Abstract
Marijuana is the most widely used illicit substance among youth and recent epidemiological data indicate that rates of marijuana use are on the rise. The purpose of this study was to examine marijuana craving trajectories among adolescents in an eight-week, placebo-controlled pharmacotherapy trial targeting marijuana cessation. All participants received contingency management and cessation counseling, and were randomized to either N-acetylcysteine (NAC 1200 mg twice daily; n = 45) or placebo (n=44). Craving for marijuana was measured using the short-form of the Marijuana Craving Questionnaire (MCQ). Results demonstrated a significant decrease in MCQ scores over time for the total sample, but no significant differential change in scores between the NAC and placebo groups. This lack of significant difference is in the setting of NAC participants submitting significantly more negative urine cannabinoid tests as compared to placebo participants. This suggests that cessation effects associated with NAC may be mediated by effects other than marijuana craving.
Keywords: marijuana, craving, N-Acetylcysteine, adolescents
1. Introduction
Marijuana is the most widely used illicit substance among youth, and its use has steadily risen in recent years. The Monitoring the Future survey indicates that 6.6% of high school seniors use marijuana daily, the highest rate in the last 30 years (Johnston et al., 2012). Marijuana use in adolescents is associated with fewer completed years of education as well as an increased risk of affective, anxiety, and psychotic disorders in vulnerable individuals (Casadio et al., 2011). Given the increase in use as well as the detrimental effects of marijuana use during adolescence, a greater understanding of ideal treatments and factors that affect treatment is needed.
Craving is one of the key elements of addiction and is, therefore, one of the primary targets of treatment (McRae et al., 2007; Gray et al., 2008; Coffey et al., 2002). A study by McRae and colleagues of treatment-seeking individuals found that individuals with marijuana dependence (n=50) reported higher craving than individuals with cocaine dependence (n=153) (McRae et al., 2007). Little is known about the trajectory of marijuana craving among adolescents receiving cessation treatment, and whether psychosocial or pharmacological therapies might specifically influence craving.
Preclinical evidence suggests that NAC reduces drug seeking and relapse via glutamate modulation in the nucleus accumbens (Baker et al., 2003; Madayag et al., 2007; Kalivis, et al., 2009). Similarly, preliminary clinical evidence suggests that NAC may play a therapeutic role in the treatment of substance use disorders (Gray et al., 2012; Knackstedt et al., 2009; Mardikian et al., 2007). Given the salience of craving in addiction, it is possible that the effects of NAC are due, at least in part, to a reduction in craving. In two studies by LaRowe and colleagues, NAC reduced self-reported craving in individuals seeking treatment for cocaine dependence (LaRowe et al., 2006; 2007).
The purpose of the current study was to evaluate the trajectory of marijuana craving among adolescents participating in a marijuana cessation trial. A recently completed randomized controlled trial of N-acetylcysteine (NAC), added to brief weekly cessation counseling and a contingency management intervention, provided the opportunity to monitor self-reported marijuana craving over the course of treatment (Gray et al., 2012).
2. Materials and Methods
2.1 Participants
Participants were 89 treatment-seeking adolescents, aged 15-21, who met DSM-IV criteria for cannabis dependence, enrolled in the parent trial, and completed sufficient questionnaires for the present study’s objective (see section 2.2). Exclusion criteria included allergy to NAC, pregnancy or lactation, use of carbamazepine or nitroglycerin within 14 days of enrollment, enrollment in additional substance abuse treatment, substance dependence other than cannabis or nicotine, and significant medical or psychiatric illness that may increase risk in the judgment of the study physician. All participants received a contingency management intervention and weekly brief (≤10 minute) cessation counseling. Participants were assessed at baseline for eligibility (which included a history and physical examination) and eligible individuals were then randomized to receive NAC 1200mg orally twice daily or placebo for 8 weeks. Further details of the parent trial are described elsewhere (Gray et al., 2012).
2.2 Measurements
Craving was assessed with the 12-item, short form of the Marijuana Craving Questionnaire (MCQ). The MCQ has been shown to be a reliable and valid form for measuring craving (Heishman et al., 2001; Heishman et al., 2009). The short form of the MCQ includes 12 items that are divided into four factors: (1) compulsivity, (2) emotionality, (3) expectancy, and (4) purposefulness. The four factors are defined as follows: (1) an inability to control marijuana use; (2) use of marijuana in anticipation of relief from withdrawal or negative mood; (3) anticipation of positive outcomes from smoking marijuana; and (4) intention and planning to use marijuana for positive outcomes. (Heishman et al., 2009). Participants rate the items using a 7-item Likert scale ranging from strongly disagree to strongly agree, and the total score ranges from 12 to 84. Participants completed the MCQ at baseline and at each weekly visit. Out of those enrolled and randomized in the parent trial (n=116), 89 participants (77%) completed the MCQ at baseline and at least once during the 8-week treatment phase. These participants were included in the current study.
2.3 Statistical Analysis
The primary aim of interest was to test for differential treatment effects on marijuana craving as measured by the MCQ between participants receiving N-acetylcysteine versus placebo. Prior to analyses, demographic and clinical characteristics were tabulated for all participants and compared between groups. Standard descriptive statistics were used to summarize the demographic and clinical data. Treatment group differences for baseline continuous variables were assessed using a Wilcoxon rank sums test, and for categorical characteristics, a Pearson’s chi square test was used. Demographic, clinical, and marijuana use characteristics were also examined for univariate predictive relationships with MCQ total score response, as well as possible confounding effects within the primary treatment analysis models.
Initial models were fit using the treatment assignment, baseline MCQ score, time, and the interaction of treatment and time. As the primary aim of the parent study was to test the effects of NAC on abstinence from marijuana use, all models were also adjusted for the time varying effect of the weekly urine cannabis testing results. Additional models were developed using an expanded range of possible covariates based on baseline imbalances between treatment groups (p<0.10) and covariates showing evidence as prognostic factors of the MCQ total score (p<0.20). To test the primary hypothesis of differential treatment effects on the MCQ total score during the study, a repeated measures analysis of variance (ANOVA) framework was used and model estimation was constructed in SAS Proc Mixed. Restricted maximum likelihood (REML; Patterson & Thompson, 1971) methods were used to estimate the fixed effects and variance components in the presence of unbalanced data. Additional analyses examined the percent change in MCQ scores from baseline to the last available visit during treatment. In addition to the MCQ total score, the four factor components of the MCQ were also tested. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, 2011). Significance for all planned comparisons was set at a 2-sided p-value of 0.05 and no correction for multiple testing was applied to reported p-values.
3. Results
Of the 116 participants who were randomized in the parent trial, 89 (77%) completed the MCQ at baseline and at least once during subsequent visits and were included in the analysis. There were no significant differences in age, gender, or race between the treatment groups (all p>0.20). Similarly, cannabis use characteristics and the number of weekly treatment visits attended during treatment did not significantly differ by group (all p>0.20). Significantly more participants in the placebo group were diagnosed with current or past psychiatric disorders (NAC = 4.4% vs. placebo =18.2%; p=0.05); this was thus adjusted for in the analyses. Univariate predictive analyses showed that age, gender, race, and the number of days using marijuana in the previous 30 days were also possibly predictive of MCQ total score over time.
Mean total MCQ scores at baseline were not significantly different between the placebo and NAC groups (Mean±SEM: 48.4±1.9 vs. 47.9±1.8; p=0.84). Although there was a significant decrease in MCQ scores over the course of the treatment, there was no significant differential change between the NAC and placebo groups (F8,524=0.61, p=0.77; Figure 1). Individual analysis of the MCQ subscales revealed similar findings; the emotionality, expectancy, and purposefulness subscales all showed a significant decrease during the treatment phase (all p<0.02) while the compulsion subscale showed no significant change during the treatment phase (p=0.61). Similar to the MCQ total score, no differences were seen in the four subscale scores between groups over the course of the treatment portion of the study. Following adjustment for additional prognostic covariates (age, gender, race, marijuana use history) and the baseline imbalance in psychiatric diagnosis, the MCQ total score as well as the subscale scores continued to show no difference between NAC or Placebo groups over time (F8,524=0.59, p=0.78; Figure 1).
Figure 1. MCQ Total score over time by treatment group.
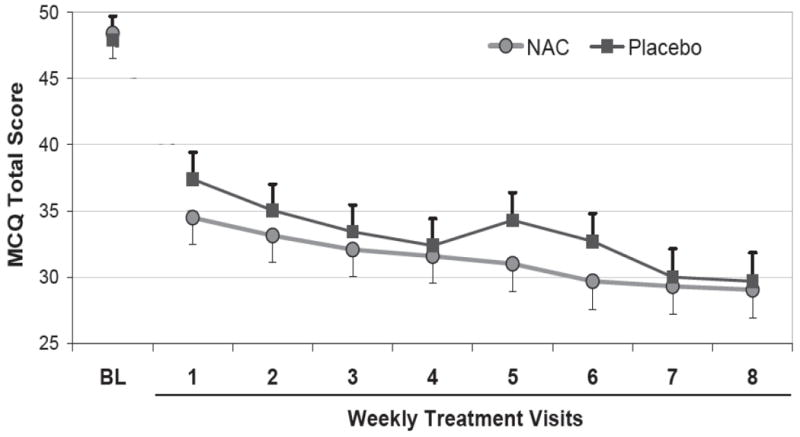
Data are shown as group level means over time and associated standard errors. All values are adjusted for time, baseline MCQ score, the interaction of treatment and time, and the time varying effect of the results of weekly urine cannabis testing. *p<0.05.
In addition to the baseline MCQ score (p<0.001), both time (visit) and weekly urine cannabinoid test results were significantly associated with the MCQ scores over time. Participants with negative urine cannabinoid tests were more likely to have lower MCQ scores measured at the same visit than those with positive tests (Δ=3.2±1.0, p=0.003). None of the other covariates attained statistical significance in the fully adjusted model.
To account for possible ceiling and floor effects of marijuana craving, the percent change from the baseline visit to the last treatment visit as well as the follow up visit were assessed between the NAC and placebo groups. Figure 2 shows the percent change from baseline to the end of treatment for the 89 participants with at least one medication visit following baseline. No significant differences were seen between the NAC and placebo treated participants (all p>0.28).
Figure 2. Percent change in MCQ Total Score and Subscale scores from the Baseline visit to the last available visit during treatment.
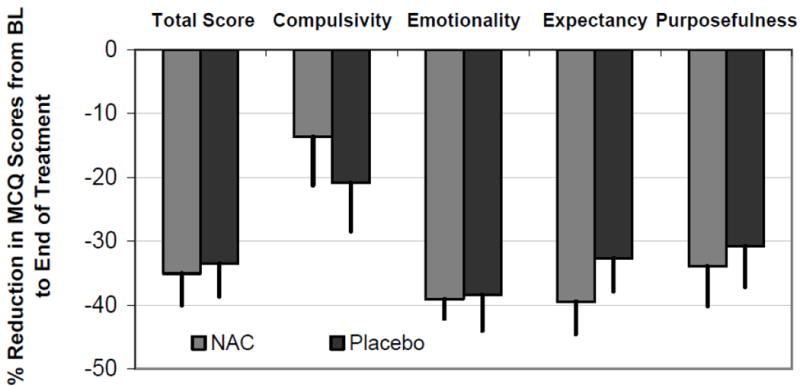
Data are shown as the mean percent change with associated standard error. All values are adjusted for the baseline MCQ score and baseline urine cannabis test result. *p<0.05.
4. Discussion
The aim of this study was to examine trends in marijuana craving in a pharmacotherapy trial for adolescents with marijuana dependence. In summary, marijuana craving as measured by the MCQ did not decrease more in the treatment group when compared with the placebo group. This was true for both the total MCQ and the individual four factor scores. This was contrary to our hypothesis, which was based on presumption that the NAC group’s significantly greater odds of negative urine cannabinoid tests in the parent trial was at least in part related to effects on craving (Gray et al., in press).
A possible explanation is that NAC does not work directly on craving, but instead exerts its effects via another clinical mechanism (clinical because on a molecular level preclinical evidence suggests glutamate modulation in the nucleus accumbens). A simpler explanation is that because all participants were treatment seekers, they were likely expecting craving to decrease during treatment. Similarly, as the participants were receiving counseling as well as contingency management, bias may have been introduced and participants may have wanted to please the investigators; if this phenomenon were present it would likely increase over time as attachment to team strengthened, thus explaining continuation of decreases in reported craving.
Although the primary aim of the parent study was to determine the efficacy of NAC for marijuana cessation, and was not powered to detect clinically relevant differences in craving, results suggest a null effect of NAC on marijuana craving during treatment.
A possible limitation of this study is that the MCQ was validated in adult non-treatment seekers whereas it was used to measure craving in our study in adolescent treatment seekers. It is also worth noting that the MCQ was used to measure ambient craving as opposed to cue-induced craving in this study (i.e., craving was measured without introducing a drug-related cue). An example of a paradigm that measures cue-induced craving, a cue (a picture, an imagery script, or a drug-related object for example) is introduced to the participant and subsequent craving is measured by self-reports and/or possible physiological measures of craving (Carter & Tiffany, 1999; Gray et al., 2008). Exploring the effect of NAC on cue-induced craving, as opposed to ambient craving, could be of interest in the future. Additionally, functional neuroimaging could be useful in adding to our understanding of NAC’s mechanism.
4.1 Conclusions
Craving for marijuana, as measured by the MCQ, decreased significantly over the treatment period, regardless of treatment group. This decrease was statistically and clinically significant. Contrary to our hypothesis, there was no significant difference in decrease in MCQ scores between NAC and placebo groups. This is despite NAC demonstrating a superior cessation effect when compared to placebo. Therefore, this study suggests NAC does not reduce craving, but may exert its treatment effect via alternative mechanisms. This is important in attempting to further understand the mechanism of NAC. In addition, the craving trajectories provide useful information when considering concurrent psychosocial treatments (CM and counseling).
Highlights.
Participants in the parent trial received either N-acetylcysteine (NAC) or placebo targeting marijuana cessation.
Marijuana craving was measured using the short form of the Marijuana Craving Questionnaire (MCQ).
Total MCQ scores decreased significantly during the trial among the total sample, but did not decrease differentially between the NAC and placebo groups.
NAC’s cessation effects may be mediated by effects other than marijuana craving.
Acknowledgments
This work was supported by National Institute on Drug Abuse grants R25 DA020537-06 (PIs: Back & Brady) and R01DA026777 (PI: Gray). Administrative and technical support was provided by National Center for Research Resources grant UL1RR029882. Thanks to Dr. Sudie Back for providing feedback.
Role of Funding Sources
NIDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Contributors
Amanda Roten wrote the introduction, material and methods (other than statistical analysis), discussion, and conclusion sections of the manuscript and was involved in developing the hypothesis for this secondary study. Nathaniel Baker provided statistical analysis and wrote the statistical analysis section of the methods as well as the results section of the manuscript. Kevin Gray was the primary investigator for the parent study. All authors contributed to and approved the final version of the manuscript.
Conflict of Interest
Kevin Gray receives funding for research from Supernus, Merck, Inc., and the National Institute on Drug Abuse. Nathaniel Baker has no conflicts of interest. Amanda Roten receives funding for research from the National Institute on Drug Abuse.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker DA, McFarland K, Lake RW, Shen H, Tang X, Toda S, Kalivas PW. Neuroadaptations in cysteine-glutamate exchange underlie cocaine relapse. Nature Neuroscience. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: The risk for schizophrenia. Neuroscience and Biobehavioral Reviews. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. Cannabis dependence in young adults: An Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: A preliminary investigation. Psychology of Addictive Behaviors. 2008;22(4):582–586. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. American Journal of Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the marijuana craving questionnaire. Drug and Alcohol Dependence. 2009;102(1-3):35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kalivas PW, LaLumiere R, Knackstedt L, Shen H. Glutamate Transmission in Addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. American Journal on Addictions. 2006;15(1):105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-Acetylcysteine? American Journal of Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. Journal of Neuroscience. 2007;27(51):13968–13978. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae AL, Hedden SL, Malcolm RJ, Carter RE, Brady KT. Characteristics of cocaine- and marijuana-dependent subjects presenting for medication treatment trials. Addictive Behaviors. 2007;32(7):1433–1440. doi: 10.1016/j.addbeh.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson HD, Thompson R. Recovery of inter-block information when block sizes are unequal. Biometrika. 1971;58:545–554. [Google Scholar]


