Abstract
Smad7 inhibits responses mediated by transforming growth factor β (TGF-β) and acts in a negative-feedback loop to regulate the intensity or duration of the TGF-β signal. However, the aberrant expression and continued presence of Smad7 may cause TGF-β resistance. Here we report that Jab1/CSN5, which is a component of the COP9 signalosome complex, associates constitutively with Smad7 and that overexpression of Jab1/CSN5 causes the translocation of Smad7 from the nucleus to the cytoplasm, promoting its degradation. Overexpression of Jab1/CSN5 increases Smad2 phosphorylation and enhances TGF-β-induced transcriptional activity. The inhibition of endogenous Jab1/CSN5 expression by small interfering RNA (siRNA) induces Smad7 expression. This study thus defines Jab1/CSN5 as an adapter that targets Smad7 for degradation, thus releasing Smad7-mediated suppression of TGF-β signaling.
Transforming growth factor β (TGF-β) superfamily members have a broad range of biological activities, including regulation of cell growth, differentiation, apoptosis, and morphogenesis (44). TGF-β family members signal through heteromeric complexes of type II and type I transmembrane serine/threonine kinase receptors. Activated type I receptor phosphorylates the downstream signal transducers, the receptor-regulated Smads (R-Smads) (14, 22, 35), which induces binding of the R-Smad to the common Smad, Smad4. After binding to Smad4, the complex translocates into the nucleus to activate transcription of various target genes (36). Nuclear Smad complexes bind to transcriptional coactivators (p300 and CBP) or corepressors (e.g., TGIF, c-Ski, and SnoN) and regulate transcription of target genes (2, 38). Another class of Smads, inhibitory Smads (I-Smads), which are represented by Smad6 and Smad7, negatively regulates TGF-β signaling by interfering with the activation of R-Smads by the serine/threonine kinase receptors (21, 24, 40). Expression of Smad7 is induced by TGF-βs, bone morphogenetic proteins (BMPs), epidermal growth factor, gamma interferon, lipopolysaccharide, tumor necrosis factor alpha, and interleukin 1β (1, 6, 40, 51).
Jun activation domain-binding protein 1 (Jab1)/CSN5 was first identified as a coactivator of the transcription factor c-Jun (11). Recent findings show that Jab1/CSN5 is the fifth component of the COP9 signalosome complex (13, 30). The COP9 signalosome is required for the proteosome-mediated degradation of HY5, a positive regulator of photomorphogenesis (42). The COP9 signalosome consists of eight subunits that exhibit significant homologies to the eight subunits of the 26S proteosome lid complex (19, 53), which suggests that the COP9 signalosome is involved in protein degradation via the ubiquitin-proteasome pathway (45, 53). Jab1/CSN5 interacts with a number of diverse proteins, including c-Jun, p27Kip1, MIF1, LFA-1, progesterone receptor, SRC-1, lutropin/choriogonadotropin receptor, and Bcl-3 (5, 9, 11, 12, 29, 49). In cells overexpressing Jab1/CSN5 ectopically, p27 is exported from the nucleus to the cytoplasm and is induced to degrade in a manner sensitive to chemical inhibitors of chromosomal region maintenance 1 (CRM1)-dependent nuclear export and the 26S proteosome. Moreover, HER2/neu was shown to facilitate the ubiquitin-mediated degradation of p27Kip1 by increasing the export of Jab1/CSN5 from the nucleus to the cytoplasm (56). Jab1/CSN5 also interacts with Smad4. Ectopic expression of Jab1/CSN5 decreased steady-state levels of endogenous Smad4 and induced its ubiquitylation, resulting in the inhibition of TGF-β-induced gene transcription (52). These results suggest that Jab1/CSN5 regulates numerous signaling pathways, including those for development, light signaling, the transcriptional transactivation activity of AP-1 and nuclear receptors, and cell cycle control.
Proteolytic degradation of intracellular proteins by the ubiquitin-proteosome pathway plays a key role in various biological processes, such as signal transduction, cell cycle progression, endocytosis, and transcriptional regulation (7, 15, 23). Ubiquitination is controlled by a multienzyme cascade that involves activities of E1 (ubiquitin-activating enzymes), E2 (ubiquitin-conjugating enzymes), and E3 (ubiquitin-protein ligases) (23). In the ubiquitin-proteasome pathway, E3 ligases play a crucial role in the recognition of target proteins and subsequent protein degradation by the 26S proteasome. The ubiquitin-proteasome pathway has also been implicated in the degradation of Smads. Smad ubiquitin regulatory factor 1 (Smurf1), an E3 ubiquitin ligase, targets the BMP-specific Smads, Smad1 and Smad5, for degradation. Smurf1 also interacts with Smad7 and induces Smad7 ubiquitination and translocation into the cytoplasm. Smurf1 forms a complex with TβRI via Smad7 to induce degradation of TβRI (16, 58). Smurf2 interacts with Smad2, as well as other R-Smads, and induces its degradation (33). Recently, Kavsak et al. (26) reported that Smurf2 binds to the TGF-β receptor complex via Smad7 and induces degradation of receptors and Smad7. Degradation of receptor complexes through the interaction with Smad7 of either Smurf1 or Smurf2 may play an important role in the negative regulation of TGF-β superfamily signaling.
In this study, we show a unique role of Jab1/CSN5 in TGF-β signaling. Jab1/CSN5 enhances TGF-β signaling by binding to Smad7 and promoting its degradation.
MATERIALS AND METHOD
Plasmids
For yeast two-hybrid screening, the open reading frame region of the human Smad7 gene was amplified by PCR by using two oligonucleotides (5′-ATGCCATGGAGTTCAGGACCAAACGATCTG-3′ and 5′-ATAGGGATCCCTACCGGCTGTTGAAGATGA-3′) and subcloned into NcoI-BamHI sites of plasmid pGBKT7 (Clontech), generating the bait plasmid, pGBKT-Smad7. To construct the Jab1 expression vector, PCR amplification was performed by using two oligonucleotides (5′-CGGGATCCATGGCGGCGTCCGGGAGCGGTAT-3′ and 5′-CGTAGCGGCCGCCTCAGAGACTGTTTAAGAGATG-3′). The full-length Jab1/CSN5 cDNA, which had been isolated in our screening and fused in frame to the GAL4 activation domain, was used as the template. The amplified fragment of Jab1/CSN5 was subcloned into BamH1-NotI sites of pcDNA-hemagglutinin (HA) (Clontech), pGEX4T-1 (Pharmacia), and mammalian glutathione S-transferase (GST) expression vector. The sequences of the PCR-generated portion of all constructs were verified by DNA sequencing. N-terminally truncated (Smad7C) and C-terminally truncated (Smad7N) mutants were kindly provided by S. Souchelnytskyi (43).
Yeast two-hybrid screening
The Smad7 bait plasmid (pGBKT-Smad7) was transformed into yeast strain AH109 for library screening. The transformants containing bait plasmid were mated with the pretransformed human placenta MATCHMAKER cDNA library purchased from Clontech. Two-hybrid screening was performed according to the manufacturer's protocol. Briefly, after the two yeast strains containing bait and pretransformed library were mixed and incubated overnight in YPDA-kanamycin media, candidates for two-hybrid interaction were initially selected on Trp−/Leu−/Ade−/His− medium, and positive interactions were further confirmed by the LacZ+ phenotype. To characterize the Smad7-interacting proteins, plasmids were recovered from yeast strains showing positive interactions, and their sequences were verified by DNA sequencing.
Cell culture, transfection, and reporter assays
Cell lines were maintained in Dulbecco modified Eagle medium or minimal essential medium supplemented with 10% fetal bovine serum. HepG2 cells were transfected with 3TP-Lux (54) or 4 × SBE-Luc (57) with or without the Smad7 expression construct (1 μg), together with an increasing amount of Jab1/CSN5 expression vector in six-well plates by using Lipofectin (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. After transfection, cells were treated with 5 ng of TGF-β1/ml for 24 h in media. All assays were performed in triplicate, and the results are presented as the means (± the standard errors) of three independent transfections.
Western blotting, GST pull-down assay, and immunoprecipitation
HepG2 cells were transiently transfected with the indicated plasmids. After 24 h, the cells were switched to 0.2% serum overnight and induced with 5 ng of TGF-β1/ml for 1 h, and then whole-cell extracts were prepared. The extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by electrotransfer to nitrocellulose membranes, probed with polyclonal or monoclonal antisera followed by horseradish peroxidase-conjugated anti-rabbit, anti-mouse, or anti-goat immunoglobulin G (IgG), and visualized by chemiluminescence according to the manufacturer's (Pierce) instructions. GST pull-down assays were performed by incubating GST beads (Pharmacia) with each extract overnight. After the GST beads were washed four times with the buffer containing 200 mM NaCl and 75 mM KCl, Western blotting was performed. Immunoprecipitation was carried out by incubation with anti-HA (Santa Cruz, Santa Cruz, Calif.) for 1 h. After the immunoprecipitates were washed four times with the buffer containing 200 mM NaCl and 75 mM KCl, Western blots were prepared. For the inhibition of protein degradation, cells were treated with the proteasome inhibitor lactacystin (10 μM) or MG132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucinal) (1 μM) after transfection. The proteasome inhibitors MG132 and lactacystin were from Sigma (St. Louis, Mo.).
Indirect immunofluorescence
COS7 cells were plated at 2 × 105 cells/coverslip onto 22-mm glass coverslips 24 h prior to transfection. The cells were transfected in complete medium by using Fugene6 (Roche) according to the manufacturer's instructions. After 12 h, the transfection medium was replaced with Dulbecco modified Eagle medium-10% fetal bovine serum. When indicated, the cells were treated with MG132 (2.5 uM) for 6 h and then fixed in cold 3.5% paraformaldehyde for 5 min, permeabilized in cold absolute methanol for 2 min, and then incubated for 5 min in 50 mM glycine to quench paraformaldehyde autofluorescence. The transfected constructs were then detected by incubation at 4°C with anti-HA rabbit polyclonal (Y-11; Santa Cruz Biotechnology) and anti-Myc mouse monoclonal (9E10; Santa Cruz Biotechnology) antibodies. After being washed in phosphate-buffered saline (three times for 5 min each), the coverslips were incubated for 1 h at room temperature with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG and tetramethyl rhodamine isocyanate-conjugated goat anti-mouse IgG secondary antibodies. The coverslips were then mounted in medium containing 4,6-diamino-2-phenylindole (DAPI) (Vector Laboratories). The cells were examined with a Leica TCS.NT laser scanning confocal microscope. A 63× oil immersion objective was used. Appropriate emission filters, settings, and controls were included to exclude bleed-through effects.
Adenoviral infections
Recombinant adenoviruses expressing Smad7 or β-galactosidase were kindly provided by Kohei Miyazono (The Cancer Institute, Tokyo, Japan) and were used at a multiplicity of infection (MOI) of 200 with single viruses as described by Fujii et al. (18).
Generation of HepG2 cell lines expressing Jab1/CSN5
Jab1/CSN5 was PCR-amplified, restriction-digested, and purified to be subcloned into the MFG vector. An IRES-NEO cassette was also subcloned into the constructs to obtain the stable transfectants.
siRNA methods
We used the small interfering RNA (siRNA) design tool (Dharmacon Inc., Lafayette, Colo.) to identify target siRNAs. The Hab1/CSN5-specific sequence was 5′-GCUCAGAGUAUCGAUGAAAdTdT-3′ (Jab1 nucleotides 184 to 202; GenBank accession number NM_006837). HepG2 cells were seeded at 30% density the day before transfection. Transfections were performed by using TransIT-TKO reagent (Mirus, Madison, Wis.) according to the manufacturer's instructions with 200 pmol of siRNA and 10 μl of transfection reagent/10-cm dish for HepG2 cells.
RESULT
Interaction of Jab1/CSN5 with Smad7
To identify proteins that interact with Smad7, a yeast two-hybrid screening was performed. The entire Smad7 protein was fused in frame to the GAL4 DNA binding domain as a bait. Using this bait, a pretransformed human placenta cDNA library was screened as described in Materials and Methods. From about 3 million transformants, 125 clones were initially screened on selective medium, showing the Trp+/Leu+/Ade−+/His+ phenotype. Fifty-two clones were further selected by β-galactosidase activity. Sequence analysis of plasmids rescued from positive clones revealed that two clones are identical, showing in-frame fusion with the GAL4 activation domain, and that the positive clones encoded human Jab1/CSN5. Jab1/CSN5 was originally identified as a coactivator of c-Jun and JunD (11). It is involved in numerous signaling pathways, including cell cycle (49) and integrin signaling (5). It is also known that Jab1/CSN5 is the fifth subunit of the COP9 signalosome complex, which phosphorylates c-Jun, IκB, p105, and p53 (4). These results suggest that Jab1/CSN5 might interact with Smad7 in vivo and play a role in the TGF-β/Smad signaling cascade.
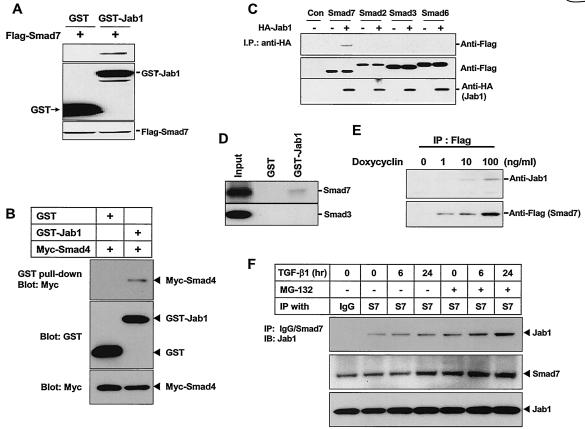
To confirm the interaction of Smad7 with Jab1/CSN5 in mammalian cells, we performed GST pull-down assays by using HepG2 cells transfected with Jab1/CSN5 in a mammalian GST fusion vector along with a Flag-tagged Smad7 expression construct. A specific interaction between Jab1/CSN5 and Smad7 was observed (Fig. 1A and C). Consistent with a previous report, Jab1/CSN5 interacted with Smad4 (Fig. 1B) (52). To examine whether Jab1/CSN5 also interacts with other Smads, HepG2 cells were transfected with an HA-tagged Jab1/CSN5 expression construct and then cells were infected with Smad2, Smad3, and Smad6 DNAs by using a recombinant adenovirus system to obtain high transfection efficiency. Since the increased interaction between Jab1/CSN5 and Smad proteins might lead to increased degradation of Smad proteins, cells were reincubated in the presence of lactacystin, which inhibits protein degradation by the proteosome, after adenovirus infection. Jab1/CSN5 interacted only with Smad7 (Fig. 1C). The interaction between Smad proteins and Jab1/CSN5 was also studied by GST pull-down assays in vitro by using 35S-labeled Smad3 and Smad7 proteins. Jab1/CSN5 interacted with 35S-labeled Smad7 but not with Smad3 (Fig. 1D). To examine whether overexpressed Smad7 interacts with endogenous Jab1/CSN5, we generated a HepG2 cell line stably expressing Flag-tagged Smad7. The transcription of Flag-Smad7 was under the control of doxycycline with an improved tetracycline-inducible system in which the expression of the reverse tetracycline repressor was driven by the EF-1α promoter (59). In the absence of doxycycline, no Smad7 was detected in total cell lysate by Western blot analysis (Fig. 1E). The level of Smad7 expression correlated with the amount of doxycycline present in the cell growth medium. The whole-cell lysates prepared from cells treated with or without doxycycline were immunoprecipitated by the anti-Flag-antibody, and the interaction of endogenous Jab1/CSN5 with Flag-Smad7 was examined by Western blot analysis using anti-Jab1 antibody. As shown in Fig. 1E, endogenous Jab1/CSN5 interacts with stably overexpressed Flag-Smad7. To see whether the interaction between endogenous Smad7 and Jab1/CSN5 increases with time over the course of TGF-β treatment, HaCat cells were treated with TGF-β1 at different time points in the presence or absence of MG132, an inhibitor of proteosome-mediated degradation. The increase in the level of Smad7 protein was observed at 24 h of TGF-β1 stimulation and compared to that at 6 h of TGF-β1 stimulation (Fig. 2F). However, MG132 treatment stabilized Smad7 protein even after 6 h of TGF-β1 stimulation. The increase in the interaction between Smad7 and Jab1/CSN5 was also observed when cells were treated with MG132, suggesting that the increased interaction between these two proteins may lead to increased degradation of Smad7.
FIG. 1.
Interaction of Jab1/CSN5 with Smad7. (A) GST-Jab1/CSN5 was transfected into HepG2 cells with the Flag-tagged Smad7 construct. Cell extracts were subjected to GST pull-down assay with glutathione-Sepharose beads followed by immunoblotting with anti-Flag antibody. Expression of GST, GST-Jab1/CSN5, and Smad7 was monitored as indicated. (B) GST-Jab1/CSN5 was transfected into HepG2 cells with the Myc-tagged Smad4 construct. Cell extracts were subjected to GST pull-down assay with glutathione-Sepharose beads followed by immunoblotting with anti-Myc antibody. Expression of GST, GST-Jab1/CSN5, and Smad4 was monitored as indicated. (C) HA-tagged Jab1/CSN5 constructs were transfected into HepG2 cells. After transfection, the cells were infected with adenoviruses carrying Smad2, Smad3, Smad6, and Smad7 at an MOI of 300. Adenovirus carrying β-galactosidase (MOI, 800) was used as the control (Con). The cells were then treated with lactacyctin (10 μM). Twenty-four h after transfection, the interaction between Smads and Jab1/CSN5 was analyzed by immunoblotting with the anti-Flag antibody after immunoprecipitation (I.P.) with anti-HA antibody. Expression of Smads was confirmed by anti-FLAG immunoblotting. Similar results were achieved in three separate experiments. (D) The interaction between Jab1/CSN5 and Smad7 or Smad3 was examined by GST pull-down assay in vitro. Bacterially expressed GST-Jab1/CSN5 and GST alone were incubated with [35S]methionine-labeled Smad7 protein. Twenty-five percent of [35S]methionine-labeled Smad7 or Smad3 protein used for the assay was applied as the control (Input). (E) Interaction of Jab1/CSN5 with Smad7 in vivo. Flag-tagged Smad7 was stably transfected into HepG2 cells. Transcription of the tagged Smad7 was under the control of doxycycline with an improved tetracycline-inducible system in which the expression of the reverse tetracycline repressor was driven by the EF-1α promoter (39). After the cells were incubated with the indicated amount of doxycycline for 24 h, they were lysed and subjected to immunoblotting with the anti-Jab1 antibody, following immunoprecipitation (IP) with anti-Flag antibody. (F) Interaction between endogenous Jab1/CSN5 and Smad7 in HaCat cells. Cells were incubated in the presence or absence of TGF-β1 (5 ng/ml) for 6 and 24 h, treated with MG-132 (2 μM) for 2 h before cell harvest, and then lysed and subjected to immunoblotting (IB) with the anti-Jab1 antibody following immunoprecipitation with anti-Smad7 antibody.
FIG. 2.
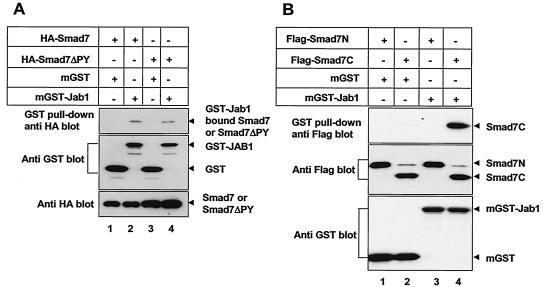
Mapping of Smad7 domains which interact with Jab1/CSN5. (A) The PY motif of Smad7 is not required for Smad7-Jab1/CSN5 interaction. 293T cells were transfected with GST or GST-Jab1/CSN5 either alone or together with wild-type or ΔPY versions of Smad7. Cell extracts were subjected to GST pull-down assay with glutathione-Sepharose beads followed by immunoblotting with anti-HA antibody. Expression of GST, GST-Jab1/CSN5, HA-Smad7, and HA-Smad7ΔPY was monitored as indicated. (B) Jab1/CSN5 associates with the C-terminal domain of Smad7. FLAG-tagged Smad7 deletion mutants (7C; amino acids 204 to 407 and the N-terminal domain of Smad7; amino acids 1 to 261) (25) were cotransfected into the 293T cells together with either GST or GST-Jab1/CSN5. Cell extracts were subjected to GST pull-down assay with glutathione-Sepharose beads followed by immunoblotting with anti-FLAG antibody. Expression of Smad7 deletion mutants, GST, and GST-Jab1 was confirmed by immunoblotting aliquots of total cell lysates (bottom panels). The higher band in lanes 2 and 4 is a nonspecific band.
We next mapped the domain of Smad7 responsible for interaction with Jab1/CSN5 in vivo. Smad7 possesses a PPXY sequence (PY motif) in its linker region, which was shown to mediate protein-protein interaction (10). Mutation of the Smad7 PY motif reduced the binding of Smurf2 to Smad7 but did not completely abolish it, suggesting that the PY motif is important for Smurf-Smad7 interaction but is not the sole determinant (26). Therefore, we examined whether the PY motif of Smad7 is required for Smad7-Jab1/CSN5 interaction. Mutation of the Smad7 PY motif did not abolish the Smad7-Jab1/CSN5 interaction, suggesting that it is not involved in the Smad7-Jab1/CSN5 interaction (Fig. 2A). We then determined the domain of Smad7-Jab1/CSN5 interaction by GST pull-down assays using GST-tagged Jab1/CSN5 along with HA-tagged deletion mutants of Smad7. Jab1/CSN5 was found to associate with the full-length and C-terminal domains of Smad7 but not with the N-terminal domain of this molecule (Fig. 2B).
Jab1/CSN5 controls the subcellular localization of Smad7
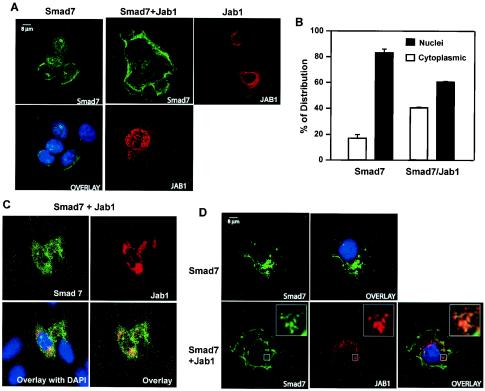
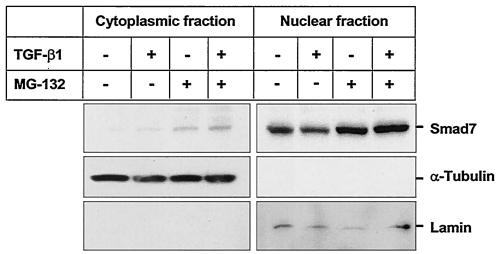
The subcellular localization of Smad7 is still unclear. Itoh et al. (25) have shown that Smad7 is localized in the nucleus and that upon ligand stimulation Smad7 accumulates in the cytoplasm of COS1 cells. However, Zhu et al. (59) have shown that Smad7 is expressed predominantly in the perinuclear region and the cytoplasm of Mv1Lu mink lung epithelial cells. Since previous studies have implicated Jab1/CSN5 as a regulator of intracellular p27Kip1 levels and localization in the cell (49), we investigated whether Jab1/CSN5 might regulate Smad7 localization. For this purpose, we examined the intracellular localization of HA-tagged Smad7 and Myc-tagged Jab1/CSN5 by indirect immunofluorescence in COS7 cells. Consistent with previous reports (16), Smad7 displayed a specific staining pattern that was present throughout the nucleus and the perinuclear region (Fig. 3A). A fraction of Jab1/CSN5 was also enriched in the same peripheral membrane regions (Fig. 3A, right panel); the rest was distributed heterogeneously in the nucleus and the cytoplasm. Cotransfection of Jab1/CSN5 and Smad7 showed extensive, although not complete, relocalization of Smad7 staining from the nucleus to the cytoplasm (Fig. 3A and B). Since Jab1/CSN5 expression in COS7 was weak, we also transfected HA-tagged Smad7 with Myc-tagged Jab1/CSN5 into HepG2 cells. As shown in Fig. 3C, when Jab1/CSN5 was coexpressed, Smad7 was predominantly found outside the nucleus and was colocalized with Jab1/CSN5. Furthermore, cotransfection of HA-tagged Smad7 and Myc-tagged Jab1/CSN5 in COS7 cells treated for 6 h with the proteosomal inhibitor MG132 showed selective colocalization of the two proteins in punctate structures in the cytoplasm (Fig. 3D). Colocalization was not complete and may reflect differences in the stoichiometry of Smad7 versus Jab1/CSN5 protein levels or may indicate that there are subcellular locations for Jab1/CSN5 from which Smad7 is excluded (Fig. 3C and D). To study whether Jab1/CSN5 plays a role in causing nuclear export of Smad7, cytoplasmic and nuclear lysates from HaCaT cells treated with TGF-β1 for 14 h were immunoblotted with anti-Smad7 antibodies. Most of the Smad7 was present in the nucleus even in the absence of TGF-β1. Treatment with TGF-β1 for 14 h resulted in a decrease in nuclear Smad7 and a minimal increase in cytoplasmic Smad7 (Fig. 4). However, treatment with MG-132, which inhibits protein degradation by the proteosome, stabilized both cytoplasmic and nuclear Smad7. Immunodetection of blots with cytoplasmic (α-tubulin) and nuclear (lamin) marker proteins confirmed the purity of the different fractions. Taken together with our biochemical analysis, these results indicate that Jab1/CSN5 favors cytoplasmic versus nuclear localization of Smad7 and that a fraction of Smad7 and Jab1/CSN5 present in a cell are in physical contact and might cooperate in regulating TGF-β signaling.
FIG. 3.
Jab1/CSN5 controls the subcellular localization of Smad7. (A) COS7 cells were transiently transfected with either HA-tagged Smad7 (0.5 μg/ml) or Jab1/CSN5 (1 μg/ml) alone or in combination with Myc-tagged Jab1/CSN5 (1.5 mg/ml), fixed in 3.5% paraformaldehyde, permeabilized with methanol, stained, and analyzed with laser scanning confocal microscopy. Smad7 was visualized by polyclonal anti-HA antibody and fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (green). Jab1/CSN5 was visualized by monoclonal Myc antibody and a tetramethyl rhodamine isocyanate-conjugated goat anti-mouse IgG (red). DAPI staining (blue) highlights the location of nuclei. (B) Quantitative analysis of Smad7 nuclear staining in the absence or presence of Jab1/CSN5. The Smad7 nuclear signal was plotted as the percentage of cells with prominent nuclear versus cytoplasmic staining ± the standard deviation duplicated from a representative experiment. For each condition, >100 cells were counted in each experiment. (C) HepG2 cells were transiently transfected with HA-tagged Smad7 (0.5 μg/ml) and Myc-tagged Jab1/CSN5 (1.5 μg/ml), fixed in 3.5% paraformaldehyde, permeabilized with methanol, stained, and analyzed with laser scanning confocal microscopy. (D) COS7 cells were transfected as described for panel A and then treated with the proteosomal inhibitor MG132 (2.5 μM) for 6 h. Colocalization of Smad7 and Jab1/CSN5 appears as yellow. Areas marked by a rectangle are enlarged and shown as insets.
FIG. 4.
Smad7 is constitutively present in the nucleus, and TGF-β1 treatment increases Smad7 in the cytoplasm. HaCaT cells treated or not treated with 5 ng of TGF-β1/ml for 14 h were incubated in the presence or absence of MG-132, an inhibitor of proteosom-mediated degradation, for 2 h before lysis. Lysates were separated into nuclear and cytoplasmic fractions and immunoblotted with antibodies against Smad7 (top panel) and, to confirm the purity of the fractions, α-Tubulin (middle panel) and Lamin (lower panel). All three blots were prepared from the same lysate and are representative of the results obtained in three separate experiments.
Down regulation of Smad7 by Jab1/CSN5
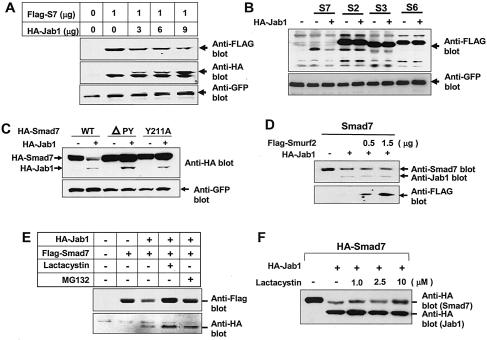
Since ectopic expression of Jab1/CSN5 induces degradation of p27Kip1, we investigated whether Jab1/CSN5 expression also affects the steady-state level of Smad proteins in 293 cells. Coexpression of Smad7 with increasing amounts of Jab1/CSN5 resulted in a dose-dependent decrease in the steady-state level of Smad7 (Fig. 5A). However, Jab1/CSN5 expression did not decrease Smad2, Smad3, or Smad6 protein levels, consistent with its inability to bind to these Smads (Fig. 5B). These data suggest that Jab1/CSN5 specifically targets the Smad7 protein for degradation while having no effect on other highly homologous Smads. We also analyzed the effect of Jab1/CSN5 on the stability of Smad7 mutant proteins Smad7ΔPY and Smad7(Y211A). Jab1/CSN5 decreased the level of wild-type Smad7 but failed to decrease levels of Smad7 mutant proteins (Fig. 5C), despite its efficient interaction with Smad7ΔPY. These results suggest that the PY motif in the Smad7 linker region is not involved in its interaction with Jab1/CSN5 but is essential for the Jab1/CSN5-mediated decrease of the Smad7 protein level. Previous studies (16, 26, 47) have shown that Smurf2 associates constitutively with Smad7, thus inducing Smad7 nuclear export and recruitment to the activated TGF-β receptor and ultimately causing degradation of TGF-β receptors and Smad7 via protesomal and lysosomal pathways (26). Therefore, we investigated whether Smurf2 synergizes with Jab1/CSN5 in controlling the Smad7 protein level. Coexpression of Smurf2 and Jab1/CSN5 together with Smad7 did not show a significant synergistic effect on the Smad7 protein level (Fig. 5D).
FIG. 5.
Jab1/CSN5 induces degradation of Smad7. (A) Ectopic expression of Jab1/CSN5 down regulates Smad7. HepG2 cells were transfected with increasing amounts of HA-Jab1/CSN5 expression vector or control plasmid together with Flag-Smad7 expression vector. A GFP expression construct was used as the control. After 24 h, cell extracts were analyzed by immunoblotting using antibodies against HA, Flag, and GFP. (B) Jab1/CSN5 specifically degrades Smad7 but not Smad2, Smad3, or Smd6. HepG2 cells were transfected with HA-Jab1 expression construct or control plasmid together with the vectors indicated at the top, and after 24 h, total cell extracts were analyzed by immunoblotting using anti-Flag antibody. (C) The PY motif in Smad7 is important for Jab1-/CSN5-mediated degradation of Smad7. 293T cells were transfected with HA-Jab1/CSN5 or control plasmid together with either wild-type (WT), ΔPY, or mutant Y211A versions of Smad7. Total cell extracts were analyzed by immunoblotting by using anti-HA antibody, and Smad7 and Jab1/CSN5 were identified on the basis of their molecular weights. A GFP expression construct was used as the loading control. (D) Effect of Smurf2 on the Jab1-mediated degradation of Smad7. A Smad7 expression construct was transfected into 293T cells with either HA-Jab1/CSN5 alone or together with increasing amounts of Flag-Smurf2 expression vector. Total cell extracts were analyzed by immunoblotting using antibodies against Smad7, Jab1, and Flag. (E and F) Degradation of Smad7 by Jab1/CSN5 is sensitive to 26S proteosome inhibitors. 293T cells transfected with Flag-Smad7 alone or together with HA-Jab1/CSN5 were incubated with lactacystin (10 μM) or MG132 (2 μM), and expression of Flag-Smad7 was analyzed by immunoblotting.
To test whether Jab1/CSN5-dependent degradation of Smad7 occurred through the 26S proteosome pathway, we assessed the turnover of Smad7 in the presence and absence of lactacystin and MG132, which inhibit protein degradation by the proteosome. Treatment with inhibitors stabilized Smad7 levels in the presence of Jab1/CSN5 (Fig. 5E), depending on the amount of lactacystin (Fig. 5F), suggesting that the proteosome contributes to the degradation of Smad7 mediated by Jab1/CSN5.
To investigate ubiquitination of Smad7, we expressed an HA epitope-tagged version of ubiquitin and evaluated ubiquitin conjugates of Smad7 by immunoprecipitation with Smad7 antiserum followed by immunoblotting with antiubiquitin antibody. In the absence of Jab1/CSN5, little ubiquitination was observed for Smad7. In the presence of Jab1/CSN, a strong ladder of high molecular weight, ubiquitin-conjugated Smad7 products were readily observed (Fig. 6). In contrast to wild-type Smad7, Jab1/CSN5 was unable to induce the ubiquitination of Smad7ΔPY. A previous study (26) showed that Smurf2 induced only a slight amount of ubiquitin-Smad7 conjugate. However, when Smurf2 was coexpressed with Smad7 and the TGF-β receptor, a strong increase in high-molecular-weight ubiquitin conjugates of Smad7 was observed, as reported previously (26). In contrast to our findings for Smurf2, we were able to detect significant Jab1/CSN5-dependent ubiquitination of Smad7 when Jab1/CSN5 was coexpressed with Smad7 alone, without the TGF-β receptor.
FIG. 6.
Jab1/CSN5 induces the ubiquitination of Smad7. 293T cells were transfected with HA-tagged ubiquitin together with combinations of Smad7, Smad7ΔPY, Jab1/CSN5, Smurf2, TβRI, or TβRII as indicated. Cell lysates were subjected to immunoprecipitation with Smad7 antiserum and boiled in sodium dodecyl sulfate and were then subjected to immunoblotting with anti-HA antibodies. Expression of the proteins was confirmed by immunoblotting the total cell lysates.
Jab1/CSN5 enhances TGF-β signaling
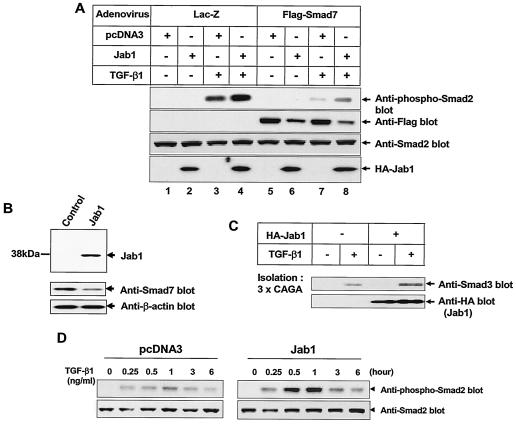
To examine whether the decrease in the Smad7 protein level by Jab1/CSN5 enhances TGF-β signaling, we infected HepG2 cells with adenoviruses carrying Smad7 cDNA and examined TGF-β1-induced Smad2 phosphorylation. Without TGF-β1 treatment, phosphorylation of Smad2 was not observed in the cells (Fig. 7A). TGF-β1 treatment increased phosphorylation of Smad2, and expression of Jab1/CSN5 enhanced Smad2 phosphorylation by approximately two- to threefold. Expression of Smad7 markedly decreased the level of Smad2 phosphorylation, whereas Jab1/CSN5 coexpression slightly blocked the negative effect of Smad7 on Smad2 phosphorylation. To confirm these findings, we generated HepG2 cells stably expressing HA-Jab1 (Fig. 7B). As expected, basal expression of endogenous Smad7 was slightly lower in HepG2-Jab1/CSN5 cells than in control HepG2 cells. Treatment of cells with TGF-β1 activates the ability of Smad3 to bind DNA. To test whether the overexpression of Jab1/CSN5 might enhance this activity, a DNA-affinity precipitation assay with biotinylated oligonucleotides containing the Smad-binding element (CAGA box) (20) was performed. Using nuclear extracts isolated from Jab1/CSN5-expressing HepG2 cells (HepG2-Jab1) and control cells with or without TGF-β1 treatment, we isolated active Smad3 by using biotinylated DNA containing the TGF-β-responsive CAGA sequence. The amount of Smad3 bound to the CAGA sequence increased in nuclear extracts from control cells after treatment with TGF-β1 (Fig. 7C). The amount of Smad3 bound to the CAGA sequence was further enhanced in nuclear extracts from HepG2 cells expressing Jab1/CSN5 after TGF-β1 treatment. We also examined the TGF-β-induced phosphorylation of Smad2 in these stable cells. TGF-β1-induced Smad2 phosphorylation in control cells was detectable 25 min after treatment and peaked at 1 h. While the kinetics of Smad2 phosphorylation was unchanged in Jab1/CSN5-expressing HepG2 cells, the level of Smad2 phosphorylation was markedly enhanced (Fig. 7D).
FIG. 7.
Jab1/CSN5 induces degradation of Smad7 and enhances TGF-β-induced phosphorylation of Smad2. (A) HepG2 cells were transfected with a HA-Jab1/CSN5 expression construct or control plasmid (pcDNA3) and then infected with adenoviruses carrying Flag-Smad7 at an MOI of 300. After a 30-min treatment with TGF-β1 (5 ng/ml), a band of 58 kDa representing phosphorylated Smad2 (Smad2P) was detected in cell extracts by immunoblotting using rabbit anti-Smad2P antibodies. Adenovirus carrying β-galactosidase (MOI, 800) was used as a control. Expression of Smad7 and HA-Jab1 was confirmed by immunoblotting. Similar results were achieved in three separate experiments with comparable outcomes. (B) Generation of HepG2 cells stably expressing Jab1/CSN5. Expression of endogenous Smad7 protein was decreased in HepG2 cells stably expressing Jab1/CSN5. (C) HepG2 cells stably expressing Jab1/CSN5 and control HepG2 cells were stimulated with 5 ng of TGF-β1/ml for 2 h, and Smad3-binding to CAGA-biotinylated DNA was examined. (D) TGF-β1-induced Smad2 phosphorylation is markedly increased in HepG2 cells stably expressing Jab1/CSN5.
To examine the role of Jab1/CSN5 in TGF-β-induced transcriptional activation, we cotransfected HepG2 cells with a Jab1/CSN5 expression construct, a Smad7 expression construct, and either the TGF-β-responsive 3TP-Lux reporter construct or SBE4-Luc, which contains four SBE (Smad binding element) sites in tandem (57). Introduction of Smad7 repressed the TGF-β-dependent activities of these reporter gene constructs (Fig. 8), whereas increasing expression levels of Jab1/CSN5 antagonized Smad7-mediated inhibition and restored TGF-β-induced transcriptional activation in a dose-dependent manner. These results suggest that Jab1/CSN5-mediated degradation of Smad7 restores TGF-β signaling.
FIG. 8.
Jab1/CSN5 relieves Smad7-mediated suppression of transcriptional activity induced by TGF-β1. HepG2 cells were cotransfected with either p3TP-Lux (B) or SBE4-Luc (A) together with various combinations of Jab1 and Smad7 expression constructs. Luciferase activity was measured 24 h after TGF-β1 stimulation.
We also performed loss-of-function studies using Jab1-specific siRNA to evaluate the specific effect of Jab1 on TGF-β signaling. We tried to reduce endogenous Jab1/CSN5 expression through RNA-mediated interference (37) by using Jab1-specific siRNA. Transfection of the Jab1 siRNA (∼100 to 200 nM) resulted in 70 to 90% decreases in protein levels and was accompanied by an increase in Smad7 expression (Fig. 9A). Since Jab1/CSN5 is also known to facilitate degradation of p27Kip1, we examined the expression of p27Kip1 after transfection of Jab1 siRNA. As expected, transfection of Jab1 siRNA markedly induced expression of p27Kip1. When transfected with Jab1-specific siRNA, transcriptional activity induced by TGF-β was repressed in HepG2 cells (Fig. 9B).
FIG. 9.
siRNA-mediated inhibition of Jab1/CSN5 induces Smad7 expression. (A) Jab1 siRNA efficiently blocks expression of endogenous Jab1/CSN5. HepG2 cells were transfected with either control (scrambled) siRNA or Jab1 siRNA, and 24 h after transfection, cell lysates were extracted for Western blot analysis. Cell lysates were immunoblotted with antibodies against Jab1/CSN5, Smad7, p27, and β-actin (loading control). (B) Suppression of endogenous Jab1/CSN5 expression by Jab1 siRNA in HepG2 cells results in a decrease of TGF-β1-dependent Smad transcriptional activity. HepG2 cells were cotransfected with a SBE4-Luc reporter vector and either control siRNA (scrambled) or Jab1 siRNA. Cells were lysed and assayed for luciferase activity 24 h after transfection.
DISCUSSION
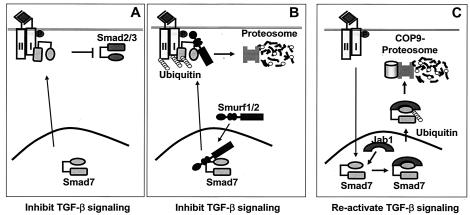
In this study, we have shown that Jab1/CSN5 binds to Smad7 and promotes Smad7 ubiquitination and degradation, thus amplifying the TGF-β signal transduction pathway. Smad7 functions as an inhibitor of TGF-β signaling. The inhibition of endogenous Jab1/CSN5 expression results in an increase in Smad7 protein. Smad7 interacts with activated TβRI, thereby preventing R-Smads from binding to and being phosphorylated by these receptors (21, 25, 40, 46). Smad7 expression is induced transiently by various stimuli, including TGF-β and gamma interferon (1, 6, 40, 51). Induction of Smad7 expression by TGF-β suggests the existence of a negative feedback loop in TGF-β signaling. This increased Smad7 level may inhibit TGF-β signaling by preventing access of Smad2 or Smad3 to the TGF-β receptor or by binding to Smurfs to target the TGF-β receptor for degradation. On the other hand, the prolonged repressed state of TGF-β signaling may also not be preferable for the cellular function. Both feedback inhibition and reactivation of TGF-β signaling may therefore normally function to balance these two pathways. Since the continuous presence of Smad7 in the cell could result in prolonged resistance to TGF-β signaling, degradation of Smad7 may be an initial step in the reactivation of TGF-β signaling. Thus Smad7-Jab1/CSN5 interaction, followed by ubiquitin-mediated degradation of Smad7, may represent the early event of a reactivation mechanism for facilitating the flux through the TGF-β signaling pathway (Fig. 10).
FIG. 10.
Models for repression of TGF-β signaling by the Smurf-Smad7 complex and reactivation of TGF-β signaling by the Jab1/CSN5-Smad7 complex. (A) Smad7 inhibits TGF-β signaling by preventing access of Smad2 or Smad3 to the TGF-β receptor. (B) Smad7 inhibits TGF-β1 signaling by binding directly to Smurfs, bringing the Smurf/Smad7 complex to TβRI, and inducing the turnover of TβRI. (C) Jab1/CSN5 enhances TGF-β signaling by binding to Smad7 and inducing degradation of Smad7 through the COP9-proteosome pathway.
Recent studies suggest that Smad7 might serve more complicated functions in the cell rather than acting simply as an inhibitor of TGF-β signaling. Smad7 also inhibits activin and BMP signals (8). Moreover, ectopic expression of Smad7 has been shown to induce apoptosis (31, 32), and TGF-β-induced apoptosis in mesangial cells is mediated through the activation of caspase 3 by Smad7 but not by Smad2 or Smad3 (41). However, others have demonstrated that overexpression of Smad7 inhibits TGF-β1-dependent apoptosis (55). Recently, Pulaski et al. (43) reported that inhibitory Smad7 could also act as a transcriptional regulator, extending this role for the first time to inhibitory Smads. These observations suggest that Smad7 is involved not only in control of signaling pathways of TGF-β family members but may also have direct effects in the nucleus, possibly regulated by other regulatory pathway(s). Jab1/CSN5-mediated degradation of Smad7 might also regulate Smad7 function in apoptosis and transcription.
HECT type E3 ubiquitin ligases, Smurf1 and Smurf2, have been reported to interact with Smad7 to gain more efficient interaction with the TGF-β receptor in order to mediate degradation (16, 26, 47). Smad7 functions as an adaptor protein to recruit Smurf to the activated receptor complex. Smad7-Smurf complex formation induces translocation of this complex from the nucleus to the plasma membrane and then induces ubiquitination and degradation of the TGF-β receptors (26). It has been shown that nuclear export of Smad7 depends on Smurf1. Smad7 was exported to the cytoplasm only in cells cotransfected with Smurf1, and only Smurf1, but not Smad7, has a functional nuclear export signal (48). In the present study, Smad7 was predominantly detected in the cytoplasm in the presence of Jab1/CSN5, suggesting that Jab1/CSN5 induces nuclear export and subsequent degradation of Smad7. In addition, Jab1/CSN5 is the direct target for the CRM1-dependent nuclear export receptor, which contains a typical leucine-rich nuclear export signal sequence (50), suggesting that transportation of Smad7 from the nucleus to the cytoplasm may be mediated by CRM1.
Jab1/CSN5 is known to regulate protein stability. Jab1/CSN5 binding to c-Jun stabilizes complexes of c-Jun with AP-1 sites, increasing the specificity of target gene activation by AP-1 proteins. Jab1/CSN5 also interacts with HIF-1α and regulates its stability, leading to HIF-1-dependent transcriptional activation (3). On the other hand, Jab1/CSN5 appears to present other proteins to the proteosome for degradation. Jab1/CSN5 directly interacts with p27Kip1 in the nucleus, exports it to the cytoplasm, and facilitates its degradation in a 26S proteosome-dependent manner (49). However, the biochemical functions and regulatory mechanisms of Jab1/CSN5 are largely unknown. A recent report has shown that Jab1/CSN5 also interacts with Smad4 and induces its degradation, resulting in the inhibition of TGF-β signaling (52). However, when HepG2 cells were transfected with Jab1 siRNA, expression of endogenous Smad4 was not increased (unpublished observation). In the present study, we have shown that Jab1/CSN5 binds to Smad7 and promotes its degradation, leading to activation of TGF-β signaling. Whether Jab1/CSN5 degrades either Smad4 or Smad7 might be indicative of the cell's overall status in the TGF-β signaling pathway. However, the upstream signaling machinery needs to be identified to see how Jab1/CSN5 differentially regulates Smad4 and Smad7.
The amino acid sequences which are required for binding to Jab1/CSN5 in the p27 molecule have been identified. These amino acid sequences are also present in other Jab1/CSN5-interacting proteins, such as c-Jun and the LFA-1 β2 subunit (50). Careful examination of the amino acid sequences of Smad4 and Smad7 revealed that Smad7 contains conserved amino acids at positions Asp-133 and Leu-155 and Asp-224 and Leu-246, whereas these amino acids are not conserved in the Smad4 amino acid sequences. However, these conserved amino acid sequences in Smad7 are not involved in the interaction of Smad7 with Jab1/CSN5 (Fig. 2C), suggesting that Jab1/CSN5 associates with either Smad7 or Smad4 through a region that is distinct from the one found in p27.
Our present study defines a novel intracellular mechanism that can function to reactivate TGF-β signaling that has been suppressed by Smad7. Upon activation by TGF-β receptors, R-Smads (Smad2 and Smad3) form a heteromeric complex with Smad4 and translocate into the nucleus, where they regulate transcription of target genes, including Smad7. After initial signal transduction, TGF-β signaling will be terminated by multiple ubiquitin-proteosome pathways controlling degradation of R-Smads, degradation of TGF-β receptors by Smurf2 via Smad7, and degradation of Smad4 by Jab1/CSN5. To ensure a subsequent round of signaling, a Jab1/CSN5-mediated degradation mechanism is activated to eliminate the antagonist of TGF-β signaling, Smad7. This is a unique mechanism to reactivate TGF-β signaling upon its suppression by the inhibitory Smad7, which could provide for precision in the control of TGF-β signaling not afforded by secreted ligands in an autocrine manner.
In numerous tumors, resistance to TGF-β is associated with inactivating mutations that can occur either in the receptors or in signaling intermediates, such as Smad4 and Smad2, and transcriptional repression of TGF-β receptors in an epigenetic mechanism (17, 20, 27, 34). Increased expression of Smad7 was observed in human pancreatic cancer, and amplified expression of Smad7 enhances tumorigenicity in pancreatic cancer (28), which may be due to the activation of Smad7 transcription. However, we cannot exclude the possibility that inefficient clearance of Smad7 protein by the ubiquitin-proteosome pathway may also contribute to enhanced tumorigenicity, as suggested in this study. Thus, clearly, it will be important in the future to elucidate how the degradation of Smad7 by Jab1/CSN5 is regulated under physiological and pathological conditions.
Acknowledgments
We thank S. Kern, J. Wrana, K. Miyazono, S. Souchelnytskyi, H. Zhu, and J. Massague for reagents and A. Roberts for the critical reading of the manuscript and advice. We also thank H. van Gelder for preparation of the manuscript.
REFERENCE
- 1.Afrakhte, M., A. Moren, S. Jossan, S. Itoh, K. Sampath, B. Westermark, C.-H. Heldin, N. E. Heldin, and P. ten Dijke. 1998. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem. Biophys. Res. Commun. 249:505-511. [DOI] [PubMed] [Google Scholar]
- 2.Attisano, L., and J. L. Wrana. 2000. Smads as transcriptional co-modulators. Curr. Opin. Cell Biol. 12:235-243. [DOI] [PubMed] [Google Scholar]
- 3.Bae, M. K., M. Y. Ahn, J. W. Jeong, M. H. Bae, Y. M. Lee, S. K. Bae, J. W. Park, K. R. Kim, and K. W. Kim. 2002. Jab1 interacts directly with HIF-1α and regulates its stability. J. Biol. Chem. 277:9-12. [DOI] [PubMed] [Google Scholar]
- 4.Bech-Otschir, D., M. Seeger, and W. Dubiel. 2002. The COP9 signalosome: at the interface between signal transduction and ubiquitin-dependent proteolysis. J. Cell Sci. 115:467-473. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi, E., S. Denti, A. Granata, G. Bossi, J. Geginat, A. Villa, L. Rogge, and R. Pardi. 2000. Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate AP-1 activity. Nature 404:617-621. [DOI] [PubMed] [Google Scholar]
- 6.Bitzer, M., G. von Gersdorff, D. Liang, A. Dominguez-Rosales, A. A. Beg, M. Rojkind, and E. P. Böttinger. 2000. A mechanism of suppression of TGF-β/SMAD signaling by NFκB/RelA. Genes Dev. 14:187-197. [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifacino, J. S., and A. M. Weissman. 1998. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Biol. 14:19-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casellas, R., and A. H. Brivanlou. 1998. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev. Biol. 198:1-12. [DOI] [PubMed] [Google Scholar]
- 9.Chauchereau, A., M. Georgiakaki, M. Perrin-Wolff, E. Milgrom, and H. Loosfelt. 2000. JAB1 interacts with both the progesterone receptor and SRC-1. J. Biol. Chem. 275:8540-8548. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H. I., and M. Sudol. 1995. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc. Natl. Acad. Sci. USA 9 2:7819-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claret, F. X., M. Hibi, S. Dhut, T. Toda, and M. Karin. 1996. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature 383:453-457. [DOI] [PubMed] [Google Scholar]
- 12.Dechend, R., F. Hirano, K. Lehman, V. Heissmeyer, S. Ansieau, F. G. Wulczyn, C. Scheidereit, and A. Leutz. 1999. The Bcl-3 oncoprotein acts as a bridging factor between NF-κB/Rel and nuclear co-regulators. Oncogene 18:3316-3323. [DOI] [PubMed] [Google Scholar]
- 13.Deng, X. W., W. Dubiel, N. Wei, K. Hofmann, and K. Mundt. 2000. Unified nomenclature for the COP9 signalosome and its subunits: an essential regulator of development. Trends Genet. 16:202-203. [DOI] [PubMed] [Google Scholar]
- 14.Derynck, R., Y. Zhang, and X.-H. Feng. 1998. Smads: transcriptional activators of TGF-β responses. Cell 95:737-740. [DOI] [PubMed] [Google Scholar]
- 15.Deshaies, R. J. 1999. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15:435-467. [DOI] [PubMed] [Google Scholar]
- 16.Ebisawa, T., M. Fukuchi, G. Murakami, T. Chiba, K. Tanaka, T. Imamura, and K. Miyazono. 2001. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 276:12477-12480. [DOI] [PubMed] [Google Scholar]
- 17.Eppert, K., S. W. Scherer, H. Ozcelik, R. Pirone, P. Hoodless, H. Kim, L. C. Tsui, B. Bapat, S. Gallinger, I. L. Andrulis, G. H. Thomsen, J. L. Wrana, and L. Attisano. 1996. MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 86:543-552. [DOI] [PubMed] [Google Scholar]
- 18.Fujii, M., K. T. Takeda, H. Imamura, T. K. Aoki, S. Sampath, M. Enomoto, M. Kawabata, H. Kato, H. Ichijo, and K. Miyazono. 1999. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol. Biol. Cell 10:3801-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glickman, M. H., D. M. Rubin, O. Coux, I. Wefes, G. Pfeifer, Z. Cjeka, W. Baumeister, V. A. Fried, and D. Finley. 1998. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94:615-623. [DOI] [PubMed] [Google Scholar]
- 20.Hahn, S. A., M. Schutte, A. T. Hoque, C. A. Moskaluk, L. T. da Costa, E. Rozenblum, C. L. Weinstein, A. Fischer, C. J. Yeo, R. H. Hruban, and S. E. Kern. 1996. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271:350-353. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi, H., S. Abdollah, Y. Qiu, J. Cai, Y.-Y. Xu, B. W. Grinnell, M. A. Richardson, J. N. Topper, M. A. Gimbrone, Jr., J. L. Wrana, and D. Falb. 1997. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell 89:1165-1173. [DOI] [PubMed] [Google Scholar]
- 22.Heldin, C.-H., K. Miyazono, and P. ten Dijke. 1997. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465-471. [DOI] [PubMed] [Google Scholar]
- 23.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 24.Imamura, T., M. Takase, A. Nishihara, E. Oeda, J.-I. Hanai, M. Kawabata, and K. Miyazono. 1997. Smad6 inhibits signaling by the TGF-β superfamily. Nature 389:622-626. [DOI] [PubMed] [Google Scholar]
- 25.Itoh, S., M. Landstrom, A. Hermansson, F. Itoh, C.-H. Heldin, N.-E. Heldin, and P. ten Dijke. 1998. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J. Biol. Chem. 273:29195-29201. [DOI] [PubMed] [Google Scholar]
- 26.Kavsak, P., R. K. Rasmussen, C. G. Caysing, S. Bonni, H. Zhu, G. H. Thomsen, and J. L. Wrana. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-β receptor for degradation. Mol. Cell 6:1365-1375. [DOI] [PubMed] [Google Scholar]
- 27.Kim, S.-J., Y.-H. Im, S. D. Markowitz, and Y.-J. Bang. 2000. Molecular mechanisms of inactivation of TGF-β receptors during carcinogenesis. Cytokine Growth Factor Rev. 11:159-168. [DOI] [PubMed] [Google Scholar]
- 28.Kleeff, J., T. Ishiwata, H. Maruyama, H. Friess, P. Truong, M. W. Buchler, D. Falb, and M. Korc. 1999. The TGF-β signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene 18:5363-5372. [DOI] [PubMed] [Google Scholar]
- 29.Kleemann, R., A. Hausser, G. Geiger, R. Mischke, A. Burger-Kentischer, O. Flieger, F. J. Johannes, T. Roger, T. Calandra, A. Kapurniotu, M. Grell, D. Finkelmeier, H. Brunner, and J. Bernhagen. 2000. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 408:211-216. [DOI] [PubMed] [Google Scholar]
- 30.Kwok, S. F., R. Solano, T. Tsuge, D. A. Chamovitz, J. R. Ecker, M. Matsui, and X. W. Deng. 1998. Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex and their abundance is differentially affected by the pleiotropic cop/det/fus mutations. Plant Cell 10:1779-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lallemand, F., A. Mazars, C. Prunier, F. Bertrand, M. Kornprost, S. Gallea, S. Roman-Roman, G. Cherqui, and A. Atfi. 2001. Smad7 inhibits the survival nuclear factor κB and potentiates apoptosis in epithelial cells. Oncogene 20:879-884. [DOI] [PubMed] [Google Scholar]
- 32.Landstrom, M., N. E. Heldin, S. Bu, A. Hermansson, S. Itoh, P. ten Dijke, and C. H. Heldin. 2000. Smad7 mediates apoptosis induced by transforming growth factor β in prostatic carcinoma cells. Curr. Biol. 10:535-538. [DOI] [PubMed] [Google Scholar]
- 33.Lin, X., M. Liang, and X.-H. Feng. 2000. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-β signaling. J. Biol. Chem. 275:36818-36822. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz, S. D., and A. B. Roberts. 1996. Tumor suppressor activity of the TGF-β pathway in human cancers. Cytokine Growth Factor Rev. 7:93-102. [DOI] [PubMed] [Google Scholar]
- 35.Massagué, J., and Y. G. Chen. 2000. Controlling TGF-β signaling. Genes Dev. 14:627-644. [PubMed] [Google Scholar]
- 36.Massagué, J., and D. Wotton. 2000. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 19:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McManus, M. T., and P. A. Sharp. 2002. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 3:737-747. [DOI] [PubMed] [Google Scholar]
- 38.Miyazono, K. 2000. Positive and negative regulation of TGF-β signaling. J. Cell Sci. 113:1101-1109. [DOI] [PubMed] [Google Scholar]
- 39.Mizushima, S., and S. Nagata. 1990. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 18:5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakao, A., M. Afrakhte, A. Moren, T. Nakayama, J. L. Christian, R. Heuchel, S. Itoh, M. Kawabata, N.-E. Heldin, C.-H. Heldin, and P. ten Dijke. 1997. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signalling. Nature 389:631-635. [DOI] [PubMed] [Google Scholar]
- 41.Okado, T., Y. Terada, H. Tanaka, S. Inoshita, A. Nakao, and S. Sasaki. 2002. Smad7 mediates transforming growth factor-β-induced apoptosis in mesangial cells. Kidney Int. 62:1178-1186. [DOI] [PubMed] [Google Scholar]
- 42.Osterlund, M. T., C. S. Hardtke, N. Wei, and X. W. Deng. 2000. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462-466. [DOI] [PubMed] [Google Scholar]
- 43.Pulaski, L., M. Landstrom, C. H. Heldin, and S. Souchelnytskyi. 2001. Phosphorylation of Smad7 at Ser-249 does not interfere with its inhibitory role in transforming growth factor-β-dependent signaling but affects Smad7-dependent transcriptional activation. J. Biol. Chem. 276:14344-14349. [DOI] [PubMed] [Google Scholar]
- 44.Roberts, A. B., and M. B. Sporn. 1990. The transforming growth factor-βs, 419-472. In M. B. Sporn and A. B. Roberts (ed.), Peptide growth factors and their receptors, part I. Springer-Verlag, Heidelberg, Germany.
- 45.Schwechheimer, C., and X. W. Deng. 2000. The COP/DET/FUS proteins—regulators of eukaryotic growth and development. Semin. Cell Dev. Biol. 11:495-503. [DOI] [PubMed] [Google Scholar]
- 46.Souchelnytskyi, S., T. Nakayama, A. Nakao, A. Morén, C.-H. Heldin, J. L. Christian, and P. ten Dijke. 1998. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-β receptors. J. Biol. Chem. 273:25364-25370. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, C., G. Murakami, M. Fukuchi, T. Shimanuki, Y. Shikauchi, T. Imamura, and K. Miyazono. 2002. Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. J. Biol. Chem. 277:39919-39925. [DOI] [PubMed] [Google Scholar]
- 48.Tajima, Y., K. Goto, M. Yoshida, K. Shinomiya, T. Sekimoto, Y. Yoneda, and K. Miyazono. 2003. Chromosomal region maintenance 1 (CRM1)-dependent nuclear export of Smad ubiquitin regulatory factor 1 (Smurf1) is essential for negative regulation of transforming growth factor-β signaling by Smad7. J. Biol. Chem. 278:10716-10721. [DOI] [PubMed] [Google Scholar]
- 49.Tomoda, K., Y. Kubota, and J.-J. Kato. 1999. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398:160-165. [DOI] [PubMed] [Google Scholar]
- 50.Tomoda, K., Y. Kubota, Y. Arata, S. Mori, M. Maeda, T. Tanaka, M. Yoshida, N. Yoneda-Kato, and J.-J. Kato. 2002. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J. Biol. Chem. 277:2302-2310. [DOI] [PubMed] [Google Scholar]
- 51.Ulloa, L., J. Doody, and J. Massagué. 1999. Inhibition of transforming growth factor-β/SMAD signalling by the interferon-γ/STAT pathway. Nature 397:710-713. [DOI] [PubMed] [Google Scholar]
- 52.Wan, M., X. Cao, S. Bai, L. Wu, X. Shi, N. Wang, and X. Cao. 2002. Jab1 antagonizes TGF-β signaling by inducing Smad4 degradation. EMBO Rep. 3:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei, N., and X. W. Deng. 1999. Making sense of the COP9 signalosome. A regulatory protein complex conserved from Arabidopsis to human. Trends Genet. 15:98-103. [DOI] [PubMed] [Google Scholar]
- 54.Wrana, J. L., L. Attisano, J. Carcamo, A. Zentella, J. Doody, M. Laiho, X.-F. Wang, and J. Massagué. 1992. TGF-β signals through a heteromeric protein kinase receptor complex. Cell 71:1003-1014. [DOI] [PubMed] [Google Scholar]
- 55.Yamamura, Y., X. Hua, S. Bergelson, and H. F. Lodish. 2000. Critical role of Smads and AP-1 complex in transforming growth factor-β-dependent apoptosis. J. Biol. Chem. 275:36295-36302. [DOI] [PubMed] [Google Scholar]
- 56.Yang, H.-Y., B. P. Zhou, M.-C. Hung, and M.-H. Lee. 2000. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J. Biol. Chem. 275:24735-24739. [DOI] [PubMed] [Google Scholar]
- 57.Zawel, L., J. L. Dai, P. Buckhaults, S. Zhou, K. W. Kinzler, B. Vogelstein, and S. E. Kern. 1998. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell 1:611-617. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, H., P. Kavsak, S. Abdollah, J. L. Wrana, and G. H. Thomsen. 1999. ASMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400:687-693. [DOI] [PubMed] [Google Scholar]
- 59.Zhu, H.-J., J. Iaria, and A. M. Sizeland. 1999. Smad7 differentially regulates transforming growth factor β-signaling pathways. J. Biol. Chem. 274:32258-32264. [DOI] [PubMed] [Google Scholar]