Abstract
Perivascular epithelioid cell tumors (PEComas) are a group of rare mesenchymal tumors that typically show both melanocytic and smooth muscle cell features. Some types of PEComa are seen at high frequency in tuberous sclerosis complex (TSC). The TSC1 and TSC2 genes are commonly mutated in both TSC-associated and sporadic PEComas, and mTOR signaling pathway activation is also common in these tumors. Preliminary reports have indicated that the mTOR inhibitors sirolimus and related drugs have activity in some patients with non-TSC-associated PEComa.
Here we report on the use of these medications in the treatment of five consecutive patients with extrarenal non-pulmonary PEComas seen at one institution. Three complete responses, one partial response and one case of progression were seen. Molecular studies identified TSC2 aberrations in four of these patients, and TFE3 translocation was excluded in the resistant case. A review of all published cases as well as those reported here indicates that partial or complete response was seen in 6 of 11 PEComas, with 5 of the 6 having a complete response. These findings highlight the consistent though incomplete activity of mTOR inhibitors in the treatment of PEComas.
Keywords: perivascular epithelioid cell tumor, PEComa, mTOR, TSC2, sirolimus, everolimus
Introduction
Sarcomas are a heterogeneous group of malignancies of mesenchymal origin. One subtype of sarcoma is the group of tumors called perivascular epithelioid cell tumors (PEComa), which are made up of cells that are of variable spindle to epithelioid morphology, and typically express both smooth muscle (actin, calponin) and melanocytic (HMB-45, Melan A, Mitf) markers.1, 2 The cell of origin of these tumors is unknown. PEComas include the more benign and relatively common renal angiomyolipoma, as well as pulmonary lymphangioleiomyomatosis (LAM), both of which are commonly seen in patients with tuberous sclerosis complex (TSC).3 However, the spectrum of PEComas also includes rarer tumors of variable malignant potential that typically involve the lung, as well as the gynecologic and gastrointestinal systems. This latter subset of PEComa is not particularly common in TSC.1–3
TSC1 and TSC2 are the two genes which cause TSC, a tumor suppressor gene syndrome, characterized by development of mostly benign tumors in multiple organ systems including skin, brain, heart, lungs, and kidneys.3 The TSC1 and TSC2 genes in TSC follow the classic Knudsen model of single allele germline inactivation combined with somatic second allele loss in TSC-associated tumors.4 Biochemical and signaling studies over the past 10 years have defined in increasing detail the critical function of the TSC1/TSC2 protein complex in the regulation of the state of activation of the mTORC1 kinase complex, through regulation of the activation state of the rheb GTPase.3 Thus, TSC lesions typically demonstrate marked enhancement of mTORC1 signaling, and this is also seen in a variety of mouse models of TSC.5–9 Sirolimus (rapamycin) and related compounds are highly effective in TSC mouse models, and this has led to a series of clinical trials in TSC patients for various tumors.10, 11 These trials have shown significant benefit for both renal angiomyolipoma and LAM, in the latter case in both patients with and without TSC.12–15 More limited studies have shown that sporadic PEComa not associated with TSC, including those with locally invasive and/or metastatic behavior, also commonly have mutation of the TSC2 gene and activation of mTORC1.5–7, 9 In addition, previous reports on a limited number of patients with non-renal abdominal PEComas described clinical benefit in those receiving sirolimus or temsirolimus.16, 17 Here we describe a consecutive series of five patients with malignant PEComa who were also treated with sirolimus or everolimus, four of whom demonstrated major clinical benefit, including two with sustained complete responses. We also explore the correlation between response and molecular and pathologic features of these tumors. We then review the current literature on treatment of malignant PEComa with this class of compounds.
Patients and Methods
Patient Selection, Treatment and Clinical Assessments
Five consecutive patients with PEComa who were seen at Memorial Sloan-Kettering Cancer Center (MSKCC) were offered off-label treatment with mTOR inhibitors. Diagnosis was made based on typical histology and positive reactivity with specific markers: SMA (smooth muscle actin) and/or CMA (common muscle actin), and HMB45 (melanocytic marker). None of the patients had signs of TSC or a personal or family history of TSC, apart from one patient who had radiographic evidence of pulmonary LAM but no other TSC features. All patients provided informed consent for treatment. Archival tumors and peripheral blood samples were collected and analyzed in accordance with a protocol approved by the MSKCC Institutional Review Board. The dosage of mTOR inhibitors was determined by the treating physician and was adjusted on the basis of trough levels or patient tolerance. Disease status was assessed by CT scans at baseline and at intervals as determined by the treating physician.
Histologic and Immunohistochemical Evaluation
Immunohistochemistry (IHC) was performed on 5 µm tissue sections prepared from formalin-fixed, paraffin-embedded tissue blocks. Slides were deparaffinized and sections were boiled with antigen retrieval solution (Dako) (pH 6.0). Endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 10 min at room temperature. 5% normal goat serum and 0.1% TritonX in phosphate-buffered saline (PBS) was used for blocking. Antibodies to phospho-S6 (Ser235/236; Cell Signaling Technology #2211; 1:200 dilution) were applied and incubated overnight at 4°C. Immunodetection was performed with DAB (Dako EnVision+ kit). Hematoxylin was used as a counterstain and an adjacent section was stained with hematoxylin and eosin.
Genetic Analyses
Formalin fixed paraffin-embedded tissue blocks were used for extraction of DNA using the QIAamp DNA FFPE Tissue Kit (Qiagen) according to the manufacturer's instructions. Normal DNA samples were prepared from blood samples for patients 1–3, after informed consent, by standard methods. For patient 4, normal liver tissue adjacent to the paraffin-embedded tumor was used as a control. The most frequently mutated exons in TSC2 (exons 10, 14, 16, 20, 23, 29, 30, 33, 36, 37, 38, 39, 40), in which over half of all TSC germline mutations are found, were amplified and sequenced bidirectionally by standard Sanger sequencing.3 Three microsatellite markers (STR3, KG8 and STR7) in the region of the TSC2 gene were used for loss of heterozygosity (LOH) analyses. Details of the markers, heterozygosity, genomic location, primer sequences, genotyping reactions and analysis have been described.7 LOH was determined to be present when there was ≥ 30% reduction of one allele in the PEComa sample in comparison to a control sample.
Fluorescence In Situ Hybridization (FISH) for TFE3 translocation
The possibility of translocation involving TFE3 on chromosome Xp11.23 was examined by FISH, using ‘break-apart’ probes specific for the 5’ and 3’ ends of TFE3, as described previously.18 One hundred nuclei showing both signals were examined in each case to avoid false-positive results.
Results
We describe the clinical features and response to treatment for each of 5 consecutive cases of extra-renal PEComa seen at MSKCC, and treated with sirolimus or everolimus. There were no manifestations of TSC in any of these patients, apart from evidence of pulmonary LAM in Patient 1. There was no family history of TSC in these patients.
Patient 1
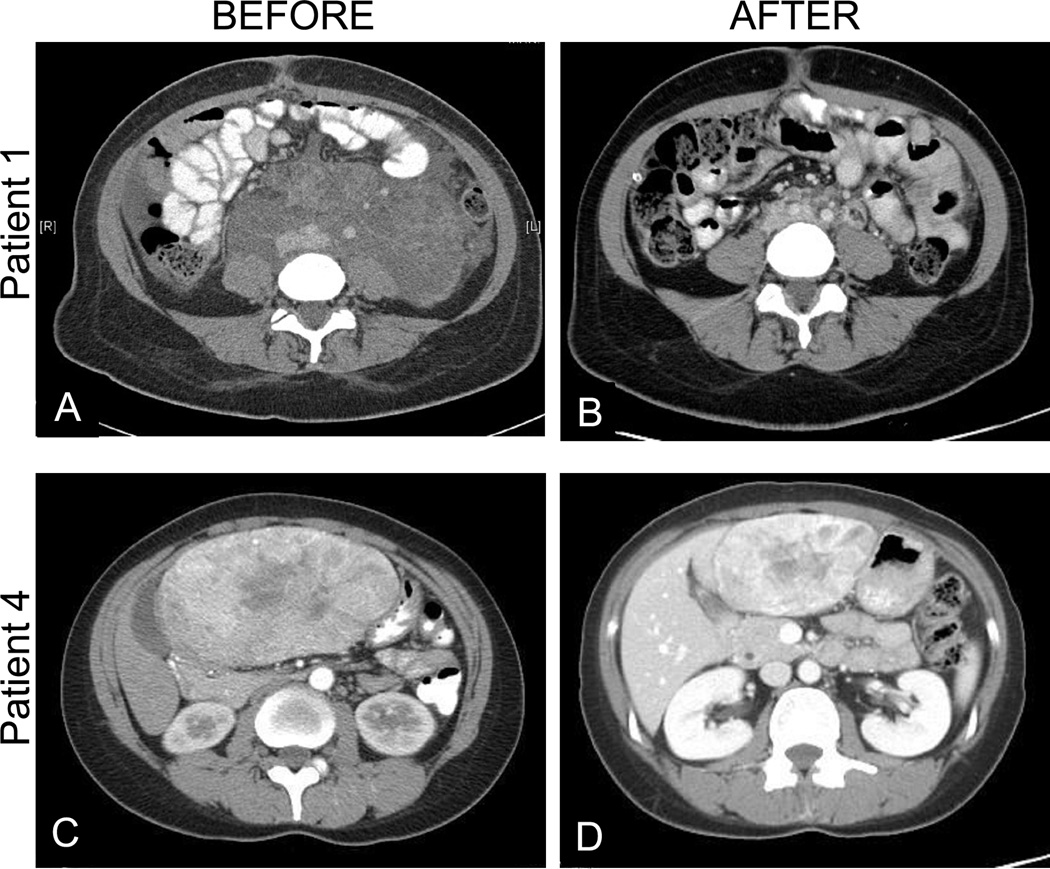
Patient 1 was a 24-year-old woman who presented in 2009 with abdominal distention. CT scan showed a 25 cm retroperitoneal mass encasing the aorta and inferior vena cava (Fig. 1A). She had a laparoscopic biopsy showing PEComa. The mass was considered unresectable, and she was treated with sirolimus 4 mg daily. Trough levels ranged from 7–17 ng/mL. As the retroperitoneal mass decreased, she developed chylous ascites. A peritoneovenous (Denver) shunt was placed. After 6 months of treatment, only residual retroperitoneal fibrosis remained with near complete disappearance of the tumor (Fig. 1B). CT chest scan showed numerous small (<6mm) cysts predominantly in the upper lobes consistent with pulmonary LAM. These lesions did not change during treatment and she had no pulmonary symptoms. She continues in complete response at 22 months of follow-up on rapamycin at 4mg PO QD.
Figure 1.
Computed tomography images of two patients (Patient 1 and Patient 4) with PEComa at baseline (A and C, respectively) and after treatment with sirolimus for 6 months (B) or everolimus for 2 months (D).
Patient 2
Patient 2 was a 40-year-old woman who presented in 2009 with bulky pelvic and retroperitoneal lymphadenopathy. Confluent lymph nodes in the bilateral iliac regions measured 6 cm in maximum diameter. CT-guided biopsy indicated PEComa. Treatment with sirolimus 4 mg daily was initiated but was reduced to 3 mg daily due to diarrhea. Trough levels ranged from 8–21 ng/mL. After 8 months of treatment, repeat abdominal and pelvic CT scan showed no evidence of lymphadenopathy, consistent with complete response. The patient developed chylous ascites requiring placement of a Denver shunt. Follow-up scans at 16 months of treatment showed that she continued to have no evidence of disease, and sirolimus continues at 3mg PO QD. Chest CT scan was normal.
Patient 3
Patient 3 was a 57-year-old man who presented with an acute abdomen in 2009, and was found to have bowel perforation due to a 10.5 cm PEComa of the small bowel, which was resected. Ten months later, multifocal intra-abdominal recurrence was apparent on follow-up scans. Treatment with sirolimus 4 mg daily was initiated. Significant reduction in the size of all lesions consistent with a partial response was seen 2 months later on follow-up scans. Sirolimus trough levels were 8–21 ng/mL with no significant toxicity. Response and treatment continue at 14 months of follow-up from initiation of sirolimus.
Patient 4
Patient 4 was a 37-year-old woman with a history of lupus erythematosus who developed abdominal pain. A large mass in the anterior liver was detected on CT (Fig. 1C). Fine-needle aspiration showed a PEComa. She was treated with everolimus 5 mg PO daily. There was no significant toxicity and drug levels were not obtained. After 2 months of treatment, CT scan showed a major decrease in the size of the mass (Fig. 1D). The tumor was then resected, and was found to be pseudo-encapsulated such that negative margins were obtained. The resected specimen showed major treatment effect including fibrosis and regions of hemorrhage (Fig. 2). At 6 months of follow-up, the patient is well and without evidence of disease. Everolimus was discontinued prior to surgery and has not been restarted.
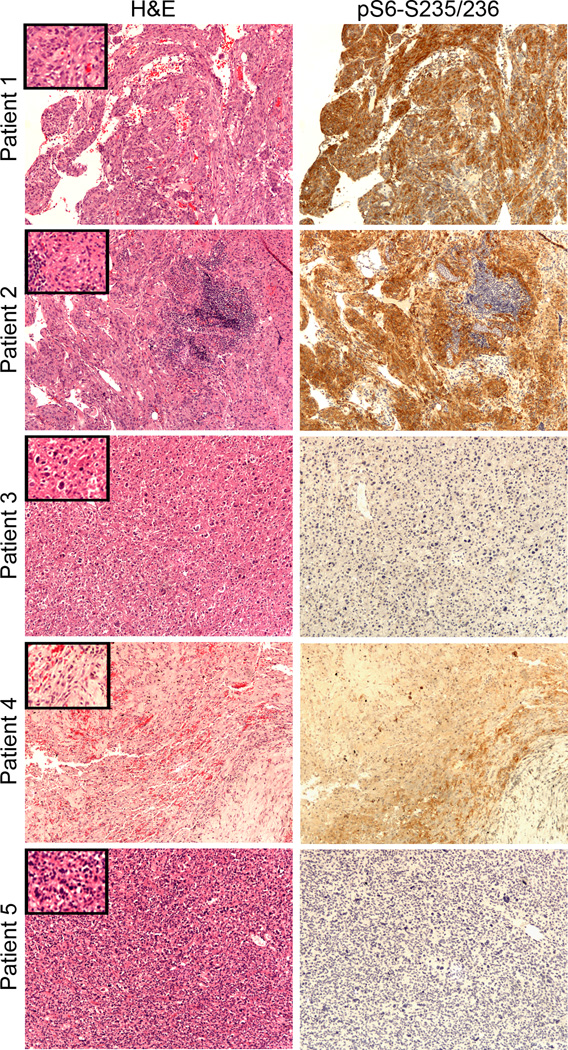
Figure 2.
Pathology and immunohistochemistry analysis of PEComas. H&E staining and immunostaining for phospho-S6 protein (Ser235/236) in all tumors samples (10× objective; insets-20×). PEComas from patients 1 and 2 are strongly pS6 positive, whereas the PEComas of patient 3, 4 and 5 are not. In patient 2 normal lymphoid tissue is negative for pS6-S235/236. In Patient 4 areas of hemorrhage, hemosiderin deposits and variable pS6 staining are apparent, following drug treatment.
Patient 5
Patient 5 was a 65-year-old man who presented in 2008 with abdominal pain and was found to have a large adrenal mass and multiple lung and soft tissue metastases on imaging studies. Biopsy of the adrenal mass showed a PEComa. Treatment with sirolimus began at 1 mg daily and was gradually increased to 4 mg daily. Although there was an initial modest decrease in SUV uptake on PET scan (for example, lung mass SUV 3.4 to 2.2, soft tissue mass SUV 11.3 to 9.8, but adrenal mass SUV 23.1 to 25.6), there was no decrease in tumor volume. During continuing treatment, progression of disease was seen. He was then treated with sorafenib 400mg daily with no benefit. The patient resumed sirolimus as a palliative approach, but without clear benefit. During this period of sirolimus treatment a subcutaneous metastasis was resected. This sample was used in the analyses described below. The patient declined further systemic therapy and died of metastatic disease 3 years after initial diagnosis.
Pathologic and Molecular Studies
mTORC activation by IHC
We assessed activation of mTORC1 in PEComa sections from these patients by IHC using the marker protein pS6-S235/236.16, 17, 19 Tissue sections from Patients 1 and 2 showed strong pS6-S235/236 staining indicating mTORC1 activation in the tumors (Figure 2). In Patient 3 the staining was negative; but this may relate to poor preservation of phosphorylated proteins due to the circumstances of the resection in the setting of bowel perforation. In Patient 4, pS6 staining was difficult to interpret due to extensive necrosis, fibrosis, and hemorrhage as a treatment response to pre-operative everolimus treatment. In Patient 5, pS6-S235/236 reactivity was also negative, likely due to concurrent sirolimus therapy at the time of resection.
Mutational analysis of TSC2
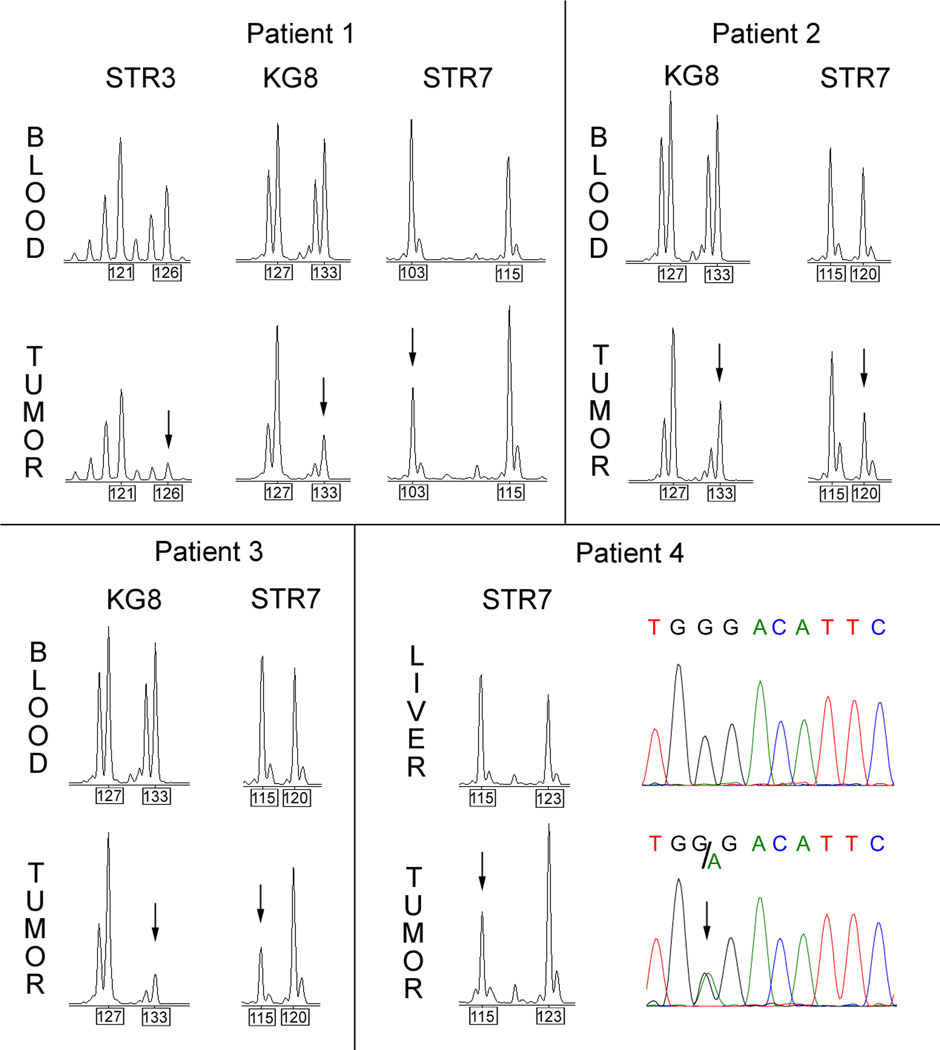
Limited analyses of the TSC2 gene were performed due to limited amounts of DNA available from these PEComas for analysis. Mutational analysis of 13 exons of TSC2 was performed on tumor DNA samples for all 5 patients. A nonsense mutation was identified in Patient 4 (c.1073G>A, p.Trp358X, Figure 3). No other significant variants were identified. Loss of heterozygosity (LOH) analysis in Patients 1 – 4 (this study could not be performed for Patient 5 due to lack of a control DNA sample) demonstrated that all four samples had reduction in the allele signal for one or more microsatellite markers near TSC2 (Figure 3 and Table 1). Allele reduction was modest in samples from Patient 2, consistent with the significant admixture of normal cells in this specimen (Figure 2). The corresponding normal DNA samples did not contain the TSC2 mutation (Patient 4), or show evidence of LOH (Patients 1–3) (Figure 3).
Figure 3.
LOH and sequencing analysis of TSC2 in PEComa. Informative microsatellite chromatograms for STR3, KG8, and STR7 markers near TSC2 are shown for Patients 1 to 4. All showed LOH in the tumor compared to normal DNA, with reduction in intensity of signal for one allele, marked with black arrows. The numbers in boxes below the graphs show the size of the alleles in base pairs. Lower right: Sequence traces of liver (control) and tumor DNA from Patient 4 for TSC2 exon 10 show normal sequence in liver and a nonsense point mutation in the tumor (c.1073G>A, p.358Trp>X).
Table 1.
Percent reduction in one allele of microsatellite markers STR3, KG8, STR7 indicating loss of heterozygosity (LOH).
| Marker | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| STR3 | 69% | NI | NI | NI |
| KG8 | 69% | 42% | 80% | NI |
| STR7 | 58% | 39% | 65% | 59% |
NI = not informative means the marker was homozygous in control DNA.
Analysis of TFE3
As TFE3 translocations have been identified in some PEComas, we performed TFE3 FISH analysis on the tumor from patient 5, the single patient that failed to respond to sirolimus/everolimus treatment, and included patient 2 as a control.20 There was no evidence of TFE3 translocation in either patient, using a break-apart FISH probe set. In addition, immunohistochemistry staining for TFE3 (clone p16, Santa Cruz Biotechnology, CA) was performed on tumor from patient 4 and it was negative.
Discussion
PEComas are a set of neoplasms that share morphologic features as well as expression of both melanocytic and smooth muscle markers, but otherwise have considerable diversity in organ of development, histologic features, severity, and clinical presentation.1, 2 Among PEComas, renal angiomyolipomas are both the most common and typically the most benign in terms of clinical presentation, although they are capable of progressive growth leading to loss of renal parenchyma and function, as well as major hemorrhagic events that can be life-threatening.3 LAM is also often an indolent slowly proliferative process, but can also progress, leading to respiratory failure, necessity for transplantation, and death.3 Extrarenal abdominal PEComas also have highly variable clinical behavior, but are typically more rapidly progressive. However, reliable prediction of malignant potential on histologic grounds is not currently possible.1, 2 Here we describe the treatment of 5 extrarenal non-pulmonary PEComas that displayed malignant behavior in the form of massive growth, major local invasion with compromise of organ function, and metastases in one instance, with sirolimus or everolimus. Four of 5 patients had major, durable responses, including 2 CRs continuing on therapy at 16–22 month follow-up. One of the other two responders was converted to a CR by resection of residual disease, and the other has a sustained PR continuing on therapy at 16 months of follow-up. One progressed. It is notable that two patients with CR in this series developed chylous ascites requiring shunting during treatment. This clinical development may be due to an abnormal sclerotic response of the lymphatic vasculature in response to treatment of PEComa that were involving this system. It is important to note that this clinical development did not herald resistance to drug treatment, and instead these patients went on to have sustained clinical responses.
Table 2 provides a compilation of all published reports of extra-renal non-pulmonary PEComa patients treated with mTOR inhibitors, including the 5 new cases reported here.16, 17, 21, 22 In aggregate these studies strongly support the model that loss of the tumor suppressor gene TSC2, or more rarely TSC1, is a common event in sporadic PEComa. We identified point mutations and/or LOH in TSC2 in four of five cases, and combined with previous analyses of TSC1/TSC2, 6 of 7 (85%) PEComas showed these hallmarks of TSC1/TSC2 involvement. IHC staining for pS-S235/236 correlated with these genetic findings in general, except in cases where prior treatment with an mTOR inhibitor or poor pathologic preservation may have compromised the reliability of this marker. Although the numbers are small, it appears that the combination of genetic findings in TSC1/TSC2 and staining for pS6-S235/236 may predict response to sirolimus/everolimus, as CRs were seen in 4 of 5 (80%) of patients in this category. Overall, 5/11 (45%) had complete response, 1/11 (9%) had a partial response, and 5/11 (45%) showed progressive disease. In addition, note that one of these patients received combination therapy with a total of three agents, and the dose of temsirolimus was likely suboptimal in this instance.
Table 2.
Review of published extrarenal PEComas treated with mTOR inhibitors.
| Case # |
Tumor type/location |
mTOR inhibitor |
Dose | Duration | Response | Other drugs (combined with mTOR inhibitor) |
TSC1/2 mutation/LOH |
pS6 IHC |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Retroperitoneal PEComa | sirolimus | 8 mg daily | 16 months | CR | - | TSC2 | + | Wagner AJ et al. 201017 |
| 2 | Uterine, metastatic PEComa | sirolimus | 2–8 mg daily | 6 months | SD, then progression | (added later) clarithromycin sorafenib | TSC1 | + | Wagner AJ et al. 201017 |
| 3 | Uterine, metastatic PEComa | temsirolimus | 25 mg IV weekly | 9+ months | CR | - | N/A | + | Italiano A et al. 201016 |
| 4 | Uterine, metastatic PEComa | temsirolimus | 25 mg IV weekly | 22 weeks | PR, then progression | - | N/A | + | Italiano A et al. 201016 |
| 5 | Retroperitoneal metastatic PEComa | temsirolimus | 10mg IV weekly* | 2 cycles (8 weeks) | progression | topotecan, bortezomib (NCT00770731) | N/A | N/A | Subbiah V et al. 201021 |
| 6 | Retroperitoneal metastatic PEComa | everolimus | 10 mg daily | 2 cycles (8 weeks) | progression | figitumumab | N/A | N/A | Quek R et al. 201122 |
| 7 | Retroperitoneal PEComa | sirolimus | 4 mg daily | 22+ months | CR | - | TSC2 | + | case described in this report |
| 8 | Retroperitoneal PEComa | sirolimus | 3 mg daily | 16+ months | CR | - | TSC2 | + | case described in this report |
| 9 | Small bowel PEComa / recurrent | sirolimus | 4 mg daily | 14+ months | PR | - | TSC2 | - | case described in this report |
| 10 | Liver PEComa | everolimus | 5 mg daily | 2 months | CR (tumor resected after 2 months of treatment) | - | TSC2 | +/− | case described in this report |
| 11 | Adrenal PEComa with metastases | sirolimus | 1–4 mg daily | 2 years | progression | - | not found | - | case described in this report |
CR-complete response, PR-partial response, SD-stable disease; N/A not available.
Note: Renal PEComa from Wagner AJ et al. 2010 was not included.
V Subbiah, personal communication
It is clear that some PEComas are resistant to sirolimus/everolimus treatment, despite optimal conventional dosage regimens, including patient 5 described here. In this patient, there was no evidence of TSC2 mutation, although analysis was limited due to lack of a normal specimen for LOH analysis. TFE3 gene rearrangement was recently reported in a small number of PEComas with unique alveolar epithelioid architecture, and we recently reported that such PEComas have normal expression of TSC2 and do not have TSC2 LOH.20, 23 We examined the PEComa of patient 5 for TFE3 rearrangement, and saw no evidence of this event. The mechanisms of resistance to mTOR inhibition in PEComa remain an important area for future research. However, it is also remarkable that sirolimus/everolimus are so effective in inducing complete remissions in a significant subset of PEComa as single agents, including patients with massive disease. These medications are thought to act as cytostatic agents, inducing cell cycle arrest rather than cell death in multiple types of TSC1/TSC2 deficient cell lines in vitro.24, 25 In addition, although these agents are effective in the treatment of several different types of tumors that occur in TSC patients, they do not induce complete responses, and lesions will typically regrow when the inhibitor is discontinued.12, 13 Nonetheless, these compounds are highly effective in mouse models of TSC, where a 99% reduction in tumor mass is seen in response to a one month treatment.11 Moreover, the resection specimen of patient #4 demonstrates that cell death was likely induced in response to everolimus. Thus, overall, among cancer patients, this degree of response to sirolimus/everolimus in PEComas is unprecedented and unexplained, and hence worthy of further clinical investigation as well.
These results clearly support the use of mTOR inhibitors in the treatment of malignant PEComa. Treatment is generally well-tolerated, and can lead to durable remissions of duration up to 2 years. Sirolimus, temsirolimus, and everolimus are structurally similar and may be therapeutically equivalent for PEComa, but this may not be the case.25, 26 All three drugs have led to complete responses as single agents in different PEComa patients. Both sirolimus and everolimus have the advantage of being orally administered, and the dosage of sirolimus can be guided by serum levels permitting adjustment in dosage to reduce side-effects when they occur. These medications represent an important therapeutic option for patients with locally invasive and metastatic PEComa.
Acknowledgements
The authors thank Paola Dal Cin (Department of Pathology, Cytogenetics Laboratory, Brigham and Women's Hospital) for providing FISH probes for TFE3 “break-apart” assay, and Shumei Wang and Chengsheng Zhang for perfoming FISH assay in the Cytogenetics Core Lab of the Dana-Farber/Harvard Cancer Center in Boston, MA.
Funding
Supported in part by Cycle for Survival, NIH NCI P01 1P01CA120964-01A1, NIH 2P30CA006516-42.
Footnotes
Conflicts of interest: DJK received a commercial research grant from Intellikine and served as a consultant to Millenium.
Impact Statement: PEComas are a rare but increasingly recognized subset of mesenchymal tumor, which can be both locally invasive and metastatic. In this report, we demonstrate that some patients with PEComa derive significant benefit, including long lasting complete responses, to mTORC1 inhibitors, and that genetic aberrations in TSC2 and pS6-positive staining correlate with response.
References
- 1.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Human pathology. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Archiv : an international journal of pathology. 2008;452:119–132. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwiatkowski DJ, Thiele EA, Whittemore VH. Tuberous Sclerosis Complexed. Weinheim, Germany: Wiley-VCH; 2010. [Google Scholar]
- 4.Henske EP, Wessner LL, Golden J, Scheithauer BW, Vortmeyer AO, Zhuang Z, Klein-Szanto AJ, Kwiatkowski DJ, Yeung RS. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am J Pathol. 1997;151:1639–1647. [PMC free article] [PubMed] [Google Scholar]
- 5.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan CC, Chung MY, Ng KF, Liu CY, Wang JS, Chai CY, Huang SH, Chen PC, Ho DM. Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): genetic evidence for the relationship of PEComa with angiomyolipoma. The Journal of pathology. 2008;214:387–393. doi: 10.1002/path.2289. [DOI] [PubMed] [Google Scholar]
- 7.Qin W, Bajaj V, Malinowska I, Lu X, MacConaill L, Wu CL, Kwiatkowski DJ. Angiomyolipoma have common mutations in TSC2 but no other common genetic events. PLoS ONE. 2011;6:e24919. doi: 10.1371/journal.pone.0024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- 9.Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Human pathology. 2007;38:1361–1371. doi: 10.1016/j.humpath.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63:5173–5177. [PubMed] [Google Scholar]
- 11.Pollizzi K, Malinowska-Kolodziej I, Stumm M, Lane H, Kwiatkowski D. Equivalent benefit of mTORC1 blockade and combined PI3K-mTOR blockade in a mouse model of tuberous sclerosis. Molecular cancer. 2009;8:38. doi: 10.1186/1476-4598-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salisbury S, Franz DN. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ, Jr, Miles D, Cutler D, Krueger D, Uppot RN, Rabenou R, Camposano S, Paolini J, et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS ONE. 2011;6:e23379. doi: 10.1371/journal.pone.0023379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies DM, de Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, Watson PC, Howe CJ, Doyle T, Pointon K, Cross JJ, Tattersfield AE, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res. 2011;17:4071–4081. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- 15.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP, 3rd, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Italiano A, Delcambre C, Hostein I, Cazeau AL, Marty M, Avril A, Coindre JM, Bui B. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol. 2010;21:1135–1137. doi: 10.1093/annonc/mdq044. [DOI] [PubMed] [Google Scholar]
- 17.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH, Demetri GD, Kwiatkowski DJ, Maki RG. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosquera JM, Dal Cin P, Mertz KD, Perner S, Davis IJ, Fisher DE, Rubin MA, Hirsch MS. Validation of a TFE3 break-apart FISH assay for Xp11.2 translocation renal cell carcinomas. Diagnostic molecular pathology : the American journal of surgical pathology, part B. 2011;20:129–137. doi: 10.1097/PDM.0b013e31820e9c67. [DOI] [PubMed] [Google Scholar]
- 19.El-Hashemite N, Zhang H, Henske EP, Kwiatkowski DJ. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- 20.Argani P, Aulmann S, Illei PB, Netto GJ, Ro J, Cho HY, Dogan S, Ladanyi M, Martignoni G, Goldblum JR, Weiss SW. A distinctive subset of PEComas harbors TFE3 gene fusions. The American journal of surgical pathology. 2010;34:1395–1406. doi: 10.1097/PAS.0b013e3181f17ac0. [DOI] [PubMed] [Google Scholar]
- 21.Subbiah V, Trent JC, Kurzrock R. Resistance to mammalian target of rapamycin inhibitor therapy in perivascular epithelioid cell tumors. J Clin Oncol. 2010;28:e415. doi: 10.1200/JCO.2010.29.4678. [DOI] [PubMed] [Google Scholar]
- 22.Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, Huftalen T, Jederlinic N, Manola J, Wagner AJ, Demetri GD, George S. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17:871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 23.Malinowska I, Weiss SW, Martignoni G, Netto GJ, Kwiatkowski D, Argani P. Perivascular Epithelioid Cell Tumors (PEComas) harboring TFE3 gene rearrangements lack the TSC2 alterations characteristic of conventional PEComas: further evidence for a biologic distinction. The American journal of surgical pathology. 2012 doi: 10.1097/PAS.0b013e31824a8a37. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews. Molecular cell biology. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Science signaling. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 26.Blay JY. Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol. 2011;22:280–287. doi: 10.1093/annonc/mdq307. [DOI] [PubMed] [Google Scholar]