Abstract
Peripheral nerve injury evokes rapid and complex changes in gene transcription and cellular signaling pathways. Understanding how these changes are functionally related is essential for developing new approaches that accelerate and improve nerve regeneration. Towards this goal we found that nerve injury induces a rapid and significant up-regulation of the transcription factor Sox11 in dorsal root ganglia (DRG) neurons. Gain and loss of function studies have shown this increase is essential for normal axon regeneration. To determine how Sox11 impacts neuronal gene expression, DRG neurons were treated with Sox11 siRNA to identify potential transcriptional targets. One gene significantly reduced by Sox11 knockdown was TRAF (tumor necrosis factor (TNF) receptor-associated factor)-associated NF-κB activator (TANK). Here we show that TANK is expressed in DRG neurons, that TANK expression is increased in response to peripheral nerve injury and that Sox11 overexpression in vitro increases TANK expression. Injury and in vitro overexpression were also found to preferentially increase TANK transcript variant 3 and a larger TANK protein isoform. To determine if Sox11 regulates TANK transcription bioinformatic analysis was used to identify potential Sox binding motifs within 5 kbp of the TANK 5’ untranslated region (UTR) across several mammalian genomes. Two sites in the mouse TANK gene were examined. Luciferase expression assays coupled with site-directed mutagenesis showed each site contributes to enhanced TANK promoter activity. In addition, chromatin immunoprecipitation assays showed direct Sox11 binding in regions containing the two identified Sox motifs in the mouse TANK 5’-UTR. These studies are the first to show that TANK is expressed in DRG neurons, that TANK is increased by peripheral nerve injury and that the regulation of TANK expression is, at least in part, controlled by the injury-associated transcription factor Sox11.
Keywords: nerve regeneration, transcription factor, TANK, Sox11, TRAF, dorsal root ganglia
Peripheral nerve injury causes rapid changes in the neuronal cell body that include the induction of transcription factors (Rossi et al., 2007; Michaelevski et al., 2010). Transcriptional reprogramming facilitates changes that impact neuronal survival, axonal outgrowth and regeneration of nerve fibers. A transcription factor that is markedly increased in response to nerve injury is Sox11, a Sry-related high-mobility group (HMG) box (Sox) protein. Sox genes in human and mouse comprise a family of approximately 20 members that are divided into subgroups based on homology within the Sry-like HMG sequence and other structural domains. Sox11 is a group C protein that has been shown to be an essential regulator of neural development, cell determination (Uwanogho et al., 1995; Hargrave et al., 1997; Wegner and Stolt, 2005; Bhattaram et al., 2010) and recently, neurogenesis in the adult brain (Haslinger et al., 2009). Gain and loss of function studies show Sox11 to be particularly important for the development of neurons in the peripheral nervous system and spinal cord, e.g., among other defects, mice that lack Sox11 display deficient sensory and sympathetic neuron growth and survival (Potzner et al., 2010; Thein et al., 2010; Lin et al., 2011).
At late stages of sensory neuron development Sox11 expression is significantly downregulated (Hargrave et al., 1997; Jankowski et al., 2006). Although a low level of expression is normally maintained in adult neurons, peripheral nerve injury elicits a rapid and significant increase in its expression. Knockdown and overexpression approaches have shown this increase is correlated with enhanced axonal regeneration in injured DRG neurons (Tanabe et al., 2003; Jankowski et al., 2006; Jankowski et al., 2009). Cultures of DRG neurons treated with small interfering RNAs (siRNA) that block Sox11 upregulation exhibit increased apoptosis and decreased neurite extension (Jankowski et al., 2006). Similarly, saphenous nerve injection of Sox11 siRNAs reduces the rate of axon regeneration in vivo (Jankowski et al., 2009). In gain of function studies, increased expression of Sox11 driven by nonreplicating herpes simplex virus (HSV) vectors in either cultured DRG neurons or in vivo led to enhanced neurite growth and axon regeneration, respectively (Jing et al., 2012).
To identify possible transcriptional targets of Sox11 we previously identified genes related to stress and injury that were modified in cells treated with Sox11 siRNAs (Jankowski et al., 2006). Among the potential targets of Sox11 was the TRAF-interacting protein TANK (TRAF family member-associated activator of NF-kappa B), which showed significant reduction in its mRNA in response to Sox11 knockdown in cultured DRG neurons (Jankowski et al., 2006). TANK (also known as I-TRAF) was first identified by two groups working independently to find binding partners of TNF-receptor associated factor (TRAF) family proteins. Cheng and Baltimore showed that TANK was a net activator of TRAF2-mediated NF-kappa B activation whereas Rothe et al. found that TANK inhibited TRAF2-dependent signaling (Cheng and Baltimore, 1996; Rothe et al., 1996). TANK has also been found to be a potent suppressor of Toll-like receptor (TLR)- mediated induction of proinflammatory cytokines (Kawagoe et al., 2009). These findings and others (Chin et al., 1999; Pomerantz and Baltimore, 1999; Li et al., 2003; Kawagoe et al., 2009; Zhang et al., 2010), have suggested TANK can either activate or inhibit TRAF-mediated signaling pathways and that this action is highly dependent on the cellular and tissue context.
Expression and functional studies of TANK and its role in immune and inflammatory signaling have been done using non-neuronal cell lines and tissues. Nothing is known of TANK expression and transcriptional regulation in neuronal systems. Our initial observation that TANK mRNA is modulated by Sox11 level suggested Sox11 transcriptionally controls TANK in injured neurons. The present study was therefore designed to examine TANK expression using a mouse model of nerve injury and neuronal cell culture models. Results indicate that TANK is expressed in nearly all DRG sensory neurons, that peripheral nerve injury causes a rapid elevation in TANK at transcriptional and translational levels and that Sox11 can regulate and drive TANK gene transcription in vitro, making TANK a likely target of Sox11 activity in response to peripheral nerve injury in the adult system.
EXPERIMENTAL PROCEDURES
Animal use and surgical procedure
Adult male C57BL/6J or Swiss Webster mice aged 6–8 weeks old (Jackson Laboratories, Bar Harbor, ME) were housed in an American Association for the Accreditation of Laboratory Animal Care-accredited facility in a temperature and humidity controlled room on a 12h light/12h dark cycle with food and water provided ad libitum. Experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Sciatic nerve cut surgery was performed under 2–3% isoflurane anesthesia (Abbott Laboratories, Abbott Park, IL). The left flank was shaved, wiped with betadine and a small incision made on the left hip just caudal to the head of the femur. The muscle was retracted using spring scissors, the sciatic nerve was completely transected and the wound closed using wound clips. All efforts were made to minimize pain and discomfort. Experiments were done in groups containing 4 mice for each timepoint analyzed and for each assay were repeated at least 3 times.
Cell culture
The mouse neuroblastoma cell line neuro2a (ATCC clone number CCL-131, Manassas, VA) was maintained in Eagle’s minimal essential medium (MEM) containing 10% fetal bovine serum (MEMS) and 1% penicillin/streptomycin in an incubator set at 37°C and 5% CO2. DRG from all spinal cord levels were cultured as previously described in (Malin et al., 2007; Jing et al., 2012).
RNA isolation and reverse transcriptase-PCR analysis (RT-PCR)
Mice were euthanized by anesthetic overdose at 1d or 3d post sciatic nerve axotomy. Naïve control mice were processed in parallel. Tissue was frozen on dry ice, homogenized in Qiagen Buffer RLT (Qiagen, Valencia, CA) using a Polytron tissue grinder and RNA extracted using RNeasy columns. RNA was reverse-transcribed using SuperScript II (Invitrogen) and used as template in 25-uL PCR reactions. Values were normalized to GAPDH since its level did not significantly change post nerve transection. Fold changes were calculated using the ΔΔCt method (target gene – GAPDH) and statistical significance determined at 1d and 3d post injury using single-variable analysis of variance (ANOVA) with Bonferroni post-hoc testing. For cultured cells, RNA was isolated using Trizol reagent (Invitrogen) and first strand cDNA synthesis performed. Real-time SYBR green labeled PCR reactions were run on an ABI Prism 7000 system (Applied Biosystems, Foster City, CA) and normalized against GAPDH. Table 1 lists PCR primer sequences used.
Table 1.
List of primers used in this study.
| Primer name | Sequence 5’-3’ |
|---|---|
| TANK 804F | GTTTCCGCCTATGGACAATGAC |
| TANK 902R | CGGTCCTGGCACACTGTCT |
| TANK 17F | ATAGTCTGCGAAGGAACGCC |
| TANK 702R | ATCATCTTTAGCCTGGGGC |
| mTANKproF | CTCAGGCTACCCCATGTGACAC |
| mTANKproR | AGGCACGTACCCATTCTGGAAGC |
| TANKmut1F | CCATGTGACACACACAAAACCCCGGCACAAAAGTGGAAGATGG |
| TANKmut1R | CCATCTTCCACTTTTGTGCCGGGGTTTTGTGTGTGTCACATGG |
| TANKmut2F | GGACATTTCCATTTTATAACCCCGAAAAATGCCCGGATATTGC |
| TANKmut2R | GCAATATCCGGGCATTTTTCGGGGTTATAAAATGGAAATGTCC |
| TANK-chip -2255F | TCTCACCCAAGTTCACTCAGGCTA |
| TANK-chip -2002R | GGCTGGTATGAATGGCTGGAGAAT |
| TANK-chip -710F | ACCGAAGAGATCGTGTCTAAGGAC |
| TANK-chip -484R | GGTACTAGTGAGAGAGGTAAAGCA |
| SOX11 113F | ACCCGGACTGGTGCAAGAC |
| SOX11 213R | CGACTGCTCCATGATCTTCCT |
| GAPDH 756F | ATGTGTCCGTCGTGGATCTGA |
| GAPDH 904R | GCTGTTGAAGTCGCAGGAGACA |
siRNA transfection
DRG neurons collected from all spinal cord levels of Swiss Webster male mice were dissociated, cultured and transfected with 50nM non-targeting (NT) siRNA or a Smartpool of Sox11 targeting siRNAs (Dharmacon; Thermo Fisher Scientific, Lafayette, CO) using Metafectine reagent (Biontex, Martinsried, Germany). Neurons were treated with NT or Sox11 siRNAs at 2h after plating and at 24h later following the protocol described in (Jing et al., 2012). Total protein was isolated at 48h post plating and western blotting performed using anti-TANK and anti-GAPDH antibodies as described below.
Protein isolation, western blotting and cell immunolabeling
Lumbar DRGs isolated from naïve mice and from the ipsilateral side of mice with nerve transections were homogenized in SDS/Tris lysis buffer containing PMSF, 1mM Na3VO4 and protease inhibitors (aprotinin, pepstatin, leupeptin, all at 1ug/ml; Sigma Biochemicals) using a hand-held tissue grinder. Lysates were centrifuged 10 min at 10,000 rpm and the supernatant protein was separated on 10% SDS-PAGE gels and transferred overnight at 4°C to Hybond-P membrane (Amersham, Piscataway, NJ). Membranes were blocked in 5% milk in phosphate buffered saline with tween-20 (PBST), incubated overnight at 4°C with primary antibodies diluted in blocking buffer, washed with PBST, incubated in secondary antibody for 1h at room temperature, washed with PBST, and antibody binding detected using the Pierce Thermo Scientific Supersignal West Pico chemoluminescent substrate (Pierce, Rockford, IL). Antibodies used were mouse anti-TANK (1:1500 for western blot; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-TANK (1:1000–1:1200 for cell labeling and western blotting; Abcam, Cambridge, MA), rabbit anti-GAPDH (1:5000; Santa Cruz Biotechnology), mouse anti-beta-tubulin III (TuJ3; 1:1000; Cell Signaling Technology, Danvers, MA), donkey anti-rabbit HRP-conjugate (Santa Cruz, 1:5000), donkey anti-rabbit Cy3 and donkey anti-mouse Cy2 (Jackson Laboratories, 1:1000). TANK blocking peptide (Abcam) was used at a dilution of 1:400. For lumbar DRG immunolabeling mice were perfused with 0.9% phosphate buffer followed by 4% paraformaldehyde. Ganglia were post-fixed 1h in 4% paraformaldehyde, sunk in 20% sucrose, embedded in Tissue-Tek OCT medium and sectioned at 20um on a cryostat. For TANK/ TuJ3/ 4',6-diamidino-2-phenylindole (DAPI) labeling, sections were thawed, washed in phosphate buffered saline (PBS), blocked 1h in PBS with 1% BSA, 0.25% Triton-X, and 0.3 M glycine and incubated in primary antibody diluted in PBS/1% BSA/0.25% Triton-X overnight at 4°C. Sections were washed, incubated 1h in secondary antibody diluted in PBS, washed, coverslipped and viewed on a Leica DM4000B fluorescent microscope. For DAPI labeling sections were incubated for two-minutes immediately following removal of the secondary antibody.
Plasmid construction and mutagenesis
The pCMV-TANK plasmid (IMAGE clone #3492937) containing a 2,084 bp mouse TANK cDNA was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Construction of the pCMV-Sox11 and pCMV-control plasmids are described in (Jing et al., 2012). For luciferase reporter assays a 2.27-kbp fragment of the TANK proximal promoter region was PCR amplified from C57BLK/6 mouse liver genomic DNA and ligated into the TOPO-TA cloning vector (Invitrogen). Plasmids were sequenced to confirm identity and orientation of the insert that was then subcloned into pGL2-Basic (Promega, Madison, WI). pGL2-TANK plasmids containing mutated Sox binding sites were created using the Stratagene Quik-Change site-directed mutagenesis kit using primers designed with PrimerX software (Table 1). Mutations generated removed the core AAA nucleotides (AACAAAG → AACCCCG). The double mutant, TANKmut1mut2-pGL2, was generated by sequential mutagenesis using the mut2 primers on the previously generated TANKmut1-pGL2 plasmid. The amino terminus FLAG-tagged Sox11 plasmid (pSox11F) is described in (Wiebe et al., 2003) and was generously provided by Dr. A. Rizzino (University of Nebraska Medical Center, Omaha, NE). HSV vectors were generated and used as described in (Jing et al., 2012).
Luciferase assay
Neuro2A cells plated in 6-well plates at 2 × 105 cells per well were transfected using either calcium phosphate or the Mirus TransIT-Neural transfection reagent (Mirus Biosciences, Madison, WI). Plasmids used were: pGL-TANK promoter vectors (1450 ng); pRL-TK (control vector for pGL constructs containing the relatively weak herpes simplex virus thymidine kinase (HSV-TK) promoter; 50 ng); pCMV-Sox11 or empty pCMV vector (up to 500 ng). Cells were harvested 24h after transfection using Promega Passive Lysis Buffer and luciferase assays performed using the Promega Dual-Luciferase Assay kit and a Turner 20/20n automatic luminometer with dual injectors. Firefly luciferase activity was used as a measure of promoter activity and Renilla (sea pansy) luciferase was used for normalization. Normalized values (RLUpGL2/RLUpRL-TK) were used for statistical analysis. For luciferase assay experiments involving dose-response curves and for luciferase assays involving multiple mutagenized vectors, data were analyzed using two-way ANOVA with Bonferroni post-hoc testing. For luciferase assay experiments involving single vectors, data were analyzed using Student’s t-test. Each experiment was performed in triplicate and repeated at least three times.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed following the Upstate Biotechnology (Waltham, MA) protocol with minor modifications. Neuro2a cells (4 × 107) were transiently transfected with pFlag-Sox11, crosslinked with 1% formaldehyde for 10 min at 37°C and then treated with 125 mM glycine to quench formaldehyde fixation. Cells were suspended in lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.0) containing protease inhibitor cocktail (Sigma) and the chromatin sheared by sonication. Extracts were pre-cleared with protein G-agarose beads preadsorbed with salmon sperm DNA, diluted in ChIP dilution buffer (Upstate Biotechnology) and incubated with 5µg anti-FLAG M2 antibody (Sigma). Immunocomplexes were collected on preadsorbed protein G-agarose beads, washed, eluted and cross-linked complexes reversed by heating at 65°C overnight. No antibody and non-immune mouse IgG (Sigma) were used as controls. DNA from immunoprecipitates was purified using spin columns and incubated with PCR primers designed to regions flanking the predicted Sox sites in the TANK proximal promoter (Table 1). Amplification was done using the following conditions: one cycle at 95°C 5 min followed by 35 cycles of 95°C 30s, 62°C 30s and 72°C 30s followed by one 72°C for 5 min incubation.
RESULTS
TANK is expressed in DRG sensory neurons
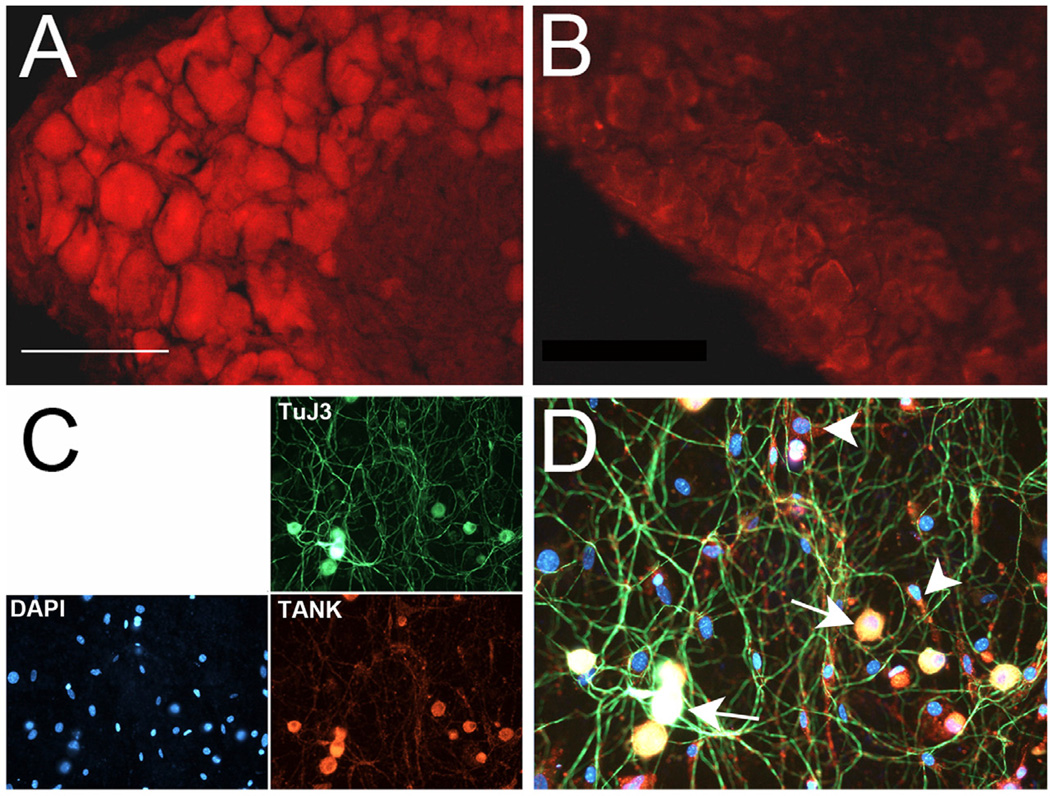
Mouse lumbar DRG were used to examine TANK protein immunoreactivity in sensory neurons. TANK labeling was primarily cytoplasmic and detected in nearly all DRG neurons (Fig. 1A), consistent with its predicted ubiquitous pattern of expression (Consortium, 2012). Pre-incubation of the TANK antibody with a TANK blocking peptide significantly blocked TANK reactivity in vivo (Fig. 1B) and in vitro (not shown). TANK immunoreactivity in dissociated and cultured DRG neurons appeared restricted to somal compartments (Fig. 1C, D). TANK labeling overlapped with the neuronal marker TuJ3 (of 105 neurons counted) and, based on this labeling and cell morphology (i.e., large rounded cells with neurites), was evident in virtually all neuronal profiles. Approximately half (123 of 262 cells counted) of the nonneuronal (TuJ3-negative) cells were also TANK-positive.
Figure 1. TANK is expressed in DRG neurons.
A. TANK antibody staining of mouse DRG shows reactivity in virtually all neurons. B. Preincubation with a TANK blocking peptide significantly reduces TANK immunoreactivity in DRG. Images were taken at the same exposure and magnification, merged into one figure and then manipulated for brightness and contrast using Photoshop. C. Dissociated DRG neurons grown 3d in culture immunolabeled with the neuronal marker anti-beta-III-tubulin (green) exhibit TANK immunoreactivity (red). DAPI (blue) labeling shows TANK expression in DRG neurons and in a subpopulation of non-neuronal cells. D. Overlap of staining shown in (C). Arrows indicated triple-labeled neurons, arrowheads indicate TANK/DAPI labeled non-neuronal cells. Bar = 100µm.
Nerve injury increases Sox11 and TANK expression
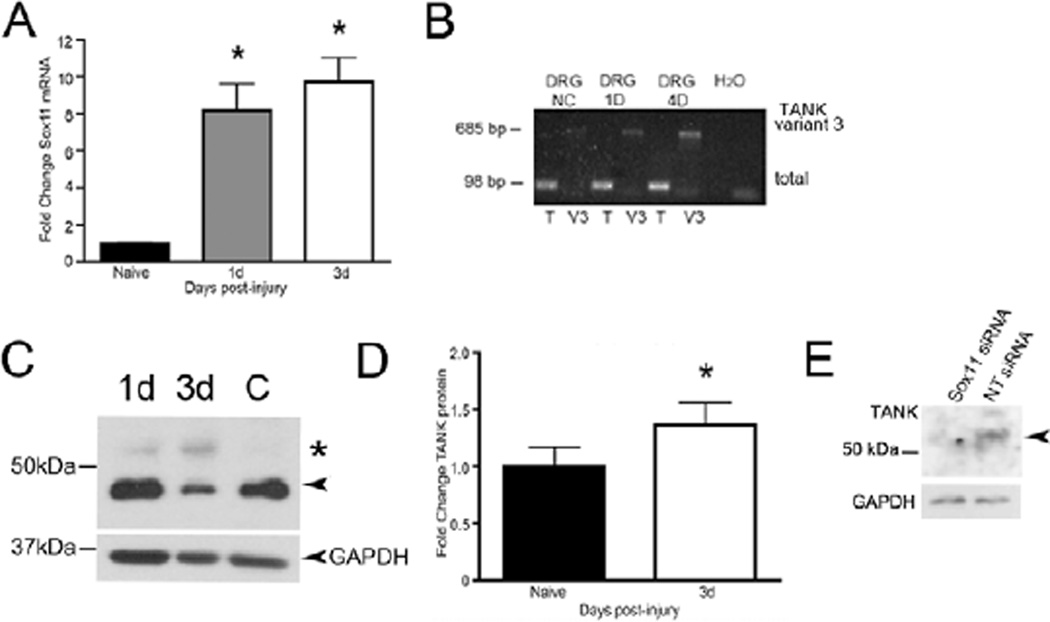
In a previous study we found that a rapid increase in Sox11 is evoked within 1d following sciatic nerve injury (Fig. 2A) (Jankowski et al., 2006). To examine if TANK could be a potential transcriptional target of Sox11 following nerve injury, the level of TANK mRNA in L3–L5 DRG isolated from naïve and sciatic nerve cut mice was compared (Fig. 2B). TANK transcription was assessed using two PCR primer sets; one set amplified all TANK transcripts (804F, 902R) and another set (17F, 702R) specifically amplified the longer transcript variant 3, which contains a 102 bp extension at the 5’ end of the TANK transcript. Interestingly, gel electrophoresis showed that in addition to an increase in total TANK transcript (98 bp product), a marked increase in the larger TANK variant 3 transcript (685 bp product) also occurred in response to injury (Fig. 2B). SYBR green RT-PCR analysis of DRG mRNA from nerve cut mice confirmed that total TANK mRNA increased to a value 220% above baseline at 1d after injury (n = 4 per group, p < 0.05 vs. naïve, ANOVA with Bonferroni post-hoc test; not shown). This analysis also found that at 3d post injury TANK mRNA remained elevated (30%) although its overall level was reduced from 1d levels.
Figure 2. Sox11 and TANK are upregulated in DRG following nerve injury.
A. Sox11 mRNA is increased in lumbar DRG in response to sciatic nerve axotomy (n = 4 per group, p < 0.001 vs. naïve, ANOVA with Bonferroni post-hoc test). B. RT-PCR products run on an ethidium bromide labeled agarose gel show increase in TANK mRNA variants. The larger TANK mRNA variant 3 (at 685 bp) is preferentially increased in lumbar DRG at 1D and 4D post axotomy. T, total TANK mRNA; V3, variant 3 mRNA. C. Western blot shows upregulation of TANK band at ~53kDa (indicted by asterisk) in DRG at 1d and 3d post axotomy, relative to naïve control (C). Arrowhead indicates 48 kDa isoform. D. Densitometric analysis using larger groups (n=8 mice per group) show the 48kDa isoform also increases in response to injury at 3d post-injury (38%, p < 0.05, vs. naïve, Student’s t-test) in DRG in response to axotomy. Values were normalized to GAPDH expression. E. siRNA-mediated knockdown of Sox11 in cultured DRG neurons reduces intensity of the TANK immunoreactive band at ~53kDa (arrowhead) relative to cultures treated in parallel with nontargeting (NT) siRNA. GAPDH is shown as loading standard. Asterisks on charts indicate significance.
Western immunoblotting was used to examine TANK protein expression following nerve injury. Analysis of protein from the L3–L5 DRG of naïve and axotomized animals showed two immunoreactive bands at ~48kDa and ~53kDa (Fig. 2C). We speculate that these bands represent TANK isoforms encoded by the long and short transcript variants, i.e., Fig. 2C indicates an increase in the 53 kDa molecular weight band in response to in vivo injury, similar to the increase in the variant 3 mRNA transcript (Fig. 2B) and TANK protein in cultured DRG neurons (below, Fig. 3F). In other assays we found the more abundant 48 kDa isoform also increased in DRG at 3d post-injury (n=8 mice per group) (Fig. 2D). Thus, both large and small TANK mRNAs and protein isoforms increase in the DRG in response to peripheral nerve injury.
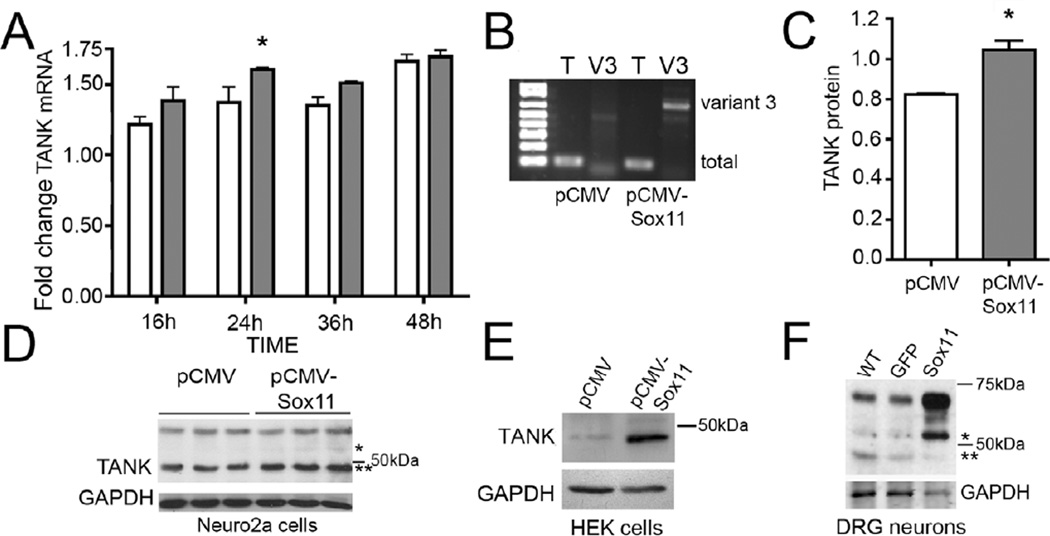
Figure 3. Sox11 increases in vitro TANK expression.
A. Neuro2a cells transfected with pCMV or pCMV-Sox11 increase total TANK mRNA expression at 24h post transfection (n = 4 per group, p < 0.05, two-way ANOVA with Bonferroni post-hoc test). Fold changes are relative to 0h baseline. TANK expression also increases overall as a function of time (n = 8 per time point, main effect using two-way ANOVA, p < 0.05). B. Neuro2a cells transfected with Sox11 plasmid also upregulate expression of TANK variant 3 mRNA. Gel shows PCR products from RT-PCR reaction using primers to detect total (T) and variant 3 (V3) mRNAs, as seen in Fig. 2B. C. TANK protein increases by 36h in pCMV-Sox11 transfected neuro2a cells (n = 3 per group, p < 0.05, Student’s t-test). Plot is for TANK variant that runs at ~48 kDa with values normalized to GAPDH. D. Image of western blot whose values are plotted in panel C. E. TANK protein is increased by Sox11 transfection in HEK cells. F. TANK protein is increased in DRG neuron cultures treated with HSV-Sox11 viral vector. In contrast to neuro2a cells, the 53kDa TANK isoform (marked by asterisk) is prominent in HSV-Sox11 infected cultured DRG neurons. This band also increases in DRG from nerve cut animals (see Fig. 2D).
A link between Sox11 and TANK expression had been suggested by previous findings that showed that reduction in Sox11 led to a decrease in TANK mRNA, i.e., treatment of cultured neurons with Sox11 siRNA reduced TANK mRNA level (Jankowski et al., 2006). To further validate this putative Sox11/TANK interaction at the translational level we assayed TANK protein in cultured DRG neurons treated with Sox11 siRNAs (Fig. 2E). Western immunoblotting shows a clear reduction in TANK protein in response to Sox11 knockdown (Fig. 2E), supporting a functional linkage between Sox11 and TANK gene expression.
TANK gene expression is modulated by Sox11 level
The injury-induced increase in TANK in vivo coupled with in vitro studies showing reduction of TANK in response to Sox11 knockdown suggested Sox11 can transcriptionally modulate TANK. To further test this possibility we measured TANK expression in neuro2a cells transfected with plasmid pCMV-Sox11. Sox11 overexpression increased TANK mRNA level at 24h post-transfection (Fig. 3A) and appears to do so primarily through upregulation of the long variant 3 transcript (Fig. 3B). Sox11 transfection also increased the 48 kDa TANK isoform in neuro2a cells (Fig. 3C, 3D) and HEK cells (Fig. 3E). Sox11 expression driven in cultured primary DRG neurons using a human herpes simplex virus (HSV) vector (Jing et al., 2012), also produced a significant increase in TANK protein relative to untreated cultures and cultures treated with a control HSV vector expressing green fluorescent protein (HSV-eGFP) (Fig. 3F). Interestingly, increased Sox11 expression in cultured DRG neurons caused a prominent increase in the 53kDa molecular weight variant of TANK, similar to that seen in response to sciatic nerve cut (Fig. 2D). This isoform was also evident in Sox11 transfected neuro2a and HEK cells but at much lower levels Fig. 3D, 3E. These data suggest that Sox11 impacts the injury-induced increase in TANK primarily through regulation of variant 3 mRNA transcription.
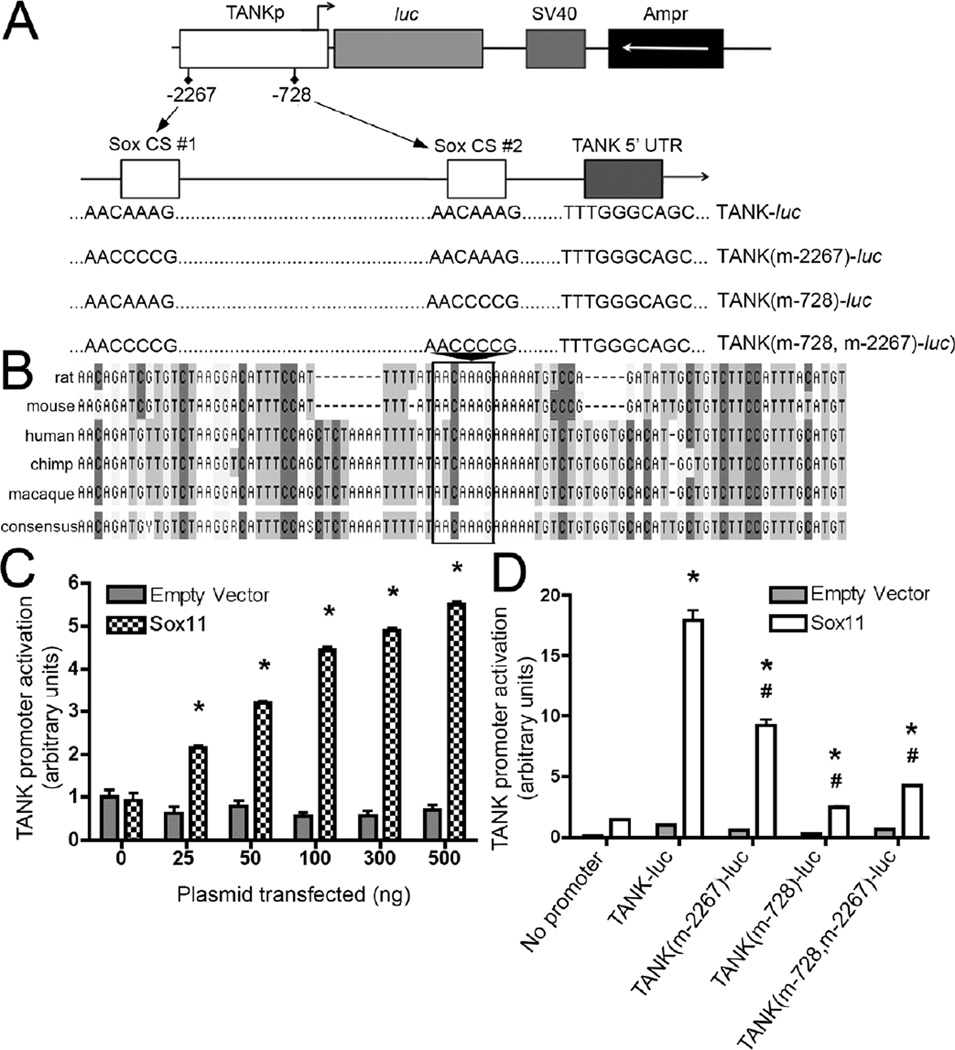
Using bioinformatic data from the National Center for Biotechnology Information we screened 5-kb of the 5’UTR of the TANK gene in several species using the general SRY/Sox binding motif (5’-(A/T)(A/T)CAA(A/T)G-3’) to identify potential Sox11 binding sites. BLAST analysis identified regions of sequence similarity and showed at least one Sox motif within the TANK 5’UTR across five of six vertebrate species examined (the exception being the common marmoset). ClustalW sequence alignment analysis revealed one cluster within 1 kb of the TANK transcriptional start site that had one Sox site in mouse and rat and two sites in human, chimpanzee and macaque. An alignment within this region is illustrated in Fig. 4B.
Figure 4. Sox11 regulates TANK promoter expression.
A. (Top) A 2.3-kb fragment of the TANK promoter was PCR cloned into the pGL2-basic vector. Forward-direction Sox binding sites at nt-2267 (SoxCS#1) and nt-728 (SoxCS#2) are indicated relative to the TANK transcript (variant 3) translational start site. (Bottom) Diagram illustrating site-directed mutagenesis constructs. Plasmid pGL2-TANK was mutated to selectively inactivate one or both Sox consensus sequence sites. B. Phylogenetic analysis of TANK promoter/5’UTR region in vertebrates was done following ClustalW analysis using MacVector software (Cary, North Carolina). Nucleotide alignment is shown for a highly conserved region within 1-kbp of the translation start site; box indicates a conserved Sox-binding motif (AACAAAG). C. Cotransfection of pGL2-TANK with pCMV-Sox11 increases TANK promoter activity in a dose-dependent manner, ranging from 2.1-fold (with 25 ng pCMV-Sox11) to 5.5-fold (with 500 ng pCMV-Sox11). TANK promoter was not activated by pCMV (empty vector) or the internal control reporter vector, pRL-TK, the activity of which remained constant for all combinations of vector + pCMV-Sox11 dosage. D. Luciferase reporter activity in neuro2a cells containing wildtype (TANK-luc) or a mutagenized TANK promoter (n = 4 per group). In this experiment TANK-pGL2/pCMV-Sox11 cotransfection caused a 17.9-fold increase in TANK promoter activity (p < 1 ×10−8, ANOVA with Bonferroni post-hoc test). Mutagenesis of the first binding site resulted in a 50% decrease in Sox11-mediated TANK promoter activation (p < 1 ×10−4, ANOVA with Bonferroni post-hoc test). Mutation of the second binding site resulted in an 85% decrease in Sox11-mediated TANK promoter activation (p < 1 × 10−6, ANOVA with Bonferroni post-hoc test), while ablation of both binding sites resulted in a 75% decrease in Sox11-mediated TANK promoter activation (p < 1 × 10−6, ANOVA with Bonferroni post-hoc test).
Sox11 activates the mouse TANK gene in a site-specific manner
To more directly assess Sox11 regulation of TANK transcription, a 2.27-kbp region of the mouse TANK 5’ untranslated region (UTR) that contains two putative Sox binding sites was evaluated using luciferase reporter assays (Fig. 4). Results show that this region is sufficient to drive luciferase expression and that, in a dose-dependent manner, cotransfection with Sox11 results in a 5.5-fold increase in promoter activity (Fig. 4C). To determine the relative contribution of the two putative Sox sites in the 2.27-kbp sequence (Fig. 4A), site directed mutagenesis was carried out. Mutagenesis of the distal (nt-2267) and proximal (nt-728) sites produced a 50% and 85% reduction in Sox11-mediated TANK promoter activity, respectively (Fig. 4D). Mutation of both binding sites resulted in a 75% decrease (Fig. 4D). Thus, Sox11 can transcriptionally activate the 2.27-kbp TANK sequence and full activity is dependent on the integrity of both Sox binding motifs.
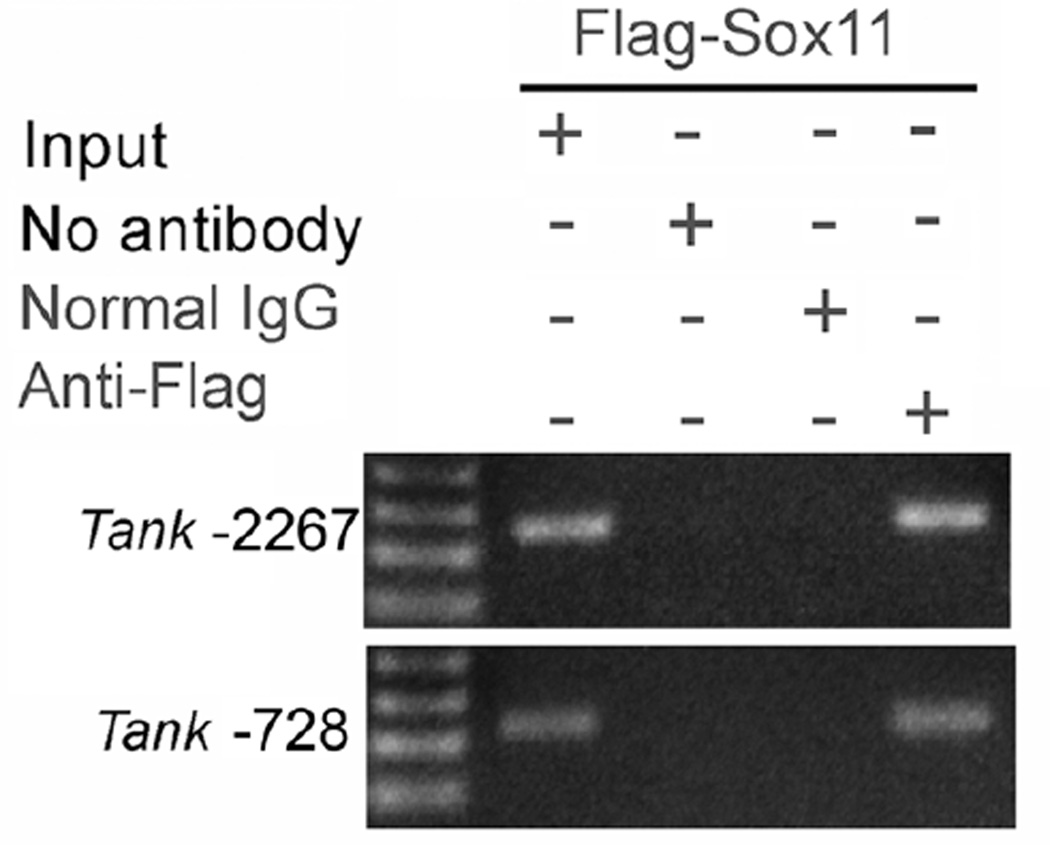
To more directly assess if Sox11 can bind Sox sites within the 2.27-kbp TANK 5’ UTR, chromatin immunoprecipitation (ChIP) assays were performed (Fig. 5). Cross-linked and sheared chromatin obtained from neuro2a cells transfected with a FLAG-tagged Sox11 was immunoprecipitated using an anti-FLAG antibody. Purified DNA/protein complexes were then assayed by PCR using primer sets designed to amplify regions flanking the two Sox11 sites (at nt-2267 and nt-728, Fig. 4) in the TANK 5’ UTR. RNA amplification shows that anti-FLAG immunoprecipitated chromatin within these regions of TANK, indicating that Sox11 is capable of binding at these sites.
Figure 5. Sox11 binds two sites on the mouse TANK promoter/5’UTR.
Primers designed against the two most proximal Sox motifs (nt-2267, nt-728) in the mouse TANK promoter/5’UTR were used in ChIP assays performed on pFlag-Sox11 transfected neuro2a cells. Lanes from left to right: Input DNA only (no IP), PCR performed after IP with no precipitating antibody, PCR after IP with control IgG antibody, PCR performed after IP with anti-FLAG IgG. Appropriate sized bands were amplified in regions flanking both putative Sox binding sites on the TANK promoter.
DISCUSSION
Here we show that primary sensory neurons express the TRAF interacting protein TANK, that peripheral nerve injury increases TANK expression in DRG at transcriptional and translational levels and that in vitro, the transcriptional control of TANK expression is regulated, at least in part, by the injury-associated transcription factor Sox11. TANK expression has not previously been examined in sensory neurons or following nerve injury. The expression of TANK in neurons and its modulation by Sox11 suggest TANK has an important role in early signaling events activated in response to axon injury. Our data also suggest the presence of at least two TANK isoforms in the DRG and that the larger of these (53 kDa) is preferentially activated by Sox11 in response to neuronal injury. The functional significance of this TANK variant is unknown at this time.
Interest in TANK gene regulation was sparked by the observation that TANK transcription and translation (this report) are decreased in cultured neurons treated with Sox11 siRNAs (Jankowski et al., 2006). TANK is a member of the TNFR-superfamily of proteins, a large and diverse family that includes the TNF receptors TNFRI and TNFRII and the p75 neurotrophin receptor (p75 NTR). TANK was first identified in a yeast two-hybrid screen for proteins that associate with TRAF2 (Cheng and Baltimore, 1996; Rothe et al., 1996) and has subsequently been shown to also interact with TRAFs 1, 3, 5 and 6 (Rothe et al., 1996; Chin et al., 1999; Li et al., 2002; Kawagoe et al., 2009). In nonneuronal cells TANK has been shown to modulate cell signaling pathways activated in response to stress and injury, particularly in regards to regulation of NFκB activation and Toll-like receptor signaling (Chin et al., 1999; Gatot et al., 2007; Kawagoe et al., 2009; Shi et al., 2011). TANK-TRAF binding appears to maintain TRAFs in an inactive state and in so doing affect downstream signaling events, e.g., TANK inhibits TRAF2-mediated NFκB activation generated by TNF activation of TNFRs (Rothe et al., 1996). In sensory neurons TRAFs have been shown to activate numerous kinases including IKK family members, p38 MAP kinase and JNKs (c-Jun terminal kinase) (Hehlgans and Mannel, 2002; Park et al., 2002; Pollock et al., 2002; Dempsey et al., 2003; Schafers et al., 2003b; Fernyhough et al., 2005). Thus, TANK-TRAF interaction in neurons could impact cellular signaling pathways. In particular, the potential TANK-TRAF6 interaction is of interest since TRAF6 is known to associate with and modulate downstream signaling of the neurotrophin receptor p75 (Khursigara et al., 1999; Roux and Barker, 2002).
The function of TANK in sensory neurons may also involve modulation of signaling activated in response to TNF. Within hours of nerve injury a significant increase in TNF and TNF receptors occurs in injured neurons (Taskinen et al., 2000; Schafers et al., 2003a; Ohtori et al., 2004; Dubovy et al., 2006; Miao et al., 2008). Thus, as in nonneuronal cell types, TANK may function to regulate the dynamics of TRAF protein association with downstream mediators in TNF signaling pathways (Cheng and Baltimore, 1996; Kaye et al., 1996; Chin et al., 1999; Chariot et al., 2002; Bonif et al., 2006; Guo and Cheng, 2007; Manna et al., 2010; Zhang et al., 2010). Following injury, this action could shift the balance of TNFR signaling in favor of pro-survival pathways. Mechanistically, the control of functional interactions between TANK and TRAFs can be regulated by ubiquination and phosphorylation (Gatot et al., 2007). Transcriptional regulation of specific TANK isoforms may also regulate the dynamics of these interactions. In support of this possibility is the observed increase in the variant 3 transcript and the 53 kDa TANK protein in response to axotomy and Sox11 overexpression.
The activation of TANK transcription adds a new dimension to the changes induced by Sox11 in response to nerve injury (Jankowski et al., 2009; Jing et al., 2012). The present data suggest a model in which increased Sox11 stimulates TANK transcription and translation, which leads to pro-survival signaling, perhaps through modulation of TRAF protein activity. In support of this model, in vitro ChIP binding and luciferase reporter assays indicate Sox11 can directly bind and activate transcription of a 2.3-kbp 5’ UTR region of the mouse TANK gene. Although of the three group C Sox proteins (Sox11, Sox4 and Sox12), Sox11 is the most potent activator of transcription, this potency is enhanced by interaction with other transcriptional modulators (Wiebe et al., 2003; Dy et al., 2008). Indeed, the transient activation of TANK transcription we measured within the 3d timeframe examined suggests other factors and feedback controls contribute to TANK transcriptional regulation.
Regulation of TANK by Sox11 is likely to occur across other species, including human, since several species have Sox binding motifs in the TANK 5’ UTR region. Within the proximal 1 kbp region, binding motifs for other transcription factors known to interact with Sox factors, such as Oct-4, Ngn1, Brns and TCF/LEF, are also present (Kuhlbrodt et al., 1998; Wiebe et al., 2003; Kim et al., 2008). These factors could cooperate or compete/interfere with Sox11 binding. Of particular interest is the potential competition for Sox binding sites from TCF/LEF family transcription factors (Chao et al., 2007; Bernard and Harley, 2010; Kormish et al., 2010). These effectors of canonical Wnt signaling, and the closest relatives of Sox-family transcription factors, have a core binding domain sequence of 5’-CAAAG-3’. This study and our recent analysis of Sox regulation of BDNF promoter activity (Salerno et al., 2012) suggest that binding sites most strongly regulated by Sox11 tended to contain the sequence 5’-AACAAAG-3’. This sequence is a potential TCF/LEF binding site as well as a Sox binding site. Other regeneration associated genes identified as transcriptional targets of Sox11, such as Sprr1a (Jing et al., 2012), do not contain this motif within proximal (5kbp) 5’UTR regions and were found to be regulated by Sox11 at later times after injury. It is possible therefore, that depending on the abundance of Sox11, which steadily increases following injury, and its binding partners, a subset of high-affinity Sox-binding sites exist that are activated immediately after injury by relatively low levels of Sox11, but which are also regulated by other feedback mechanisms. Likewise, there may be another subset of lower-affinity Sox-binding sites that require a higher abundance of Sox protein for transcriptional activation. The feasibility of this possible mechanism and better understanding of Sox11 function following nerve injury will be revealed as the regulatory and functional properties of Sox11 in injured adult neurons is further defined.
Highlights.
Peripheral nerve injury increases Sox11 and TANK (TRAF-associated NF-κB activator) in DRG
In vitro overexpression of Sox11 increases TANK; Sox11 knockdown decreases TANK expression
TANK mRNA variants and protein isoforms are expressed in DRG neurons in response to injury
ChIP shows Sox11 binds the TANK promoter, suggesting Sox11 directly regulates TANK transcription
ACKNOWLEDGEMENT
We thank Dr. Michael Jankowski for helpful technical advice and Pamela K. Cornuet for outstanding technical assistance. These experiments were supported by the National Institutes of Health through grants NS059003 (KMA), T32DK063922, UL1RR024153 and UL1TR000005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bernard P, Harley VR. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. Int J Biochem Cell Biol. 2010;42:400–410. doi: 10.1016/j.biocel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Bhattaram P, Penzo-Mendez A, Sock E, Colmenares C, Kaneko KJ, Vassilev A, Depamphilis ML, Wegner M, Lefebvre V. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun. 2010;1:9. doi: 10.1038/ncomms1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonif M, Meuwis MA, Close P, Benoit V, Heyninck K, Chapelle JP, Bours V, Merville MP, Piette J, Beyaert R, Chariot A. TNFalpha- and IKKbeta-mediated TANK/I-TRAF phosphorylation: implications for interaction with NEMO/IKKgamma and NF-kappaB activation. Biochem J. 2006;394:593–603. doi: 10.1042/BJ20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AT, Jones WM, Bejsovec A. The HMG-box transcription factor SoxNeuro acts with Tcf to control Wg/Wnt signaling activity. Development. 2007;134:989–997. doi: 10.1242/dev.02796. [DOI] [PubMed] [Google Scholar]
- Chariot A, Leonardi A, Muller J, Bonif M, Brown K, Siebenlist U. Association of the adaptor TANK with the I kappa B kinase (IKK) regulator NEMO connects IKK complexes with IKK epsilon and TBK1 kinases. J Biol Chem. 2002;277:37029–37036. doi: 10.1074/jbc.M205069200. [DOI] [PubMed] [Google Scholar]
- Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- Chin AI, Shu J, Shan Shi C, Yao Z, Kehrl JH, Cheng G. TANK potentiates tumor necrosis factor receptor-associated factor-mediated c-Jun N-terminal kinase/stress-activated protein kinase activation through the germinal center kinase pathway. Mol Cell Biol. 1999;19:6665–6672. doi: 10.1128/mcb.19.10.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TU. Reorganizing the protein space a the Universal Protein Resource (UniProt) Nucleic Acids Res. 2012;40:D71–D75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Dubovy P, Jancalek R, Klusakova I, Svizenska I, Pejchalova K. Intra- and extraneuronal changes of immunofluorescence staining for TNF-alpha and TNFR1 in the dorsal root ganglia of rat peripheral neuropathic pain models. Cell Mol Neurobiol. 2006;26:1205–1217. doi: 10.1007/s10571-006-9006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy P, Penzo-Mendez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins--Sox4, Sox11 and Sox12--exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36:3101–3117. doi: 10.1093/nar/gkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernyhough P, Smith DR, Schapansky J, Van Der Ploeg R, Gardiner NJ, Tweed CW, Kontos A, Freeman L, Purves-Tyson TD, Glazner GW. Activation of nuclear factor-kappaB via endogenous tumor necrosis factor alpha regulates survival of axotomized adult sensory neurons. J Neurosci. 2005;25:1682–1690. doi: 10.1523/JNEUROSCI.3127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatot JS, Gioia R, Chau TL, Patrascu F, Warnier M, Close P, Chapelle JP, Muraille E, Brown K, Siebenlist U, Piette J, Dejardin E, Chariot A. Lipopolysaccharide-mediated interferon regulatory factor activation involves TBK1-IKKepsilon-dependent Lys(63)-linked polyubiquitination and phosphorylation of TANK/I-TRAF. J Biol Chem. 2007;282:31131–31146. doi: 10.1074/jbc.M701690200. [DOI] [PubMed] [Google Scholar]
- Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–11826. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- Hargrave M, Wright E, Kun J, Emery J, Cooper L, Koopman P. Expression of the Sox11 gene in mouse embryos suggests roles in neuronal maturation and epithelio-mesenchymal induction. Dev Dyn. 1997;210:79–86. doi: 10.1002/(SICI)1097-0177(199710)210:2<79::AID-AJA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Haslinger A, Schwarz TJ, Covic M, Chichung Lie D. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci. 2009;29:2103–2114. doi: 10.1111/j.1460-9568.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- Hehlgans T, Mannel DN. The TNF-TNF receptor system. Biol Chem. 2002;383:1581–1585. doi: 10.1515/BC.2002.178. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143:501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Wang T, Huang S, Glorioso JC, Albers KM. The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a. Exp Neurol. 2012;233:221–232. doi: 10.1016/j.expneurol.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe T, Takeuchi O, Takabatake Y, Kato H, Isaka Y, Tsujimura T, Akira S. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat Immunol. 2009;10:965–972. doi: 10.1038/ni.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye KM, Devergne O, Harada JN, Izumi KM, Yalamanchili R, Kieff E, Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci U S A. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- Kim DK, Han SB, Hong ST, Choi YJ, Sun W, Geum D, Kim H. Expression of Sox11 and Brn transcription factors during development and following transient forebrain ischemia in the rat. Neurosci Lett. 2008;433:259–264. doi: 10.1016/j.neulet.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Enderich J, Hermans-Borgmeyer I, Wegner M. Cooperative function of POU proteins and SOX proteins in glial cells. J Biol Chem. 1998;273:16050–16057. doi: 10.1074/jbc.273.26.16050. [DOI] [PubMed] [Google Scholar]
- Li C, Ni CZ, Havert ML, Cabezas E, He J, Kaiser D, Reed JC, Satterthwait AC, Cheng G, Ely KR. Downstream regulator TANK binds to the CD40 recognition site on TRAF3. Structure. 2002;10:403–411. doi: 10.1016/s0969-2126(02)00733-5. [DOI] [PubMed] [Google Scholar]
- Li C, Norris PS, Ni CZ, Havert ML, Chiong EM, Tran BR, Cabezas E, Reed JC, Satterthwait AC, Ware CF, Ely KR. Structurally distinct recognition motifs in lymphotoxin-beta receptor and CD40 for tumor necrosis factor receptor-associated factor (TRAF)-mediated signaling. J Biol Chem. 2003;278:50523–50529. doi: 10.1074/jbc.M309381200. [DOI] [PubMed] [Google Scholar]
- Lin L, Lee VM, Wang Y, Lin JS, Sock E, Wegner M, Lei L. Sox11 regulates survival and axonal growth of embryonic sensory neurons. Dev Dyn. 2011;240:52–64. doi: 10.1002/dvdy.22489. [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Manna SK, Babajan B, Raghavendra PB, Raviprakash N, Sureshkumar C. Inhibiting TRAF2-mediated activation of NF-kappaB facilitates induction of AP-1. J Biol Chem. 2010;285:11617–11627. doi: 10.1074/jbc.M109.094961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao P, Madec K, Gong Y, Shen H, Eisenstat D, Melanson M, Gu X, Leong C, Klowak M, Namaka M. Axotomy-induced up-regulation of tumor necrosis factor-alpha in the dorsal root ganglia. Neurol Res. 2008;30:623–631. doi: 10.1179/174313208X289606. [DOI] [PubMed] [Google Scholar]
- Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, Geschwind DH, Pilpel Y, Burlingame AL, Fainzilber M. Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal. 2010;3:ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- Park JG, Yuk Y, Rhim H, Yi SY, Yoo YS. Role of p38 MAPK in the regulation of apoptosis signaling induced by TNF-alpha in differentiated PC12 cells. J Biochem Mol Biol. 2002;35:267–272. doi: 10.5483/bmbrep.2002.35.3.267. [DOI] [PubMed] [Google Scholar]
- Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42:93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potzner MR, Tsarovina K, Binder E, Penzo-Mendez A, Lefebvre V, Rohrer H, Wegner M, Sock E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;137:775–784. doi: 10.1242/dev.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007;81:1–28. doi: 10.1016/j.pneurobio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Rothe M, Xiong J, Shu HB, Williamson K, Goddard A, Goeddel DV. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc Natl Acad Sci U S A. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Salerno KM, Jing X, Diges CM, Cornuet PK, Glorioso JC, Albers KM. Sox11 modulates brain-derived neurotrophic factor expression in an exon promoter-specific manner. J Neurosci Res. 2012;90:1011–1019. doi: 10.1002/jnr.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS, Geis C, Shubayev VI. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003a;347:179–182. doi: 10.1016/s0304-3940(03)00695-5. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003b;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HX, Liu X, Wang Q, Tang PP, Liu XY, Shan YF, Wang C. Mitochondrial ubiquitin ligase MARCH5 promotes TLR7 signaling by attenuating TANK action. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002057. e1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen HS, Olsson T, Bucht A, Khademi M, Svelander L, Roytta M. Peripheral nerve injury induces endoneurial expression of IFN-gamma, IL-10 and TNF-alpha mRNA. J Neuroimmunol. 2000;102:17–25. doi: 10.1016/s0165-5728(99)00154-x. [DOI] [PubMed] [Google Scholar]
- Thein DC, Thalhammer JM, Hartwig AC, Crenshaw EB, 3rd, Lefebvre V, Wegner M, Sock E. The closely related transcription factors Sox4 and Sox11 function as survival factors during spinal cord development. J Neurochem. 2010;115:131–141. doi: 10.1111/j.1471-4159.2010.06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wiebe MS, Nowling TK, Rizzino A. Identification of novel domains within Sox-2 and Sox-11 involved in autoinhibition of DNA binding and partnership specificity. J Biol Chem. 2003;278:17901–17911. doi: 10.1074/jbc.M212211200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang J, Zhang Y, Yuan Y, Guan W, Jin C, Chen H, Wang X, Yang X, He F. The scaffold protein TANK/I-TRAF inhibits NF-kappaB activation by recruiting polo-like kinase1. Mol Biol Cell. 2010;21:2500–2513. doi: 10.1091/mbc.E09-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]