Abstract
Considerable recent work has shown that the hippocampus is critical for remembering the order of events in distinct experiences, a defining feature of episodic memory. Correspondingly, hippocampal neuronal activity can ‘replay’ sequential events in memories and hippocampal neuronal ensembles represent a gradually changing temporal context signal. Most strikingly, single hippocampal neurons – called time cells – encode moments in temporally structured experiences much as the well-known place cells encode locations in spatially structured experiences. These observations bridge largely disconnected literatures on the role of the hippocampus in episodic memory and spatial mapping, and suggest that the fundamental function of the hippocampus is to establish spatio-temporal frameworks for organizing memories.
The temporal organization of memory
‘Acts of recollection, as they occur in experience, are due to the fact that one thought has by nature another that succeeds it in regular order.’
Aristotle ([1], p. 351)
‘Organization of knowledge in the episodic [memory] system is temporal. One event precedes, cooccurs, or succeeds another in time.’
Endel Tulving ([2], p. 225)
The idea that everyday memories are organized in time dates from at least Aristotle, and Tulving emphasized temporal organization when he introduced the modern conception of episodic memory as distinct from semantic memory. Furthermore, many studies have identified the hippocampus as a core brain area that supports the capacities for recollection (e.g., [3]) and episodic memory [4]. Therefore, it stands to reason that the hippocampus might play an important role in the temporal organization of memories. In this review, I will summarize research that supports this idea and will consider neural coding mechanisms that underlie the temporal organization of events within episodic memories. In addition, I will relate the findings on temporal representation to a large body of data that implicates the hippocampus in spatial representations. Taken together, these two strands of research suggest the existence of fundamental information processing mechanisms that support memory for spatio-temporal context.
The hippocampus plays an essential role in memory for the order of events within episodes
The role of the hippocampus in the temporal organization of memory has been examined in two general paradigms, memory for serial order within lists and performance in disambiguating overlapping event sequences. The earliest studies on memory for serial order showed that amnesic patients of various etiologies were impaired in reproducing the ordering of 15 words, but the deficit was attributed to potential compromise of the functionality of prefrontal cortex, for which damage also resulted in a deficit in temporal ordering [5]. Subsequently, however, studies on individuals with selective hippocampal damage identified impairments in recalling or recognizing the order of words in lists or word pairs, even though they could recognize familiar words and word pairings [6], and in remembering the order of objects obtained while driving through a virtual reality town, even though they could recognize the familiar objects [7].
Correspondingly, in normal human subjects the hippocampus is activated while remembering the order of objects received during the virtual reality driving game [8]. Another study showed striking selective activation of the hippocampus when humans reconstructed the correct ordering of scenes previously viewed in a movie clip, as compared to when they inferred the logical order of related scenes from the same movie ([9]; Figure 1a). Using a different approach that exploited the well-known observation of enhanced neural responses to novel stimuli, Kumaran and Maguire [10] demonstrated hippocampal activation when subjects were presented with items out of order late in a familiar series; activation was not observed in the hippocampus (although it was observed in entorhinal/perirhinal cortex) when an entirely novel ordering of familiar pictures was presented. Thus, hippocampal activation signaled a mismatch of expectation in temporal order per se, and not merely a novel sequence of pictures.
Figure 1.
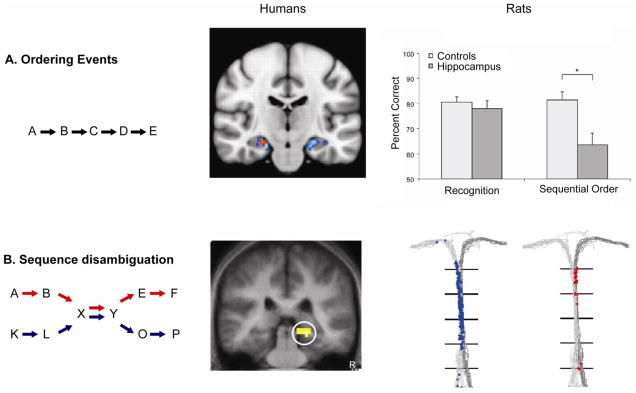
Involvement of the hippocampus in the temporal organization of memory in humans and rats. (a) Left: memory for serial order in lists. Middle: activation of the hippocampus in humans remembering the order of scenes in a movie. Reproduced, with permission, from [9]. Right: performance of normal rats and rats with selective hippocampal damage on memory for the order of odors versus recognition of odors from a list (p = 0.009). Reproduced, with permission, from [13]. (b) Left: disambiguation of overlapping sequences. Red and blue arrows indicate the two sequences. Middle: activation of the hippocampus during learning of overlapping face sequences. Reproduced, with permission, from [23]. Right: firing patterns of a hippocampal neuron as a rat traverses the maze stem on left-turn (blue dots represent spikes) and right-turn (red dots) trials. Gray lines are paths through the maze on both types of trials. Reproduced, with permission, from [41].
In addition to retrieval of ordered memories, activation of the hippocampus during the encoding of sequential events predicts subsequent memory for temporal order. Tubridy and Davachi [11] reported that hippocampal activation during the encoding of word triplets predicted subsequent accuracy of memory for word order. Notably, there was no difference in encoding activation for word triplets subsequently remembered out-of-order versus forgotten triplets, indicating that hippocampal encoding activation signaled memory for order per se, not memory for the words themselves. In another study, Staresina and Davachi [12] showed that the level of encoding activation increased with the demand to bridge a temporal (and spatial) gap between color and object pairings, independent of memory success. Thus, the level of hippocampal activity reflected the degree of temporal integration more so than memory for the pairings per se. These combined findings provide compelling evidence of strong engagement of the hippocampus during encoding and retrieval of the temporal organization of memories.
These observations on humans are complemented by studies in animals that explore whether rats can remember the order of once-experienced sequences of events and investigate whether the hippocampus is involved in temporal order memory. To address these questions, Fortin et al. ([13]; see also [14]) examined whether rats could remember once-presented sequences of odors and compared their ability to remember the order of odors versus their ability to recognize odors that had appeared within lists regardless of order. On both the order and recognition tests, rats initially were rewarded for sampling each of a list of 5 odors. Five minutes later, on the order test, they were presented two non-adjacent odors from the list and were required to choose the less recently experienced odor to obtain another reward. On the recognition test, the rats were presented with one odor from the list and another odor not on the list, and were required to choose the odor not on the list. Rats performed well above chance on temporal order memory and better when the lag between previously presented items was larger. Rats with selective hippocampal damage were impaired at all lags and performance was above chance only for the largest lag (Figure 1a). By contrast, on the recognition test, rats with hippocampal damage performed as well as normal rats and the selective impairment in order memory compared to intact recognition was striking, even when overall accuracy in normal animals was matched between tasks. Furthermore, rats with hippocampal damage showed the same better performance on memory for more recently experienced items in the recognition task (i.e., a recency effect), which indicates intact recognition following hippocampal damage and suggests that the performance of normal rats in temporal order memory was not based solely on differences in the relative recency of the odors.
Using a similar procedure in monkeys, Templeton and Hampton [15] presented convincing evidence that memory for order is not based on differences in the strengths of earlier and later experienced items, or by list position, and confirmed that accuracy in order judgments was improved by greater separation of items within a list. Another study showed that mice also remember the order of serially presented odors, which is reflected in preferential exploration of earlier over later presented odors within familiar lists. This form of temporal order memory, but not memory for the odors themselves, is dependent on the hippocampus [16]. The same normal mice did not show a preference for odors experienced much less recently between lists that were widely separated in time, which shows that their memory for order within lists was not based on recency, but rather on serial order within a list. In another study that explored memory for a list of odors and the locations where they were experienced, Ergorul and Eichenbaum [17] found that normal rats used a combination of odor and location information to remember the order of odors experienced in trial-unique lists, and that rats with hippocampal damage were impaired in temporal order memory, even as probe tests showed spared memory for the odors and for the locations alone.
Another series of studies has examined the role of the hippocampus in learning action sequences in humans and animals. In particular, several studies have examined performance on the serial reaction time test, a task in which subjects tap a series of keys as they are lit on a keyboard gradually becoming faster in repeating sequences as compared to random ordered sequences. These studies have shown that humans can use either explicit recall of sequences or implicit habit learning to facilitate the reproduction of these action sequences. The hippocampus is activated during serial reaction time learning, regardless of whether subjects are consciously aware of their memory for the sequence, which indicates that hippocampal activation reflects the acquisition of temporal organization per se, and not explicit memory for the sequence [18]. Studies in rats have shown that selective hippocampal damage impairs learning complex sequences of nose-pokes to different locations [19], anticipatory (‘explicit’) actions towards maze arms that are sequentially presented in a radial maze [20], and flexible expression of sequences of body turns that designate arm choices on a complex maze [21]. The broad range of studies showing an essential role of the hippocampus in memory for stimulus and action sequences, in humans and animals, indicates a fundamental role in the temporal organization of memories.
The hippocampus plays an essential role in disambiguation of overlapping memories
Many of our experiences overlap with the contents of events that occur in sequential experiences. For example, we often walk through common streets on the way to different locations and we often visit with the same people to have diverse discussions, yet we usually are able to recall each ordered experience distinctly. The role of the hippocampus in disambiguating overlapping sequences has been studied in rats trained on odor sequences that overlapped (Figure 1a), implemented as a series of choices between two odors for which the first correct choice indicated each of the remaining correct choices [22]. Importantly, some of the odors were common to both sequences, so subjects were required to remember the ongoing sequence through the overlapping elements in order to make the final choices. Rats with hippocampal damage were impaired at the critical choice just after the overlapping elements only when a delay was imposed between those choices. Thus, other brain systems can support reproduction of overlapping continuous sequences, but the hippocampus is required when the sequence is halted and then must be remembered to continue.
In a parallel fMRI study with humans, Kumaran and Maguire ([23]; Figure 1b) reported strong hippocampal activation during the encoding of overlapping, but not non-overlapping, sequences of faces and this activation predicted accurate sequence retrieval. The hippocampus is also activated during the retrieval of overlapping (as well as non-overlapping) sequences of faces [24] and is activated during retrieval of overlapping routes through a virtual maze [25]. Taken together, these studies show that the hippocampus plays a central role in temporal organization that distinguishes overlapping memories, in humans and animals, and in both spatial and non-spatial memory.
Neuronal networks in the hippocampus ‘replay’ sequences of events in memories
There is substantial evidence that hippocampal neural ensembles represent the order of events from previous experiences. Much of the data come from studies where rats repetitively traverse the same route through space, during which hippocampal place cells (see Glossary) activate sequentially. In this paradigm, numerous studies have reported forward and reverse ‘replays’ of the place cell sequences, both when animals are asleep and during periods of quiet wakefulness (e.g., [26]). Hippocampal replays are synchronized with cortical replays [27], and interfering with replay retards subsequent memory in maze learning [28], which suggests a role for replay in memory consolidation. Furthermore, when rats are engaged in vicarious trial and error of maze choices, hippocampal neurons replay possible choice paths [29], which suggests that replays reflect remembering past outcomes. In addition to memories for spatial sequences in rats, in humans, neurons throughout the hippocampal region replay firing sequences during recall of scenes in film clips [30]. Furthermore, selectively within the hippocampus, neuronal ensembles develop reliable firing sequences as human subjects gradually learn a sequence of scenes in a film [31]. These findings provide compelling evidence that hippocampal neuronal ensembles can encode and retrieve the temporal structure of sequential events.
How do neuronal networks in the hippocampus organize events in time?
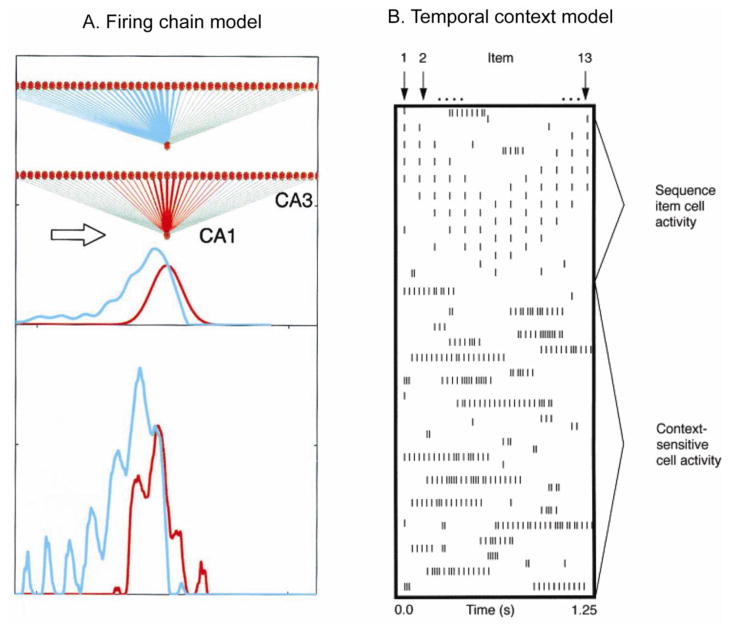
Two kinds of models have been proposed to explain the underlying neural representation that supports the temporal organization of hippocampal sequence representations. Neuronal ensembles might represent temporal order by constructing a firing chain via enhancements of direct connections between neurons that represent temporally adjacent events [32,33] or hippocampal ensembles might represent a gradually evolving temporal context to which memories are bound at appropriate times [34–36].
Consistent with the chaining model, Jensen and Lisman [32] proposed that serial events are linked via long-term potentiation (LTP), and sequence representations are fit within cycles of the theta rhythm. Consistent with this kind of model, when rats run through a sequence of locations repeatedly, the place fields of hippocampal neurons expand backward from their centers [33]. The backward expansion of the place fields could reflect each place cell being driven by an earlier firing place cell through LTP-mediated enhanced connectivity (Figure 2a). Also in support of this view, Dragoi and Buzsáki [38] reported that the correlated firing of hippocampal neurons with overlapping spatial fields is greater than that expected if place cells fired independently, driven by an external pacemaker. Although chaining of neuronal activations can most straightforwardly explain sequence representation, the disambiguation of overlapping sequences (described above as a key capacity supported by the hippocampus) is more difficult to explain with a simple chaining model. To the extent that sequence representations are composed solely of neurons that are directly driven by specific events, chains containing identical events cannot support divergent outcomes.
Figure 2.
Models of temporal representation in the hippocampus. (a) Firing chain model of Mehta et al.[33]. As a rat moves consistently from left to right (arrow), CA3 place cells that fire in order (orange dots) activate a CA1 cell, and the strength of each synapse is proportional to the thickness of the red lines. After repeated directional activation, LTP of the CA3-to-CA1 synapses results in a synaptic matrix that is asymmetric (blue lines and blue curve). Reproduced, with permission, from [33]. (b) Temporal context model of Wallenstein et al. [35]. At the top are spiking patterns of different neurons representing 13 items that occur sequentially; at the bottom are longer firing context neurons that bridge across items within the sequence. Reproduced, with permission, from [35].
Alternatively, temporal context models offer mechanisms that can both represent the order of sequential events and disambiguate overlapping sequences. Levy [34] and Wallenstein et al. [35] offered models based on a proposed existence of hippocampal ‘context’ neurons that fire for periods that bridge sequential events, thus constituting the temporal context signal to which specific events are associated at appropriate moments. Thus, for example, in the Wallenstein et al. [35] model, distinct events are encoded by neurons that fire transiently as those events occur, and these representations are bridged by extended and overlapping activations of context cells to organize the ordering of events (Figure 2b). Howard et al. [37] postulated that ensembles of hippocampal context cells might arise as leaky integrators that incorporate events prior to, including, and following each sequential event, together composing a representation of experiences that surround each event and bridge events. Alternatively, or in addition, the context neurons may be derived from a pre-existing temporal framework. Based on overwhelmingly large storage requirements for representing contexts directly from environmental cues, McNaughton et al. [39] proposed the existence of pre-configured contextual representations to which specific behavioral events were linked to compose spatial memory representations. Consistent with this proposal, Dragoi and Tonegawa [40] recently found that hippocampal ensembles tend to ‘pre-play’ sequences that will subsequently reflect serial place cell firings in a new environment, which suggests that pre-existing ensemble structure contributes to new sequence coding. These combined findings provide preliminary support for the notion that neural ensembles in the hippocampus create contextual representations based on pre-existing population connectivity, which then integrates across experiences to create a temporal organization for memories.
Within temporal context models, disambiguation of overlapping memories is accomplished by different neuronal populations that represent each of the overlapping memories. These models predict the existence of neuronal ensembles that differ in firing patterns during sequence production of distinct memories, even when the overt behaviors, places, and times in the sequence are identical. Indeed, there is substantial evidence of hippocampal context neurons that distinctly represent the same overt events that are common to different memories through differential activity depending on preceding and succeeding events. This phenomenon has been demonstrated best in variants of spatial alternation tasks in which rats traverse the ‘stem’ of a T-maze, then alternately choose between left and right turn arms (Figure 1b). On each trial the rat must remember its last choice and maintain this information as it traverses the stem that is common to both left- and right-turn paths that follow. Several studies have observed that hippocampal place cells fire differentially as the rat passes through successive locations on the maze stem, depending on either the previous choice or the succeeding choice ([41–44]; see [45], for a review). Similarly, in the non-spatial domain, as rats disambiguate overlapping odor sequences, hippocampal principal neurons fire differentially in anticipation of, or during presentation of the ambiguous odors, depending on the ongoing sequence [46]. Conversely, Barnes et al. [47] reported that the abnormal acquisition of multiple contextual representations for the same experience can explain impairments in retrieving the appropriate memories in aged rats.
There is also growing evidence that hippocampal neuronal ensembles compose a gradually changing representation of the flow of time, which is independent of explicitly identifiable locations or events that might directly drive neural activations. Early evidence came from a study in which ensembles of CA1 neurons were recorded as rats performed the above-described task, wherein rats encode and remember unique sequences of odors [13]. The firing patterns of ensembles gradually changed over entire testing sessions, and even over a few minutes in which individual sequences were encoded [48]. In the latter, the extent of ensemble change predicted subsequent memory success. This study revealed the existence of a temporal signal important to memory, but, because the specific items in memory were different in each sequence, could not identify whether ensembles encoded specific memories. This was accomplished in a study where rats ran in a running wheel in between trials in spatial alternation [49]. The main finding was that different hippocampal neurons fired sequentially associated with different subsequent memory choices. Ensembles of these cells encoded time passing, even when the position of the animal was relatively constant.
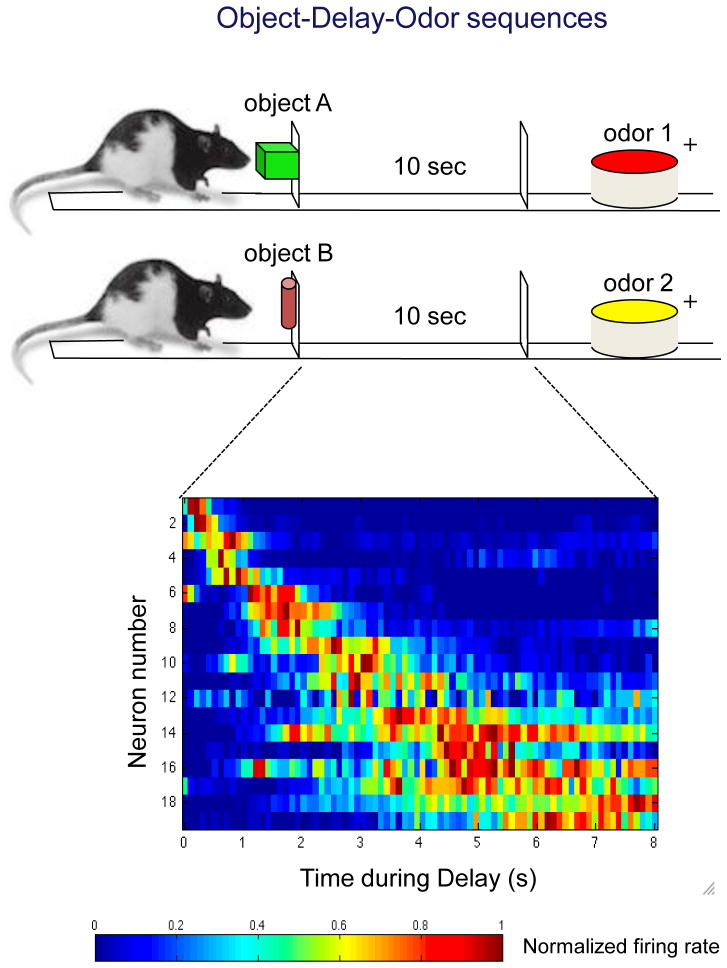
Subsequent studies on hippocampal cells that encode time have been pursued in hippocampal area CA1, which has been shown to be critical to bridging temporal gaps in diverse behavioral tasks [50–52]. In one of these tasks, rats learn associations between specific objects and odors separated by a 10 s interval. Learning to bridge these associations across a delay requires CA1, but not CA3, involvement ([50]; Figure 3). Using this paradigm, MacDonald et al. [53] reported that hippocampal neurons fire for brief moments in sequence, together filling the empty interval. These ensemble firing patterns reflected the passage of time, even after accounting for variations in the precise location of the rat, its head direction, and speed. Because these neurons seem to encode moments in a temporally-structured period, much as place cells encode locations in a spatially-structured environmental context, they were dubbed ‘time cells’ (Figure 3). Subsequent studies have provided evidence of cells that integrate time as well as distance traveled in rats running in place on a treadmill at different speeds (Kraus et al., 2011, Society for Neuroscience Annual Meeting Abstract 731.16). Also, time cells bridge empty delays in a matching to sample task in head-fixed rats, thus precluding the possibility that time-related activity is due to small variations in location, coincident or planned movement, or path integration (MacDonald et al., 2012, Society for Neuroscience Annual Meeting Abstract 203.11). Therefore hippocampal neurons can ‘tell time’ independent of their role in encoding space and other aspects of behavioral events. This role in temporal processing may extend to influencing the accuracy and precision of timing in a broad range of situations [54].
Figure 3.
Time cells in the hippocampus. Above: in the object-delay-odor sequence task, rats are initially presented with one of two objects and then, following a 10 sec delay, must respond to one of two odors to receive reward. Below: during the delay, hippocampal neurons fire sequentially; the normalized firing rate of each neuron is shown in successive rows. Reproduced, with permission, from [50].
The firing of patterns of hippocampal time cells differed during intervals that bridged distinct object-odor pairs, which suggests the existence of unique temporally organized ensembles that encode each memory ([49, 53]; MacDonald et al., 2012 Society for Neuroscience Annual Meeting Abstract 203.11]). Other studies have shown that time cells bridge the delay between trials in a maze task [55], that the appearance of these time cells evolves with learning [55], and that time cells appear only when rats must use memory in T-maze alternation as compared with running in a wheel when memory is not required [49]. These findings indicate that ensembles of time cells constitute a representation of specific learned sequences.
Other aspects of time cell firing patterns indicate that these neurons fire independently and not as elements of a firing chain. In the object-odor association paradigm, when the delay between the object and odor presentations was elongated, the sequence of cell firings that represented the original early component of the delay was not preserved and extended with additional neuronal activations, as might be expected in a firing chain [53]. Instead, after the delay was elongated, some neurons maintained their firing patterns at a fixed time anchored to the beginning or end of the delay, whereas other neurons that fired in the original sequence ‘re-timed’, that is, either turned off or fired at a different moment, and new cells joined the ensemble at various times, consistent with the view that the elongated delay required a reformulated temporal context representation. In addition, in the delayed matching to sample task used to examine time cell activity in head-fixed rats (MacDonald et al., 2012, Society for Neuroscience Annual Meeting Abstract 203.11) and in other paradigms [49,55], different time cell sequences that represent distinct memories involve combinations of neurons that fire at the same time and others that fire at moments unique to a particular memory.
The partial re-timing observed across all these studies is consistent with a temporal context signal containing common elements that encode the common temporal structure and distinct elements that encode the differences in memories. Furthermore, Naya and Suzuki [56] reported that hippocampal neurons in monkeys performing a visual paired associate task, where the stimuli are separated in time, provide a temporal signal during the interstimulus interval. Neurons in higher order visual areas (inferotemporal and perirhinal cortex) encode the visual cues, and entorhinal neurons encode both the visual cues and time, whereas the hippocampal neuronal ensemble signals only the temporal organization, without specificity for the items held in memory. It is not clear why the studies on rats observed memory-specific time cell sequences whereas Naya and Suzuki observed the same time cell sequence for all memories, but a major distinction between the protocols is that the tasks used for rats all involved a small number of highly repetitive sequences, whereas the monkeys performed a task that involved a large number of sequences that rarely repeated. Possibly, after many exposures, stimulus sequences are embodied in the regularities of the temporal structure, whereas when diverse stimulus sequences seldom repeat, only the reliable temporal structure of the task is represented by the hippocampus. These findings strongly support the idea that hippocampal time cells have the properties of ‘context cells’ proposed in temporal context models [34, 35, 37].
Time cells and place cells
Many studies have provided considerable evidence that the activity of hippocampal neurons signal an animal’s location within an environment – these neurons are called place cells [57]. There is strong evidence of spatial representation by hippocampal neurons in humans, as well [58]. How can the observations on temporal representation by the hippocampus be reconciled with its well-known role in spatial representation? There are two main features of time cells and place cells which suggest that they are not distinct ‘cell types’, but, rather, whether their activity is associated with time or space reflects different dimensions of the context in which learning occurs.
First, the properties of hippocampal time cells parallel the defining characteristics of place cells. A general characterization of time cells is that they parse temporally defined periods into representations of specific moments (‘time fields’), much as place cells parse spatially defined environments into representations of specific locations (place fields). Moreover, alterations of the major temporal dimension of a task causes a partial ‘re-timing’ of hippocampal time cells, much as alterations of spatial cues cause partial ‘re-mapping’ of place cells [53]. In addition, both time cells and place cells encode specific events within their spatial [59] and temporal ([53]; MacDonald et al., 2012, Society for Neuroscience Annual Meeting Abstract 203.11) frameworks, respectively. Although there are multiple origins of temporal signals in the brain [60,61], place and time cells may also be similar in that a source of both spatial and temporal information to hippocampal cells may be the medial entorhinal cortex (Kraus et al., 2012, Society for Neuroscience Annual Meeting Abstract 203.14). Finally, another similarity of time cells and place cells can be found in the view that ensembles of place cells may be pre-configured and then applied to represent spatial dimensions [39]. Similarly, according to temporal context models, time cells reflect a sequential configuration of the passage of time, onto which specific events are serially attached [40].
Second, time cells typically incorporate spatial information [53], just as place cells incorporate information about past and future events [45]. Furthermore, in the task where rats run on a treadmill in the midst of traversing a T-maze, many of the same neurons that fire selectively at moments on the treadmill also have spatially specific activity as the animal moves through its paths on the maze (Kraus et al., 2011, Society for Neuroscience Annual Meeting Abstract 731.16). The observation of predominant temporal or spatial coding in particular reports is likely more a reflection of different experimental designs that emphasize time or place representation, respectively, rather than a fundamental distinction between time cells and place cells.
Concluding remarks
There is a wealth of evidence from diverse experimental approaches in humans and animals that the hippocampus plays a central role in the temporal organization of memories and supports the disambiguation of overlapping episodes. Ensembles of hippocampal time cells may represent a gradually changing temporal context signal that integrates information across events and thereby both separates distinct episodes and defines the temporal structure within each unique episode. The evolving hippocampal temporal context representation may incorporate particular events at specific times itself or may contain only the temporal structure for unique experiences, which guides the timing of event representations in cortical areas to which the hippocampus is connected. Finally, a synthesis of emerging knowledge about time cells and place cells suggests that the same population of hippocampal neurons encodes both the spatial and temporal regularities of experience, creating a framework that organizes the spatio-temporal context of experience and puts each event into its time and place (see Box 1 for a list of key outstanding questions).
Box 1. Questions for future research.
What is the source of temporal information to the hippocampus?
What are the mechanisms of the development of temporal context representations that distinguish overlapping memories?
How are representations of specific events incorporated into or driven by temporal context representations?
Acknowledgments
This work was supported by NIMH grant MH095297.
Glossary
- Context cells
theoretical neurons whose activity bridges between events and therefore could link their representations in order. These may pre-exist or arise independent of specific events, or could arise as an integration of event information over periods of time
- Long-term potentiation (LTP)
the enhancement of efficacy of the synaptic connections between neurons; occurs as a result of repetitive combined pre-synaptic and post-synaptic activation
- Place cells
neurons in the hippocampus that fire when an animal (or human) is at a particular location in a spatially defined environment
- Time cells
neurons that fire at a specific moment within a temporally structured episode. Time cells and place cells are not distinct ‘cell types’; the same population of hippocampal neurons encodes both the spatial and temporal regularities of experience, and whether the activity of a cell is associated with time or space is dependent on the dimensions of the context in which learning occurs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aristotle (ca. 350 B.C.) On Memory and Reminiscence. In: Beare JI, translator; Ross WD, editor. The Works of Aristotle. Vol. 3. Clarendon Press; 1930. [Google Scholar]
- 2.Tulving E. Précis of Elements of Episodic Memory. Behav Brain Sci. 1984;7:223–268. [Google Scholar]
- 3.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 5.Shimamura AP. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 6.Mayes AR, et al. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn Neuropsychol. 2001;18:97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- 7.Spiers HJ, et al. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem. 2007;14:645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortin NJ, et al. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesner RP, et al. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- 15.Templer VL, Hampton RR. Cognitive mechanisms of memory for order in rhesus monkeys. Hippocampus. 2012 doi: 10.1002/hipo.22082. http://dx.doi.org/10.1002/hipo.22082. [DOI] [PMC free article] [PubMed]
- 16.Devito LM, Eichenbaum H. Memory for the order of events in specific sequences: contributions of the hippocampus and medial prefrontal cortex. J Neurosci. 2011;31:3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ergorul C, Eichenbaum H. The hippocampus and memory for ‘what,’ ‘where,’ and ‘when’. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schendan HE, et al. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 19.Ergorul C, Eichenbaum H. Essential role of the hippocampal formation in rapid learning of higher-order sequential associations. J Neurosci. 2006;26:4111–4117. doi: 10.1523/JNEUROSCI.0441-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeCoteau WE, Kesner RP. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav Neurosci. 2000;114 (6):1096–108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- 21.Fouquet C, et al. A new approach for modeling episodic memory from rodents to humans: the temporal order memory. Behav Brain Res. 2010;215 (2):172–9. doi: 10.1016/j.bbr.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 22.Agster KL, et al. The hippocampus and disambiguation of overlapping sequences. J Neurosci. 2002;22 (13):5760–8. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Ross RS, et al. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus. 2009;19 (9):790–9. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown TI, et al. Which way was I going? Contextual retrieval supports the disambiguation of well-learned overlapping navigational routes. J Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10 (1):100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 28.Jadhav SP, et al. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelbard-Sagiv H, et al. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paz R, et al. A neural substrate in the human hippocampus for linking successive events. Proc Natl Acad Sci U S A. 2010;107:6046–6051. doi: 10.1073/pnas.0910834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Mehta MR, et al. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25:707–715. doi: 10.1016/s0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 34.Levy WB. A Computational approach to hippocampal function. In: Hawkins RD, Bowers GH, editors. Computational Models of Learning in Simple Neural Systems. Academic Press; 1989. pp. 243–305. [Google Scholar]
- 35.Wallenstein GV, et al. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 36.Rolls ET. A computational theory of episodic memory formation in the hippocampus. Behav Brain Res. 2010;215:180–196. doi: 10.1016/j.bbr.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Howard MW, et al. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychol Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 39.McNaughton BL, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 40.Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood ER, et al. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 42.Frank LM, et al. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 43.Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- 44.Ainge JA, et al. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J Neurosci. 2007;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro ML, et al. Representing episodes in the mammalian brain. Curr Opin Neurobiol. 2006;16:701–709. doi: 10.1016/j.conb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Ginther MR, et al. Hippocampal neurons encode different episodes in an overlapping sequence of odors task. J Neurosci. 2011;31:2706–2711. doi: 10.1523/JNEUROSCI.3413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes CA, et al. multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- 48.Manns JR, et al. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastalkova E, et al. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesner RP, et al. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav Neurosci. 2005;119:781–786. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- 51.Farovik A, et al. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem. 2010;17:12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh J, et al. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald CJ, et al. Hippocampal ‘time cells’ bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin B, Troger AB. Exploring the 4th dimension: hippocampus, time, and memory revisited. Front Integr Neurosci. 2011;5:36. doi: 10.3389/fnint.2011.00036. http://dx.doi.org/10.3389/fint.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gill PR, et al. Hippocampal episode fields develop with learning. Hippocampus. 2011;21:1240–1249. doi: 10.1002/hipo.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 57.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 58.Maguire EA, et al. Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komorowski RW, et al. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. J Neurosci. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 61.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]