Abstract
Elevated immunoglobulin free light chain (FLC) level and abnormal FLC ratio is commonly seen in multiple myeloma (MM) and have prognostic implications. We hypothesized that presence of IgH translocations leads to unbalanced production of light chains and more extreme abnormalities of FLC and may explain the prognostic value of FLC. We studied 314 patients with newly diagnosed MM enrolled on a phase-III trial, in whom results of FISH testing and serum FLC were available. Cytogenetic analyses and FLC estimates were performed on stored samples and results correlated with clinical data. The median ratio (FLC-ratio) and the absolute difference (FLC-diff) between the involved and uninvolved FLC was higher among those with IgH translocations, especially t(14;16). In multivariate analysis, the prognostic value of FLC estimates on progression free and overall survival were independent of high-risk IgH translocations t(4;14) and t(14;16). A combination of the risk factors; either abnormal FLC estimate and/or the presence of high-risk IgH translocation, achieved better prognostic stratification. In conclusion, patients with IgH translocations tend to have higher FLC levels and abnormal ratios, but the prognostic effect of FLC is only partially explained by translocation status. A system including both these risk factors allows better prediction of outcome.
Keywords: Multiple myeloma, translocations, free light chain, prognosis, survival
INTRODUCTION
The monoclonal protein secreted by clonal plasma cells in multiple myeloma typically is made up of an intact immunoglobulin consisting of heavy chains, most commonly IgG or IgA, bound to a kappa or lambda light chain.(1, 2) Normal plasma cells produce mostly intact immunoglobulins with relatively little excess free light chain whereas the majority of myeloma cells produce an intact immunoglobulin along with a relative excess of free light chains with an intact immunoglobulin while a minority (15–20%) secrete only a light chain with no identifiable heavy chain, a condition termed as light chain myeloma.(3) However, in the majority of patients with a heavy chain varying amounts of free light chain is present in the serum, unbound to a heavy chain. The free light chain (FLC) assay allows a nephelometric measurement of this free kappa and lambda light chains that circulate as monomers or dimers, not bound to the immunoglobulin heavy chain.(4–6) Estimation of the free light chain levels and their ratio has utility in screening, prognostication and treatment response assessment in nearly all the monoclonal plasma cell disorders including multiple myeloma, smoldering myeloma, MGUS, plasmacytomas and light chain amyloidosis.(7–13)
Translocations involving the immunoglobulin heavy chain (IgH) locus on chromosome 14 can be detected using FISH in over half of the patients with myeloma.(14–16) The five recurrent translocations with identifiable partner chromosomes include t(11;14), t(4;14), t(14;16), t(6;14) and t(14;20) which lead to dysregulation of specific genes which are thought to play a role in the pathogenesis of myeloma and its manifestations.(17) Presence of certain IgH translocations such as t(4;14) and t(14;16) has been shown to be associated with a poor outcome in patients with myeloma.(14, 18–20) During B-cell maturation and plasma cell development the IgH chain region undergoes switch recombination events leading to a class switch and formation of IgG or IgA instead of an IgM. Previous studies in patient cells and human myeloma cell lines have suggested that absence of a functional rearrangement on at least one of the IgH alleles is the basis of light chain myeloma.(21, 22) In these studies, presence of a heavy chain was associated with a functional recombination in one of the IgH loci with the other allele often involved in a translocation. Given these, we hypothesized that presence of an IgH translocation may be associated with compromised production of heavy chains and lead to unbalanced, excess production of clonal light chains. In addition, we wanted to examine if the prognostic value of abnormal FLC ratio in myeloma can be explained by the presence of associated genetic abnormalities.
MATERIALS AND METHODS
Patients
From February 1988 to May 1992, 653 patients with previously untreated multiple myeloma from 36 ECOG institutions were enrolled on the phase III clinical trial E9486 and its companion ancillary study E9487, and their outcomes have been previously reported.(23) Serum free light chains were run on stored sera obtained at baseline in 495 patients as part of a previous study examining the value of disease response using free light chain levels.(11) Among these patients, 314 were previously studied using FISH for presence or absence of IgH translocations, p53 abnormalities and deletion 13 and were included in the current analysis.21 Eligibility and treatment details have previously been described.(23) In brief, patients with previously untreated myeloma and measurable disease defined as either a serum M-protein greater than or equal to 1.0 g/dL or urine monoclonal light chain excretion exceeding 200 mg in 24 hours or serially measurable soft tissue plasmacytomas or bone marrow plasmacytosis greater than or equal to 20% were enrolled. Patients entering the study were stratified according to age, creatinine, M-protein type, and clinical stage, and randomized to 1 of the 3 study treatment regimens. The first regimen was VBMCP (vincristine, carmustine [BCNU], melphalan, cyclophosphamide, and prednisone), administered in 5-week cycles. The second regimen was VBMCP plus recombinant alpha-2 interferon (IFN). Patients younger than 70 years were also randomized to a third regimen, VBMCP and high dose cyclophosphamide. Treatment was continued for a maximum of 2 years or until disease progression if the latter occurred first. The baseline characteristics of the 314 patients studied here were no different from the larger cohort of patients when all relevant biologic and prognostic factors are considered.
Free light chain measurements
The FLC assay (FREELITE™, The Binding Site Ltd., Birmingham, U.K.) was retrospectively and centrally performed on a Dade Behring BNII automated nephelometer using 0.5 ml of stored thawed serum.(4, 6) This assay consists of 2 separate measurements: one to quantitate kappa FLC and the other to quantitate lambda FLC. In addition to reporting the kappa and lambda FLC, the assay report also contains the FLC κ/λ ratio (diagnostic range: 0.26–1.65). Out of the 314 patients, 17 (5%) patients had normal kappa/lambda FLC ratio (0.26 ~1.65). If kappa/lambda ratio was >1.65, kappa was assigned as the involved light chain, and if the ratio was <0.26, lambda was assigned as the involved light chain. The involved light chain type was determined by IFE for patients with a normal FLC ratio. The involved vs. uninvolved light chain ratio (FLC-ratio) was calculated, and the kappa/lambda ratio was used as the involved/uninvolved ratio for patients with normal KL ratio. Since the FLC-ratio can be affected both by the tumor burden as well as the degree of suppression of the uninvolved immunoglobulin and the uninvolved free light chain we also included the absolute difference between the involved and the uninvolved free light chain (FLC-diff) in the analyses.
FISH analysis
As part of the clinical trial, bone marrow research samples were collected prospectively with Institutional review board (IRB) approval and informed consent. Aspirate samples were enriched for mononuclear cells using the Ficoll method and cytospin slides were stored for future use (at −70°C). FISH analysis was performed as previously described and the results for the entire group of patients have been previously published. To detect t(14;16)(q32;q23) we used the same 14q32 chromosome probes previously described by us,(19) in combination with 2 bacterial artificial chromosome (BAC) clones (356D21 and 484H2; Research Genetics, Huntsville, AL) that localize to 16q23, and BAC clones 10205 and 10206 described by Chesi et al.(24) To test for 17p13.1, we used a locus specific probe (LSI) p53 probe from Vysis (Downers Grove, IL). We used standard hybridization, validation, and scoring procedures as described previously by us.(19) We scored 100 cells for each one of the abnormalities and recorded the percentage of cells with abnormal patterns (with special attention to the number of fusions detected for the translocations).
Statistical methods
For each individual type of translocation, a Wilcoxon Rank Sum test was applied to assess the differences in FLC level (ratio, and difference between involved and uninvolved light chain) between groups with and without the translocation. The FLC levels were also dichotomized by different cutoff points, and the association between the FLC groups and translocation status was assessed using Fisher’s exact test. The test was two-sided and the significance level was 0.05. The overall and progression free survival were examined using Kaplan Meier product limit method and the difference between the groups was assessed using a log rank test. Cox-proportional hazards model was used for best cutoff point determination.
RESULTS
Are Free light chain levels and ratios higher in patients with IgH translocations?
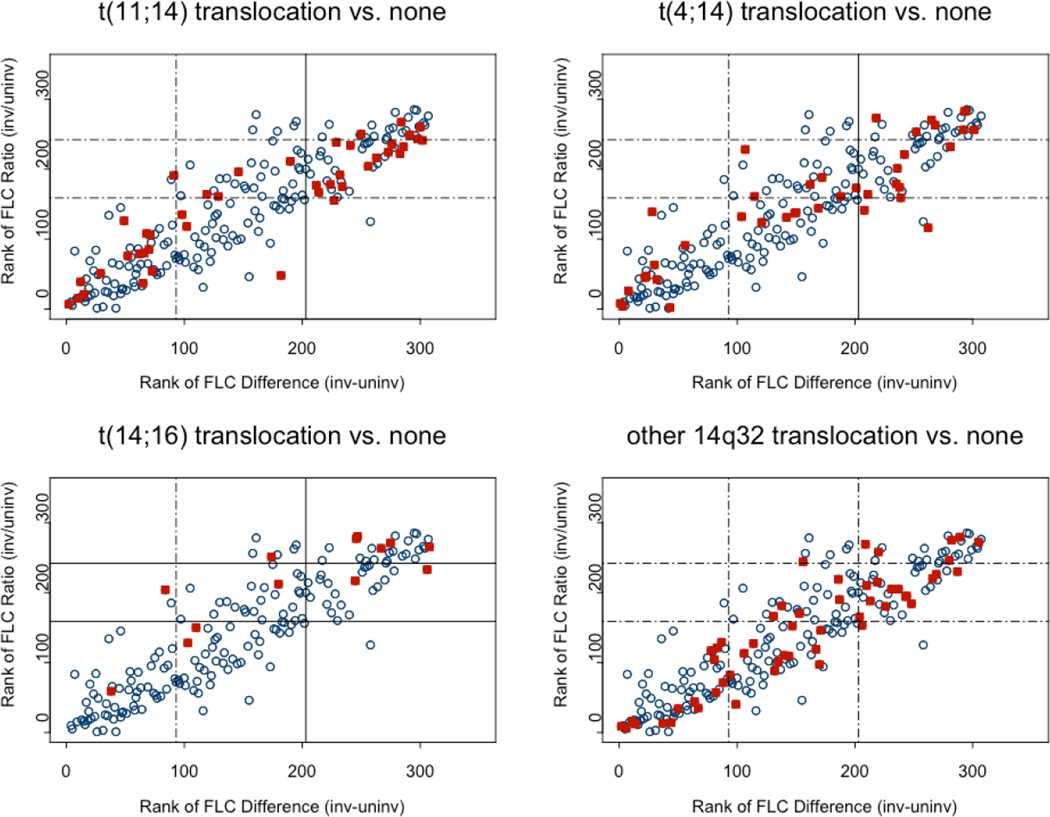
We first examined if the FLC-ratio or the FLC-diff were different among patients with IgH abnormalities detected by FISH compared to those without any of these abnormalities. Among the 314 patients included in the study, there were 147 patients (47%) who had a translocation involving the IgH region and the distribution is as shown in Table 1. The median FLC-ratio as well as the median FLC-diff was higher among the group of patients with any IgH translocation abnormality compared to those with none. When each of the IgH translocations were examined individually, the trend was similar for all the three translocations but the difference was statistically significant compared to those with no IgH translocations only among the patients with t(14;16). Given the wide range of values for the FLC-ratio and FLC-diff, we also examined the relationship between IgH translocations and FLC estimates using log transformed FLC-ratio and FLC-diff (Figure 1). With increasing rank of FLC-ratio or FLC-diff, the there was an increase in the proportion of patients with any of the three known IgH translocations, an association that was significant at the FLC-ratio cutoffs shown in the figure. In contrast, patients with an IgH abnormality but no definite partner chromosome identified had FLC values similar to those with no IgH abnormalities. Among the 314 patients, 47 patients (15%) had light chain myeloma, defined as the lack of a heavy chain on immunofixation of the serum or urine. Among the patients with light chain myeloma 57% (27/47) had an IgH translocation, and among the non light chain group 45% (120/267) had an IgH translocation (p = 0.12, Fisher’s exact test).
Table 1.
Relationship between FLC estimates and genetic abnormalities in patients with newly diagnosed myeloma
| n | FLC ratio (involved/uninvolved) |
Absolute FLC difference (involved-uninvolved) |
||||
|---|---|---|---|---|---|---|
| Median (range) | P * | Median (range) | P* | |||
| Overall | 314 | 80 (0.3, 27021) |
39 (0.02,3369) |
|||
| Any IgH translocation | Yes | 147 | 108 (0.3, 15200) |
0.03 | 62 (0.1, 3369) |
0.05 |
| No | 167 | 50 (0.3, 27021) |
26 (0.4, 3019) |
|||
| t(11;14) | Yes | 42 | 120 (0.9, 3110) |
0.22 | 79 (0.04,1978) |
0.19 |
| No IgH translocation | 167 |
50 (0.3, 27021) |
25 (0.2, 3019) |
|||
| t(4;14) | Yes | 39 | 104 (0.3, 15200) |
0.19 | 63 (0.02,1869) |
0.21 |
| No IgH translocation | 167 | 50 (0.3, 27021) |
25 (0.2, 3019) |
|||
| t(14;16) | Yes | 13 | 714 (8.7, 10000) |
0.001 | 244 (2.3,3369) |
0.04 |
| No IgH translocation | 167 | 50 (0.3, 27021) |
25 (0.2, 3019) |
|||
| Other 14q32 | Yes | 53 | 80 (0.8, 7870) |
0.55 | 39 (0.04,2399) |
0.29 |
| No IgH translocation | 167 | 50 (0.3, 27021) |
25 (0.2, 3019) |
|||
| P53 mutation | Yes | 8 | 421 (2.2, 27021) |
0.48 | 75 (0.8, 1869) |
0.84 |
| No | 306 | 78 (0.3, 17400) |
39 (0.02, 3369) |
|||
| Del Chrom13 | Yes | 157 | 108 (0.3, 27021) |
0.03 | 62 (0.02, 2399) |
0.07 |
| No | 157 | 50 (0.3, 15200) |
25 (0.04, 3369) |
|||
Wilcoxon Rank-Sum test
Figure 1.
Distribution of FLC-ratio (involved vs. uninvolved ratio, ) and FLC-diff (absolute difference between involved and uninvolved) among patients. Solid squares represent patients with t(4;14) or t(14;16) or t(11;14) or other 14q32 translocation; open circles represent patients without t(4;14), t(14;16), t(11;14) and any other 14q32 translocations. The horizontal dashed lines refer to FLC ratio original values of 100 and 1000, and vertical dashed lines refer to FLC difference original values of 10 and 100. Solid line indicates significant association (P≤0.05) between the dichotomized FLC groups and translocation status.
Is there a relationship between other genetic abnormalities and elevated FLC?
We further examined if there were differences in the FLC values based on the presence or absence of p53 (17p-) abnormality or deletion 13 as assessed by FISH. While there was no correlation between presence of p53 abnormalities and FLC measurements, patients with deletion13 had higher levels of FLC-ratio and FLC-diff compared to patients with no chromosome 13 abnormalities (Table 1). However, this was explained by the co-existence of IgH translocations in the majority of these patients since the values were not very different for patients with chromosome 13 abnormalities with out an associated IgH translocation compared to those with no chromosome 13 abnormalities (p=0.67 for FLC-diff; p=0.51 for FLC-ratio). In addition, we also noticed a significant association between the type of involved light chain and presence of IgH translocations, with over representation of lambda light chain in that group (p=0.006).
Can the prognostic value of FLC in multiple myeloma be explained by concurrent presence of high-risk IgH translocations?
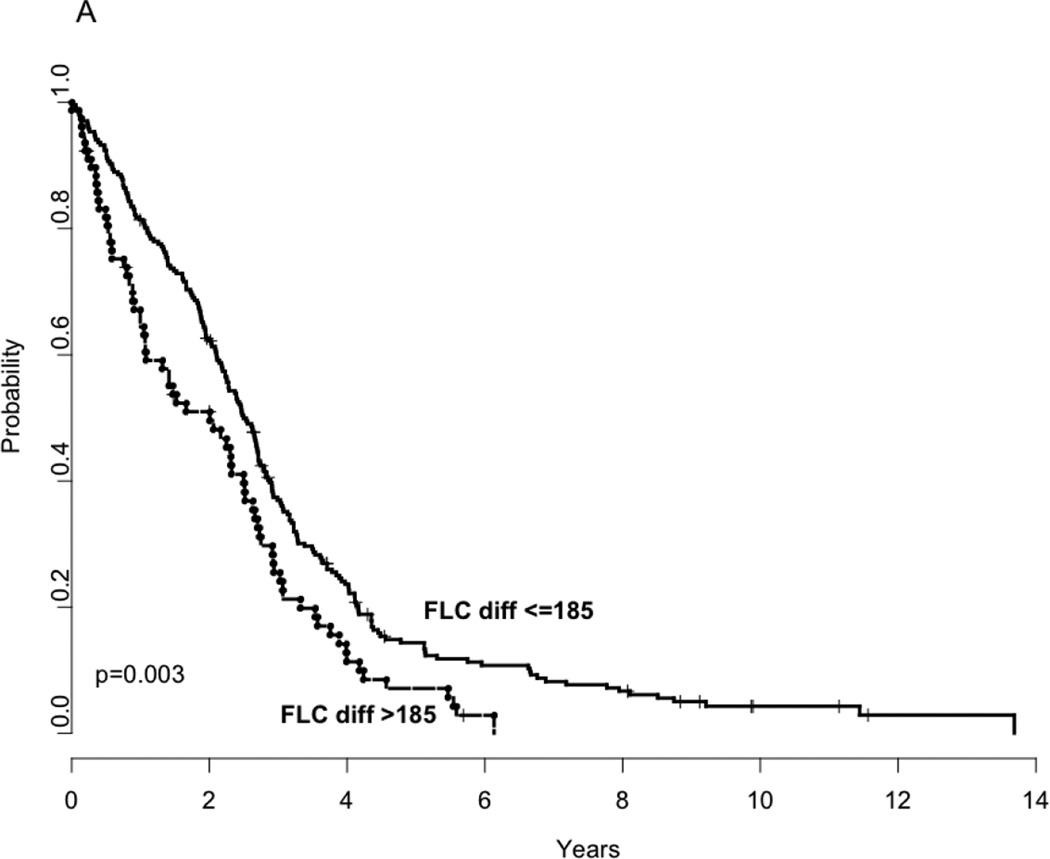
Given previous studies showing the adverse prognostic impact of abnormal FLC estimates in myeloma, we examined if it can be explained on the basis of simultaneous presence of high-risk IgH translocation abnormalities. First, we examined the relationship of FLC-diff and FLC-ratio to progression free and overall survival. Since both the values have a very wide range, we first log10 transformed both FLC-diff and ratio and used this as a continuous variable for examining their effect on PFS and OS. As shown in Table 2, both FLC-diff and FLC-ratio were prognostic for progression free and overall survival. In order to identify optimal cut-points for FLC-diff and FLC-ratio (using actual values, not log transformed) for survival analysis, we used an outcome-oriented method, i.e., maximizing the loglikelihood value using Cox proportional hazards model in a univariate fashion. For the FLC-diff, 185 was considered as the best cutoff point for both PFS and OS and for the FLC-ratio 277 was determined to be the best cutoff. (Table 2, Figure 2 A–D).
Table 2.
Impact of FLC-diff and FLC-ratio on progression-free and overall survival
| Progression Free Survival | Overall Survival | |||
|---|---|---|---|---|
| Prognostic Factor | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value |
| FLC-diff (log) | 1.2 (1.06, 1.36) | 0.0039 | 1.23 (1.09, 1.39) | 0.0009 |
|
FLC-diff >185 vs. FLC-diff ≤185 |
1.51 (1.15, 1.98) | 0.003 | 1.61 (1.24, 2.09) | 0.0004 |
| FLC-ratio (log) | 1.23 (1.10, 1.39) | 0.0005 | 1.21 (1.08, 1.36) | 0.0012 |
|
FLC-ratio >277 vs. FLC-ratio ≤277 |
1.57 (1.22, 2.02) | 0.0005 | 1.53 (1.20, 1.96) | 0.0007 |
Figure 2.
Kaplan Meier curves demonstrating comparison of progression free survival based on FLC estimates; FLC difference >185 vs. <=185; (Panel A), overall survival based on FLC estimates; FLC difference >185 vs. <=185 (Panel B), progression free survival based on FLC estimates; FLC ratio>277 vs. <=277 (Panel C) and overall survival based on FLC estimates; FLC ratio>277 vs. <=277 (Panel D).
The prognostic value of IgH translocations among the patients in this trial has been previously reported and was further confirmed within this subset. As previously demonstrated, presence of t(4;14), and t(14;16) were associated with inferior outcomes compared to patients without any IgH translocations, while those with t(11;14) and other 14q32 abnormalities had outcomes comparable to those without translocations (data not shown).
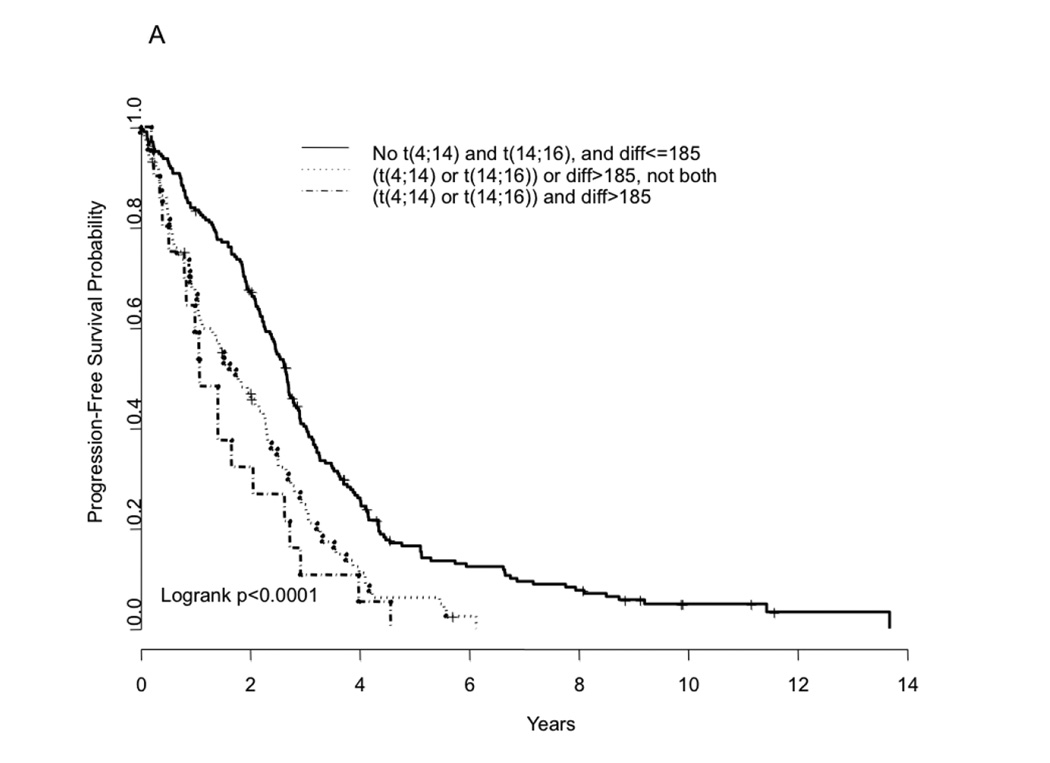
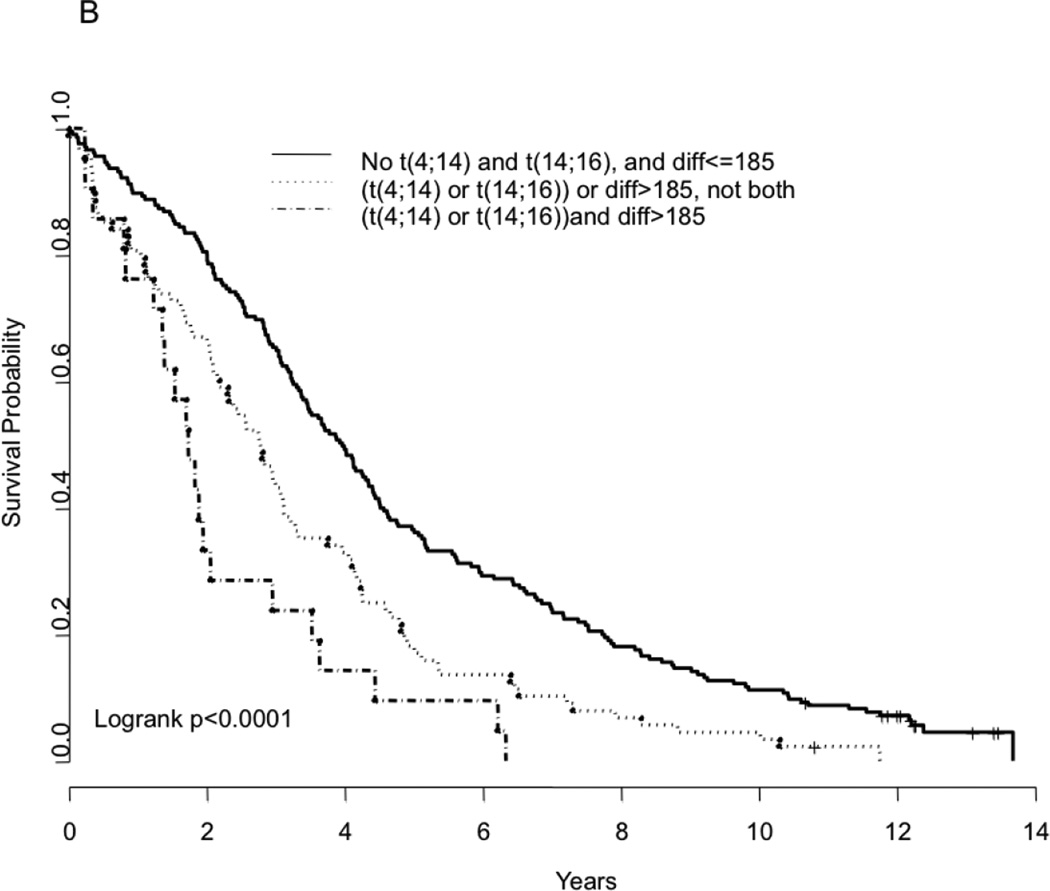
In a multivariate analysis, we examined if the prognostic value seen with FLC-diff and FLC-ratio were independent of the presence of IgH translocations t(4:14) and t(14;16), which are associated with a worse outcome. The impact of these translocations on outcome in this dataset was similar to that previously reported (data not shown). The presence of t(4;14) or t(14;16) correlated with FLC-diff, with 13% of those with FLC-diff ≤185 and 27% of those with FLC-diff > 185 having the two translocations (P= 0.008). Using this cutoff for grouping, the prognostic value of the FLC-diff on progression free and overall survival appears to be independent of these high-risk IgH translocations (P-value=0.03 and 0.003, respectively for PFS and OS) (Table 3). Similar results were also obtained when FLC-ratio was used in place of FLC-diff, in terms of its association with presence of t(4:14) and t(14;16) as well as its effect on the PFS and OS relative to the presence of translocations (Table 3). We then examined the outcome among patients grouped according to whether they had one or more of the abnormalities; neither the FISH abnormality nor FLC abnormality, either one of the abnormalities, or both. Presence of either one of the abnormalities (FLC-diff >185 or one of the two translocations) has a negative impact on the outcome, both PFS and OS (Figure 3). More importantly, presence of both abnormalities resulted in a much inferior outcome (PFS and OS) compared to having neither one of the abnormalities (Figure 3A–B). The median overall survival for the three groups were 3.97 years, 2.19 years and 1.74 years respectively (P < 0.0001) in the presence of none, either one, or both risk factors (FLC and one of the translocations). These finding were similar when the FLC-ratio was used instead of FLC-diff (Figure 3C–D).
Table 3.
Impact of translocation status and FLC estimates on the progression and overall survival
| Prognostic Factor | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
|---|---|---|---|---|---|
| Cox model for FLC ratio | Progression free survival | Overall Survival | |||
| FLC-ratio | Inv/uninv>277 vs. Inv/uninv<=277 |
1.48 (1.14, 1.91) | 0.0028 | 2.09 (1.53, 2.84) | 0.0023 |
| t(4;14) or t(14;16) | Yes vs. no | 2.13 (1.55, 2.92) | <0.0001 | 1.47 (1.15, 1.89) | <0.0001 |
| Cox model for FLC difference at | Progression free survival | Overall Survival | |||
| FLC-diff | Inv-uninv>185 vs. Inv-uninv<=185 |
1.36 (1.03, 1.79) | 0.032 | 1.49 (1.15, 1.95) | 0.003 |
| t(4;14) or t(14;16) | Yes vs. no | 2.10 (1.52, 2.90) | <0.0001 | 2.03(1.49, 2.77) | <0.0001 |
Figure 3.
Kaplan Meier curves demonstrating comparison of progression free survival based on FLC estimates (FLC difference >185 vs. <=185) and/or the presence or absence of the translocations t(4;14) or t(14;16) (Panel A); overall survival based on FLC estimates (FLC difference >185 vs. <=185) and/or the presence or absence of the translocations t(4;14) or t(14;16) (Panel B); progression free survival based on FLC estimates (FLC ratio>277 vs. <=277) and/or the presence or absence of the translocations t(4;14) or t(14;16) (Panel C); and overall survival based on FLC estimates (FLC ratio>277 vs. <=277) and/or the presence or absence of the translocations t(4;14) or t(14;16) (Panel D).
DISCUSSION
The humoral immune system is an important component of the body’s defense and consists of terminally differentiated plasma cells secreting antibodies with specificity for a variety of antigens. The normal development of plasma cells from the B cells systematically provides the opportunity for development of diversity, both in terms of the spectrum of antigens the antibodies can respond to and also in terms of the defense mechanisms it can bring to bear by virtue of the different classes of antibodies it can generate.(25, 26) The pathogenesis of plasma cell disorders is intricately intertwined with the normal B-cell development. During the normal development of plasma cells from the early stage B cells, the genes encoding for the immunoglobulin heavy as well as the light chains undergo a series of genetic modifications including the VDJ recombination that allows random segments of the V (variable), D (diversity) and J (joining) regions to come together to form a functional recombination, followed by somatic hypermutation (SHM) that generates point mutations and consequently diversity in the variable region and class switch recombination (CSR) involving the constant region that allow generation of specific types of heavy chains.(16, 27) While the primary heavy chain produced by the B cell is an IgM, and production of IgD can result from alternate splicing/ termination the production of an IgA, IgG or IgE require the CSR of the heavy chain gene.
Normal immunoglobulin production and secretion by plasma cells however require a balanced production of immunoglobulin heavy and light chains. Given the complex process of genetic rearrangements that underlie the normal plasma cell development process, it is not surprising that errors can occur that can potentially have pathologic consequences. The normal counterpart for the clonal cells in myeloma represent the terminally differentiated plasma cell, since the these cells typically have undergone SHM and CSR, both hallmarks of post germinal center B cells.(28) Several lines of evidence exists to suggest that unbalanced production of the heavy or light chains can lead to lack of intact immunoglobulin molecules and preferential secretion of the heavy or light chains.(29) While excess of heavy or light chain can occur depending on the affected component, this appears to affect the heavy chain less commonly.(30) While there are likely to be several reasons for this observation, including inability of the endoplasmic reticulum to secrete the heavy chain that is unbound to a light chain leading to an unfolded protein response and intracellular degradation of the heavy chain through a proteasome mediated pathway as well as toxicity of non-secreted full size heavy chain.(29, 31, 32) Light chains unbound to a heavy chain seem less susceptible to such degradation mechanisms and are more likely to be secreted alone in the absence of an accompanying heavy chain.(33, 34) Such an absence of heavy chain can potentially result from several mechanisms including lack of a functional rearrangement of the heavy chain, mutations involving the heavy chain gene resulting in early termination of transcription, secondary VDJ rearrangements, instability of the heavy chain mRNA or inability of the cell to secrete the heavy chain.
Light chain myeloma, constituting 15–20% of all myelomas is characterized by absence of a detectable heavy chain in the serum or urine. Studies suggest that this finding is likely to result from the lack of a functional rearrangement in the heavy chain gene.(21, 22, 35) Typically, only one of the alleles undergoes functional rearrangement in any given plasma cell. Szczepanski et al, studied 12 patients with light chain myeloma, and found monoallelic J(H) gene rearrangements in 10 patients, biallelic rearrangement in 1 patient, and biallelic deletion of the JH and Cμ regions in 1 patient.(22) The authors concluded that in many of the light chain myeloma patients, illegitimate IGH class switch rearrangement or illegitimate deletion of the functional VDJ allele is responsible for the failure of heavy chain secretion. Magrangeas et al, in a another study of patients with light chain myeloma and myeloma cell lines, using FISH, demonstrated that lack of heavy chain production was a direct result of the absence of functional rearrangement on one of the alleles, while the other have undergone a translocation.(21) In comparison, in patients with non-light chain myeloma with IgH translocations, one of the heavy chain genes remains functional while the other has undergone translocation. Translocations involving the IgH gene is found in over half of all patients with multiple myeloma, while the other half of the patients usually have trisomies of odd numbered chromosomes, leading to a hyperdiploid clone.(14, 15, 20, 36, 37) As is typically seen, only 15% of our study population had light chain disease and the presence of an IgH translocation was not significantly higher in this group compared to common myeloma. This is in agreement with the previous reports showing no association between light chain myeloma and presence of IgH translocations.(38) In the original publications we found t(11;14)(q13;q32) associated with light chain lambda myeloma. In the current study, we demonstrate an association between presence of IgH translocations and elevated free light chains and extreme light chain ratios. The proportion of patients with these translocations are clearly higher among the patients with very high absolute levels of involved free light chain, and as expected in those with a high ratio for the involved to uninvolved free light chain. While involved to uninvolved FLC ratio can be due to high levels of the involved FLC or suppression the uninvolved FLC, figure 2 suggests that most of the association with presence of translocations is due to the elevation of the abnormal FLC, since very few of the translocated patients are seen in the upper left or lower right quadrants. It is not clear how translocation involving a heavy chain locus would lead to elevation in the free light chain excretion; however, several hypotheses can be raised. It is unlikely that this results from a translocation that occurs on the functionally rearranged heavy chain locus, since that would have most likely led to complete disruption of the heavy chain production and consequent light chain myeloma. Molecular analysis of the IgH translocations suggests that this mostly occurs on the non-productive IgH allele. It is possible that the presence of a translocation is an indicator of genomic instability in the clonal myeloma cells that can potentially lead to somatic mutations or other processes influencing the functionally rearranged allele leading to decreased production of the heavy chain. Moreover, the lack of a complete concordance between presence of a translocation and the elevation of the FLC also point towards this being due to the cumulative effect of several events rather than any single abnormality.
Presence of IgH translocations, especially t(4;14) and t(14;16) have been associated with poor outcome following conventional or high dose therapies.(18, 19, 39–41) The current study sought to answer the question whether the prognostic effect of elevated free light chain in myeloma and other plasma cell disorders can be explained by the presence of high-risk IgH translocations. While the group of patients with elevated FLC level and ratio clearly were more likely to carry the high-risk IgH translocations, the prognostic effect of FLC was independent of the translocation abnormality. More importantly, we could combine the two markers to develop a better risk stratification system allowing better estimates of outcome among these patients. One could speculate that the prognostic value of FLC seen in MGUS and in SMM may also partially result from enrichment of the high-risk group for these genetic abnormalities. However, the mechanisms behind the independent effect of FLC on the outcome of patients with plasma cell disorders remains unanswered. It has been suggested that the heavy chain following proteasomal degradation in the cell may be presented on the cell surface as potential antigens for development of an immune response, which may keep the clonal population under check.(42, 43) Progressive acquisition of somatic mutations and other genetic recombination events in the malignant plasma cell, may eventually lead to decreased production of the heavy chain and escape from the immune surveillance as well as “light chain escape”.
In conclusion, elevated free light chain levels are associated with presence of IgH translocations and these patients are more likely to harbor the high-risk genetic abnormalities. Both the free light chain estimates as well as the results of the FISH testing contribute to the risk stratification in patients with newly diagnosed myeloma. Routine FISH testing and free light chain estimation for patients with newly diagnosed myeloma will allow us to confirm these and allow better risk stratification of the patients. Additional work needs to be done to help us better understand the molecular basis for unbalanced light chain excretion by myeloma cells and the current finding of the association with IgH translocation abnormalities should form the basis for this work.
Acknowledgments
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA13650 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Contributions: S.K designed research, performed research, analyzed data, and wrote the paper. L.Z. analyzed data. E.B. designed research. A.D., R.F, J.A.K., M.S., R.D., K.H., S.V., A.R.B., R.A.K., P.R.G., S.V.R. performed research and analyzed data.
Disclosures: S.K., A.D., R.F., L.Z., E.B., M.S., R.D., K.H., S.V., R.A.K., M.O., P.R.G. have nothing to disclose. A.R.B. is an employee of the Binding Site LTD. J.A.K. receives reagents from the Binding Site Ltd.
REFERENCES
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004 Oct 28;351(18):1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005 May 20;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003 Jan;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001 Apr;47(4):673–680. [PubMed] [Google Scholar]
- 5.Katzmann JA, Abraham RS, Dispenzieri A, Lust JA, Kyle RA. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem. 2005 May;51(5):878–881. doi: 10.1373/clinchem.2004.046870. [DOI] [PubMed] [Google Scholar]
- 6.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002 Sep;48(9):1437–1444. [PubMed] [Google Scholar]
- 7.Rajkumar SV, Kyle RA, Therneau TM, Clark RJ, Bradwell AR, Melton LJ, 3rd, et al. Presence of monoclonal free light chains in the serum predicts risk of progression in monoclonal gammopathy of undetermined significance. Br J Haematol. 2004 Nov;127(3):308–310. doi: 10.1111/j.1365-2141.2004.05169.x. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008 Jan 15;111(2):785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingli D, Kyle RA, Rajkumar SV, Nowakowski GS, Larson DR, Bida JP, et al. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood. 2006 Sep 15;108(6):1979–1983. doi: 10.1182/blood-2006-04-015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dispenzieri A, Lacy MQ, Katzmann JA, Rajkumar SV, Abraham RS, Hayman SR, et al. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006 Apr 15;107(8):3378–3383. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dispenzieri A, Zhang L, Katzmann JA, Snyder M, Blood E, Degoey R, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008 May 15;111(10):4908–4915. doi: 10.1182/blood-2008-02-138602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, Sachanas S, Tzenou T, Papadogiannis A, et al. Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Haematol. 2007 May;137(3):240–243. doi: 10.1111/j.1365-2141.2007.06561.x. [DOI] [PubMed] [Google Scholar]
- 13.van Rhee F, Bolejack V, Hollmig K, Pineda-Roman M, Anaissie E, Epstein J, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007 Aug 1;110(3):827–832. doi: 10.1182/blood-2007-01-067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004 Feb 15;64(4):1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 15.Chng WJ, Glebov O, Bergsagel PL, Kuehl WM. Genetic events in the pathogenesis of multiple myeloma. Best practice & research. 2007 Dec;20(4):571–596. doi: 10.1016/j.beha.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez D, van der Burg M, Garcia-Sanz R, Fenton JA, Langerak AW, Gonzalez M, et al. Immunoglobulin gene rearrangements and the pathogenesis of multiple myeloma. Blood. 2007 Nov 1;110(9):3112–3121. doi: 10.1182/blood-2007-02-069625. [DOI] [PubMed] [Google Scholar]
- 17.Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005 Sep 10;23(26):6333–6338. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007 Apr 15;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003 Jun 1;101(11):4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca R, Debes-Marun CS, Picken EB, Dewald GW, Bryant SC, Winkler JM, et al. The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood. 2003 Oct 1;102(7):2562–2567. doi: 10.1182/blood-2003-02-0493. [DOI] [PubMed] [Google Scholar]
- 21.Magrangeas F, Cormier ML, Descamps G, Gouy N, Lode L, Mellerin MP, et al. Light-chain only multiple myeloma is due to the absence of functional (productive) rearrangement of the IgH gene at the DNA level. Blood. 2004 May 15;103(10):3869–3875. doi: 10.1182/blood-2003-07-2501. [DOI] [PubMed] [Google Scholar]
- 22.Szczepanski T, van 't Veer MB, Wolvers-Tettero IL, Langerak AW, van Dongen JJ. Molecular features responsible for the absence of immunoglobulin heavy chain protein synthesis in an IgH(−) subgroup of multiple myeloma. Blood. 2000 Aug 1;96(3):1087–1093. [PubMed] [Google Scholar]
- 23.Oken MM, Leong T, Lenhard RE, Jr, Greipp PR, Kay NE, Van Ness B, et al. The addition of interferon or high dose cyclophosphamide to standard chemotherapy in the treatment of patients with multiple myeloma: phase III Eastern Cooperative Oncology Group Clinical Trial EST 9486. Cancer. 1999 Sep 15;86(6):957–968. [PubMed] [Google Scholar]
- 24.Chesi M, Bergsagel PL, Shonukan OO, Martelli ML, Brents LA, Chen T, et al. Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998 Jun 15;91(12):4457–4463. [PubMed] [Google Scholar]
- 25.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006 Oct;6(10):741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005 Mar;5(3):230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Woo CJ, Iglesias-Ussel MD, Ronai D, Scharff MD. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev. 2004 Jan 1;18(1):1–11. doi: 10.1101/gad.1161904. [DOI] [PubMed] [Google Scholar]
- 28.Bakkus MH, Heirman C, Van Riet I, Van Camp B, Thielemans K. Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood. 1992 Nov 1;80(9):2326–2335. [PubMed] [Google Scholar]
- 29.Decourt C, Galea HR, Sirac C, Cogne M. Immunologic basis for the rare occurrence of true nonsecretory plasma cell dyscrasias. J Leukoc Biol. 2004 Sep;76(3):528–536. doi: 10.1189/jlb.0803382. [DOI] [PubMed] [Google Scholar]
- 30.Cogne M, Guglielmi P. Exon skipping without splice site mutation accounting for abnormal immunoglobulin chains in nonsecretory human myeloma. European journal of immunology. 1993 Jun;23(6):1289–1293. doi: 10.1002/eji.1830230615. [DOI] [PubMed] [Google Scholar]
- 31.Morrison SL, Scharff MD. A mouse myeloma variant with a defect in light chain synthesis. European journal of immunology. 1979 Jun;9(6):461–465. doi: 10.1002/eji.1830090609. [DOI] [PubMed] [Google Scholar]
- 32.Haas IG, Wabl MR. Immunoglobulin heavy chain toxicity in plasma cells is neutralized by fusion to pre-B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7185–7188. doi: 10.1073/pnas.81.22.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaloff CR, Haas IG. Coordination of immunoglobulin chain folding and immunoglobulin chain assembly is essential for the formation of functional IgG. Immunity. 1995 Jun;2(6):629–637. doi: 10.1016/1074-7613(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 34.Dul JL, Aviel S, Melnick J, Argon Y. Ig light chains are secreted predominantly as monomers. Journal of immunology (Baltimore, Md. 1996 Oct 1;157(7):2969–2975. [PubMed] [Google Scholar]
- 35.Sakai A, Kawano MM, Tanabe O, Kuramoto A. A possible mechanism of inability of immunoglobulin heavy-chain production in Bence-Jones type myeloma cells. Int J Hematol. 1993 Dec;59(1):31–40. [PubMed] [Google Scholar]
- 36.Chng WJ, Ketterling RP, Fonseca R. Analysis of genetic abnormalities provides insights into genetic evolution of hyperdiploid myeloma. Genes, chromosomes & cancer. 2006 Dec;45(12):1111–1120. doi: 10.1002/gcc.20375. [DOI] [PubMed] [Google Scholar]
- 37.Avet-Loiseau H. Role of genetics in prognostication in myeloma. Best practice & research. 2007 Dec;20(4):625–635. doi: 10.1016/j.beha.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Avet-Loiseau H, Facon T, Grosbois B, Magrangeas F, Rapp MJ, Harousseau JL, et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood. 2002 Mar 15;99(6):2185–2191. doi: 10.1182/blood.v99.6.2185. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca R, Harrington D, Oken MM, Dewald GW, Bailey RJ, Van Wier SA, et al. Biological and prognostic significance of interphase fluorescence in situ hybridization detection of chromosome 13 abnormalities (delta13) in multiple myeloma: an eastern cooperative oncology group study. Cancer Res. 2002 Feb 1;62(3):715–720. [PubMed] [Google Scholar]
- 40.Gertz MA, Lacy MQ, Dispenzieri A, Greipp PR, Litzow MR, Henderson KJ, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and −17p13 in myeloma patients treated with high-dose therapy. Blood. 2005 Oct 15;106(8):2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaughnessy J, Jr, Tian E, Sawyer J, McCoy J, Tricot G, Jacobson J, et al. Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 deletion in multiple myeloma: early results of total therapy II. British journal of haematology. 2003 Jan;120(1):44–52. doi: 10.1046/j.1365-2141.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarti D, Ghosh SK. Induction of syngeneic cytotoxic T lymphocytes against a B cell tumor. II. Characterization of anti-idiotypic CTL lines and clones. Cell Immunol. 1992 Oct 15;144(2):443–454. doi: 10.1016/0008-8749(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 43.Halapi E, Werner A, Wahlstrom J, Osterborg A, Jeddi-Tehrani M, Yi Q, et al. T cell repertoire in patients with multiple myeloma and monoclonal gammopathy of undetermined significance: clonal CD8+ T cell expansions are found preferentially in patients with a low tumor burden. European journal of immunology. 1997 Sep;27(9):2245–2252. doi: 10.1002/eji.1830270919. [DOI] [PubMed] [Google Scholar]