M. Nicoll, B. Nagar, M. E. Gorre, R. L. Paquette, J. Kuriyan, and C. L. Sawyers. 2002. Multiple BCR-ABL domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor, imatinib (STI571), in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2:117-125. [DOI] [PubMed] [Google Scholar]
- 43.Smith, K. M., R. Yacobi, and R. A. Van Etten. 2003. Autoinhibition of Bcr-Abl through its SH3 domain. Mol. Cell 12:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun, X., P. Majumder, H. Shioya, F. Wu, S. Kumar, R. Weichselbaum, S. Kharbanda, and D. Kufe. 2000. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J. Biol. Chem. 275:17237-17240. [DOI] [PubMed] [Google Scholar]
- 45.Tanis, K. Q., D. Veach, H. S. Duewel, W. Bornmann, and A. J. Koleske. 2003. Two distinct phosphorylation pathways have additive effects on Abl family kinase regulation. Mol. Cell. Biol. 23:3884-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tybulewicz, V., C. Crawford, P. Jackson, R. Bronson, and R. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 47.Wang, J. Y. J. 1998. Cellular responses to DNA damage. Curr. Opin. Cell Biol. 10:240-247. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Y., A. L. Miller, M. S. Mooseker, and A. J. Koleske. 2001. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin binding domains to bundle actin. Proc. Natl. Acad. Sci. USA 98:14865-14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watt, S. A., G. Kular, I. N. Fleming, P. Downes, and J. M. Lucocq. 2002. Subcellular localization of phosphatidylinositol-4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ. Biochem. J. 363:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin, H. L., and P. A. Jamney. 2003. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65:761-789. [DOI] [PubMed] [Google Scholar]
- 51.Yu, H. H., A. H. Zisch, V. C. Dodelet, and E. B. Pasquale. 2001. Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene 20:3995-4006. [DOI] [PubMed] [Google Scholar]