Abstract
Introduction
While rheumatoid arthritis (RA) medications may affect survival in RA, few studies consider the propensity for medication use, which may reflect selection bias in treatment allocation in survival models. We examined the relationship between methotrexate use and mortality in RA, after controlling for individual propensity scores for methotrexate use.
Methods
We studied 5626 patients with RA prospectively for 25 years to determine the hazard for death associated with methotrexate use, modeled in time-varying Cox regression models. We used the random forest method to generate individual propensity scores for methotrexate use at study entry and during follow-up in a time-varying fashion; these scores were included in the multivariate model. We also examined if selective discontinuation of methotrexate immediately prior to death altered the hazard for mortality, and examined the association of duration of methotrexate use with survival.
Results
During follow-up 666 patients (12%) died. Methotrexate use was associated with reduced risk of death (adjusted hazard ratio 0.30; 95% confidence interval 0.09, 1.03). Selective methotrexate cessation immediately before death did not account for the protective association of methotrexate use with mortality. Only methotrexate use longer than one year was associated with lower risks of mortality, but associations were not stronger with longer durations of use.
Discussion
Methotrexate use was associated with a 70% reduction in mortality risk in RA.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease associated with reduced survival, with standardized mortality ratios ranging from 1.3 to 3.0 (1–6). In one population-based cohort, survival had not improved over a four decade span, despite new treatment options and changes in patterns of RA medication prescribing practices (7).
Medications used to treat RA may influence survival. In one study, methotrexate (MTX) use was reported to reduce the risk of all-cause mortality by 60% and death from cardiovascular disease by 70% (8). Others have shown that patients who had a therapeutic response to MTX had better survival than those who did not (8,9). Factors such as disease severity and comorbidities influence medication selection in RA. This propensity for selecting one medication over another is especially important to consider when determining the relationship between medication use and outcomes, as patients with more severe RA but less comorbidity might have been more likely to be treated with MTX, particularly in the years shortly after its adoption. MTX use has expanded over time, and is now prescribed across the full spectrum of disease severity and at doses higher than commonly used twenty years ago. This raises the question of whether previous results underestimate the protective association of MTX as currently used.
Protective associations with medication use are important to identify, but may be spurious, particularly for associations with serious health outcomes such as mortality, due to a commonly-overlooked bias. Medication use may be altered in the setting of escalating comorbidities and imminent death. If medications for RA are selectively discontinued in the months prior to death, medication use will appear protective, as deaths are enriched in the subgroup of patients who have stopped the medication (10). Ongoing medication use in this situation is a surrogate for relative wellness.
We sought to determine the relationship between the use of MTX and mortality in patients with RA, with attention to two issues not previously emphasized: risk-adjustment for the propensity for initiating and continuing MTX, and assessment of possible bias in the association due to selective discontinuation of MTX immediately prior to death. The propensity adjustment was necessitated by the vast changes in MTX use and prescribing patterns over the 25 years of this study. We also explored the relationship between cumulative duration of MTX use and mortality to determine whether longterm use was associated with survival.
Methods
Study design and enrollment
Patients with RA from ten North American rheumatology practices were recruited to participate in the Arthritis, Rheumatism, and Aging Medical Information Systems study between 1981 and January, 2005. Seven university centers and three community practice sites participated. The purpose of this prospective observational study was to assess longitudinal changes in the treatment, costs, and outcomes of patients with RA. To be eligible, patients needed to be age 18 or older and to fulfill the 1987 American College of Rheumatology criteria for RA (11). The diagnosis was verified by study rheumatologists at each site or, in 6% of cases, by review of outside medical records. The study established an open cohort, with participant enrollment and drop-out occurring over 25 years.
Participants were asked to complete a mailed questionnaire biannually that asked about sociodemographic characteristics, health status, including the Health Assessment Questionnaire (HAQ) Disability Index and a pain visual analog scale, comorbidities, medication use, physician visits, and other health care utilization (12,13). Participants were followed from study entry to death, withdrawal from the study, or to July 31, 2006. Death was ascertained by communication with next-of-kin or by searching the National Death Index. Patients who died more than 1 year after returning their last questionnaire were treated as censored rather than dead, because information on their MTX use was not available through the time of death. Patients who were missing follow-up data for more than 12 months (3 or more consecutive questionnaire cycles) were censored from the time-varying analyses during the time data were not available. If data were available subsequently, the patient was then re-established as a subject with left-truncated data. The outcome was all-cause mortality.
Statistical methods
Because patients may begin or discontinue medications over time, the association between MTX use and mortality was estimated using Cox regression models with time-varying covariates. In these models, information on the use of individual medications was updated with each 6-month questionnaire cycle. We also used the time-varying model to adjust the association between MTX and mortality for potential confounding factors. The following variables were included in the multivariate models as time-invariant covariates: age at study entry, gender, ethnicity, education level, duration of RA at entry, and calendar year at entry. We also included the following time-varying covariates in each model, updating the status of the variable every 6 months with new data obtained with each new questionnaire: body-mass index (BMI), pain score, HAQ Disability Index, presence of each of 10 comorbid conditions during the preceding 6 months, presence of a visit to a rheumatologist in the preceding 6 months, use of disease-modifying antirheumatic drugs (DMARDs) other than MTX, anti-TNF medications, use of cyclooxygenase-2 (COX-2) selective nonsteroidal anti-inflammatory drugs (NSAIDs), and use of non-selective NSAIDs. The 10 comorbid conditions were hypertension, coronary artery disease, congestive heart failure, stroke, lung disease, diabetes, cancer, gastrointestinal disease, liver disease, and infections. Because the associations of BMI and HAQ Disability Index with death were not linear over time, we modeled BMI and the HAQ Disability Index using restricted cubic splines to capture the non-linear variations in the strengths of associations of these variables with mortality (14). Models were stratified by study site and year of study entry to account for variation among patients enrolled at different centers and potential cohort effects.
Propensity scores
Because patients selected to receive a particular medication likely have different clinical and demographic characteristics than those who do not receive that medication, adjustment for selection bias in treatment allocation is also needed for proper comparison of outcomes between medication groups. The goal of this adjustment is to make the treatment groups as comparable as possible in factors associated with receipt of medication so that the comparisons approximate those of a randomized allocation of medication. A common method to do this is to compute propensity scores and use these as model covariates. A propensity score is a predictor, ranging in value from 0 to 1, that estimates the probability that an individual patient should have received a particular medication, based on how similar his or her clinical and demographic characteristics are to the characteristics of other patients in the cohort who did receive the medication.
We used random forests to compute propensity scores in this study (see Supplemental data) (15). Random forests are scalable and accurate for prediction problems in a wide variety of contexts. They have been recommended as a means for performing more robust, model-free propensity estimation (16). In this analysis we used all demographic and clinical characteristics as potential predictors; parsimony in variable selection does not aid prediction in random forests. Each random forest was based on 500 classification trees.
Two separate propensity scores were calculated for likelihood of treatment with MTX. Because some patients entered the study on MTX, a propensity score based on baseline variables was computed to capture the likelihood of entering the study on the medication. To capture variations in prescribing of MTX during the time of observation, a second, time-varying propensity score was computed using data on variables obtained during the follow-up. This was particularly important because the characteristics of the patients who were prescribed MTX, for example, in 1990 were different from those who received it in 2002, based on changing patterns of use. The time-varying propensity scores were updated every 6 months with new information from each questionnaire cycle. Models estimating MTX associations with mortality included both MTX propensity scores.
Simulation analysis
One potential explanation for an apparent protective association between MTX use and survival is that MTX is selectively discontinued as patients approach the end of life due to the development or progression of illness or serious comorbidities, thus becoming nonusers immediately prior to death. An observed protective effect may then be attributed not to beneficial effects of the medication but to the fact that relatively healthier patients were more likely to continue on the drug. We assessed this possibility directly using a simulation approach. The simulation tested the association between MTX use and mortality under the assumption that rates and patterns of discontinuation of MTX shortly before death were not different from the rates and patterns of discontinuation of MTX among those who did not die. We then compared the observed result with the distribution of results from the simulations to test how likely it would be that the observed result was plausible, given no differential drop-off in MTX use prior to death.
First, among all patients who did not die (i.e. were censored) and who reported MTX use in their penultimate questionnaire, we fitted a Random Forest model to predict their use of MTX at their last questionnaire, given clinical and demographic information at their penultimate questionnaire. Next, we used this fitted model to predict the probability of MTX use at the last questionnaire for the patients who subsequently died and who reported MTX use at their penultimate questionnaire. This procedure generalized the patterns of MTX use among the censored patients at the end of their observation time to the patients who died. We then used these predicted probabilities to simulate MTX use (present or absent) at the last questionnaire for each patient with MTX use at their penultimate questionnaire who subsequently died. We repeated this simulation 5000 times. For each simulated data set, we estimated our previous multivariate Cox model, which provided 5000 estimates of the hazard ratio of the MTX association with mortality. The simulation thus provided a sampling distribution of the hazard ratio for MTX under the condition that the pattern of MTX use at the end of observation was not biased between dead and censored patients. We then compared the distribution of these estimates to that obtained from our original data to determine the likelihood that the observed association was influenced by differential discontinuation of MTX prior to death.
Duration of MTX use and mortality
To determine whether there were survival differences based on how long patients received methotrexate, we examined associations between cumulative duration of MTX use and mortality. Since there were temporal gaps in observed data for some patients, we imputed MTX use for the missing intervals. We assumed that if a patient was using MTX both before and after a missing time interval, then they were using it during the interval as well. Otherwise, we chose the conservative approach of assuming the patient was not using MTX in the missing interval. Thus, for each patient we updated the cumulative duration of MTX use from study entry at each 6-month questionnaire, and used this as a time-dependent covariate in the multivariate Cox regression analysis. We categorized the cumulative exposure into 5 categories: 0 years, 0.1–1 year, 1.1–2 years, 2.1–5 years and >5 years.
Missing data
There were no missing data on medication use at baseline, but there were missing data on some clinical and demographic variables at baseline, ranging from 1 to 6%. Since our outcome was overall survival, we fitted a survival Random Forest model to the available data and imputed missing data based on the fitted model (17). Missing data during follow-up were imputed using last value carried forward (LVCF) or taking into account time (e.g. for age) for a maximum of 12 months (2 questionnaire cycles).
Results
Patient characteristics and follow-up
We enrolled 5626 patients with RA. Patient characteristics at study entry are shown in Table 1. Patients were primarily middle-aged, female, and white, with a median duration of RA of 7.1 years at study entry. The median follow-up was 4.2 years (interquartile range [IQR] range 1.7 – 9.6 years). During 40,722 patient-years of observation, 666 patients (12%) died.
Table 1.
Characteristics of Study Participants by MTX Use
| Overall (N=5626) | Ever MTX* (N=2920) | Never MTX** (N=2706) | |
|---|---|---|---|
| Age (y), mean ± SD | 57.1±13.8 | 57.2±13.4 | 59.3±14.0 |
| Gender | |||
| Women | 75% (4239) | 78% (2288) | 72% (1951) |
| Men | 25% (1387) | 22% (632) | 28% (755) |
| Ethnicity | |||
| White | 90% (5045) | 90% (2621) | 90% (2424) |
| Non-white | 10% (581) | 10% (299) | 10% (282) |
| Education level (y), mean ± SD | 12.70±2.65 | 12.95±2.48 | 12.4±2.8 |
| RA duration at study entry (y), mean ± SD | 10.58±10.26 | 9.43±9.27 | 11.8±11.1 |
| Year of study entry, median (IQR) | 1992 (1984,1997) | 1994 (1987,1997) | 1989 (1983, 1997) |
| BMI, mean ± SD | 26.51±5.25 | 26.73±5.67 | 26.31±4.89 |
| Rheumatology consult during obs. | 70% (3921) | 76% (2223) | 64% (1727) |
| HAQ score (0–3), mean ± SD | 1.136±0.782 | 1.228±0.765 | 1.137±0.814 |
| Pain score (0–3), mean ± SD | 1.252±0.785 | 1.278±0.778 | 1.234±0.792 |
| Follow-up time (mos.), mean ± SD | 81.4±71.1 | 93.2±73.9 | 69.0±65.6 |
| Hypertension | 20% (1119) | 26% (770) | 20% (534) |
| Coronary artery disease | 4% (198) | 4% (129) | 4% (114) |
| Lung disease | 10% (590) | 18% (520) | 10% (264) |
| Intestinal disease | 15% (830) | 22% (647) | 14% (376) |
| Liver disease | 1% (48) | 1% (26) | 1% (26) |
| Diabetes mellitus | 4% (227) | 5% (152) | 4% (107) |
| Cancer | 4% (204) | 7% (190) | 3% (87) |
| Congestive heart failure | 1% (44) | 2% (51) | 1% (21) |
| Stroke | 1% (56) | 1% (40) | 1% (27) |
| Infections | 10% (544) | 11% (329) | 8% (215) |
| No. of comorbidities, mean ± SD | 1.13±1.39 | 1.55±1.54 | 1.06±1.41 |
| On prednisone | 40% (2245) | 53% (1556) | 32% (875) |
| On TNF inhibitors | 2% (105) | 3% (92) | 1% (36) |
| On COX-2 inhibitors | 2% (139) | 5% (144) | 2% (49) |
| On NSAIDs | 64% (3604) | 67% (1968) | 60% (1622) |
| On non-MTX DMARDs | 43% (2399) | 39% (1145) | 41% (1116) |
Data from first phase these subjects report being on MTX
Data from phase of entry of each subject never on MTX
The prevalence of MTX use increased over the study period, reaching a plateau of 45%–50% in the late 1990’s. Among all patients, 2920 patients (52%) used MTX at some time during the study, and 28% of these patients entered the study on MTX. Forty-seven percent of patients who were treated with MTX were also taking prednisone at the time of initiation of MTX therapy. Among MTX users, the median duration of use during the study period was 2.5 years (IQR 1 year, 5.5 years). Median year of entry for all patients, MTX users and MTX never users was 1992, 1994 and 1989 respectively The duration of RA at study entry was shorter for ever users of MTX (median 6.7 years) than for those who never used MTX (median 9.0 years).
In general, MTX users were had a greater number of indicators of ill health than nonusers. They were more likely to see a rheumatologist during observation, had higher mean HAQ scores at entry, and were more likely treated with prednisone at entry (See Table 1). In addition, MTX users were more likely to have several comorbidities and, when comorbidities were totaled for individual patients, had on average 50% more comorbidities than the MTX never users.
The propensity score for MTX use at baseline was highly accurate. The correlation between observed MTX use and MTX use as predicted by the random forest model was 0.99 (see Supplemental Figure 1).
Associations of MTX use with mortality
In the time-varying univariate Cox regression model, MTX use was strongly associated with a reduction in risk of death (hazard ratio [HR] 0.48, 95% confidence interval [CI] 0.40, 0.59). Given the large variation in patterns of MTX use over the study period, a time-varying multivariate Cox regression model that included propensity score adjustments was generated to enable a fair comparison of the MTX effect across the long duration of this study. In this model, MTX use was associated with a 70% reduction in mortality (HR 0.30, 95% CI 0.09, 1.03) (Table 2). Older age, male gender, greater BMI, HAQ Disability Index, and prednisone use were also associated with poorer survival, as were hypertension, lung disease, diabetes, cancer, and congestive heart failure. Results were similar when this multivariate survival analysis was performed in the subset of patients who were not on MTX at study entry (i.e. examination of only incident MTX users; HR 0.42, 95% CI 0.31, 0.57).
Table 2.
Association of methotrexate use with mortality, by multivariate Cox regression model with time-dependent covariates, adjusted for both baseline and time-dependent methotrexate propensity scores.
| Hazard Ratio | 95% Conf Interval | P value | |
|---|---|---|---|
| Age at study entry | 1.06 | (1.05,1.07) | <0.001 |
| Education level | 1.00 | (0.97,1.03) | 0.94 |
| Gender (M vs F) | 2.57 | (2.15,3.08) | <0.001 |
| Ethnicity (Non-white vs white) | 0.73 | (0.49,1.09) | 0.12 |
| Disease duration at study entry | 1.00 | (0.99,1.00) | 0.33 |
| BMI | * | * | <0.001 |
| Nonlinear | * | * | <0.001 |
| HAQ score | * | * | <0.001 |
| Nonlinear | * | * | 0.003 |
| Pain score | 0.97 | (0.86,1.09) | 0.56 |
| Rheumatology consult | 0.94 | (0.79,1.13) | 0.54 |
| MTX | 0.30 | (0.09,1.03) | 0.06 |
| Time-dependent MTX propensity | 2.91 | (0.58,14.56) | 0.19 |
| Prednisone | 1.72 | (1.44,2.05) | <0.001 |
| NSAIDs | 0.91 | (0.77,1.08) | 0.28 |
| Non-MTX DMARDs | 0.93 | (0.77,1.12) | 0.45 |
| TNF inhibitors | 0.61 | (0.26,1.45) | 0.26 |
| COX2 inhibitors | 1.11 | (0.673,1.84) | 0.68 |
| Hypertension | 1.21 | (1.00,1.45) | 0.05 |
| Coronary disease | 1.03 | (0.80,1.32) | 0.82 |
| Lung disease | 1.43 | (1.18,1.72) | <0.001 |
| Intestinal disease | 1.00 | (0.83,1.20) | 0.97 |
| Liver disease | 1.29 | (0.67,2.48) | 0.45 |
| Diabetes mellitus | 1.29 | (0.98,1.71) | 0.07 |
| Cancer | 1.39 | (1.10,1.75) | 0.005 |
| Congestive heart failure | 2.31 | (1.74,3.06) | <0.001 |
| Stroke | 1.16 | (0.75,1.81) | 0.51 |
| Infections | 0.65 | (0.53,0.80) | <0.001 |
| MTX propensity at study entry | 0.67 | (0.42,1.08) | 0.10 |
BMI and HAQ scores are both modeled nonlinearly as restricted cubic splines, and as such have no single hazard ratio estimate (see Supplemental figure 3). Their p-values are based on a Chi-square test with 4 d.f., and the test for nonlinearity is a Chi-square test with 3 d.f.
Because some clinical factors may predict both MTX use and survival (e.g. functional limitations), we repeated the analysis using covariate values of the previous questionnaire cycle (6 months earlier), rather than contemporaneous values, for computing propensity scores, with similar results (HR 0.32, 95% CI 0.19, 0.56). In another sensitivity analysis, we used the inverse probability of treatment weight method to create treatment groups with similar background characteristics for comparison (18). In this model, MTX use remained associated with improved survival, although the strength of association was attenuated somewhat (HR 0.65, 95% CI 0.52, 0.79; P <.0001).
The prevalence of MTX use varied over the course of the study. To determine whether the association of MTX with mortality also varied over the study duration, we split the dataset at the point when roughly half of the deaths had occurred, i.e. at April 1991, when 60% of deaths had occurred, producing two study groups based on observation periods: one from study initiation (July 1981) to April 1991, defined as the “early period”, and the other from May 1991 to study conclusion (December, 2006), the “late period”. In multivariate propensity-adjusted analyses, MTX had no association with mortality in the “early period” study (HR 2.099, 95% CI 0.24, 18.33). In the “late period” study, MTX use had a strongly protective association with mortality (HR 0.07, 95% CI 0.009, 0.569).
Because the protective association of MTX may in part be due to the fact that MTX is discontinued in the months prior to death, as comorbidities develop, we tested this possibility using a simulation study previously described (see Methods). Using this approach, the median HR for the association of MTX with survival was 0.28 (IQR 0.19–44), in the simulation of no differential discontinuation in MTX use prior to death. The observed HR estimate of 0.30 was not significantly different from the simulated distribution of results under the assumption of no differential discontinuation of MTX shortly before death (p = .55 one-tailed), indicating that differential discontinuation of MTX was unlikely to account for the protective association. (See Supplemental Data.)
Associations of cumulative MTX use with survival
To determine the relationship between cumulative MTX use and survival, we tested associations with the duration of MTX use with time-varying multivariate Cox regression models. These analyses, shown in Figure 1, indicate that the protective association was evident only after more than 1 year of MTX use, and did not increase with longer duration of use.
Figure 1.
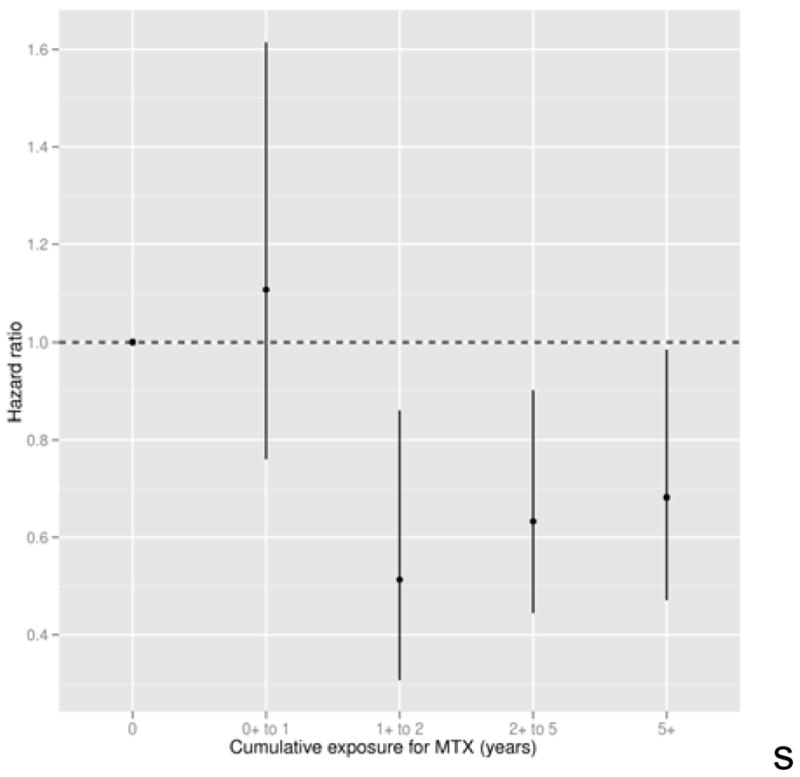
Hazard ratios and 95% confidence intervals for the association of duration of methotrexate use (as a time-dependent covariate) with survival, based on a multivariate Cox model. All covariates in the base model (shown in Table 2) were included except time-varying propensity for methotrexate use.
Discussion
Here we report the association between MTX and mortality while considering propensity scores for medication use in addition to the traditionally recognized factors that influence survival. Our hypothesis was that, after taking into account the propensity for prescribing MTX and other important covariates, MTX use would be associated with improved survival in patients with RA. Ideally the propensity for prescribing medications can be considered when studying the association between drug use and outcomes of interest, as prescribing patterns by practitioners influences the likelihood of selecting one treatment option over another. Propensity scores help to minimize the problem of confounding by indication, allowing for adjustment of these nuances that are difficult to fully account for by adjusting for sociodemographics, general and RA-related characteristics in regression modeling. In our cohort, several characteristics differed between MTX users and nonusers, underscoring the value of propensity scores in determining the relationship between MTX use and survival.
The protective relationship between MTX use and mortality previously has been described in patients with RA (8). Analyzing data from a cohort of over 1,000 RA patients, Choi et al. used a weighted pooled Cox logistic regression model to adjust for confounding by indication and found a reduction in risk of cardiovascular and all-cause death associated with MTX use (HR 0.4) though only the lower risk of cardiovascular death was statistically significant (8). Krause and colleagues described a reduction in mortality in patients with severe RA who had >50% improvement in disease activity over a year of MTX therapy (SMR 1.47), whereas those with only a 20–50% improvement fared less well (SMR 1.85) (9). RA patients discontinuing MTX in the first year of treatment had the highest mortality (SMR of 5.56), further underscoring the protective relationship between MTX use and mortality. Our results are consistent with these earlier findings. However, Landewe et al. (19) reported an increased risk of all-cause mortality in MTX users with RA; in contrast to our cohort, this study included only those RA patients with prevalent cardiovascular disease and/or hypertension, and observation extended only through 1995.
In our analyses, MTX use was associated with improved overall survival in RA. This reduction in risk of death persisted in our analyses after adjusting for propensity for MTX use and factors reflecting RA activity, namely the pain score and HAQ Disability Index. Thus these findings indicate that MTX has a favorable association with survival, independent and beyond the influence of the drug on RA activity. Interestingly, when we examined the relationship between MTX and mortality in the “early” and “late” periods of observation, we found that MTX use was only associated with reduced risk of death in the “late period.” Although reasons for this difference are unclear, it may be related to a difference in the dose of MTX used, which were likely to be higher later in the study period. In the multivariate regression model, adjusted for disability and other covariates, infection was strongly associated with reduced mortality (HR 0.65). However, further analyses (data not shown) indicated a strong interaction between infection and HAQ scores. There was no association between infection and mortality within strata of disability, suggesting that the protective association was an artifact generated by the strong association between the disability score and infection in this study population (“Simpson’s paradox”). Of note, our results for MTX did not change when regression models included number of rheumatology visits among the variables controlled for in analysis. Our adjustment indicates that improved survival associated with MTX use is not a surrogate for access to or visits to a rheumatologist, as our model takes into account comorbidities and rheumatology consultations.
We also examined if the protective association of MTX use might have been a consequence of selective discontinuation of MTX in patients shortly before death. In the case of selective discontinuation, medication use is not associated with improved survival, but rather prospects for longer survival are associated with continued medication use. This possibility should be considered whenever protective associations are found for medication and when the outcomes of interest (e.g. serious illness leading to mortality) have the potential to cause clinicians to discontinue medications for conditions that are not directly life-threatening. Although this bias has been recognized in the epidemiological literature (10), solutions to test for this bias have not been proposed. We developed a simulation analysis to directly test the potential influence of selective discontinuation of MTX on its association with mortality and found that this potential bias did not seem to be an explanation for the protective relationship between MTX use and death.
In examining the association of duration of MTX use and survival, the protective association was evident only among those with more than 1 year of use. Shorter use was not associated with any difference in mortality risk compared to non-use. However, there was no evidence of a graded association among patients with durations of use longer than 1 year. This pattern is more consistent with a threshold than with a cumulative response, which might imply that the mechanism of the association requires continued administration of the medication and is not due to tissue accumulation, deposition or structural modification due to the medication.
The precise mechanism(s) for such a reduction in risk of mortality with MTX therapy are not clear. Methotrexate use has been associated with a reduction in morbidity and mortality from cardiovascular disease in patients with RA (8,20–23). Methotrexate is a potent anti-inflammatory drug, and perhaps anti-inflammatory effects on the vasculature deter atherogenesis and/or thrombosis (24). In RA patients, MTX use improves coronary flow reserve (25) and is associated with reduction in risk of congestive heart failure (26), though in one small study, MTX appears to have no effect on brachial artery flow-mediated dilatation (27). Thus the impact of MTX on vascular function remains unclear and warrants further scrutiny, though its favorable effects on lipids, C-reactive protein, and markers of oxidative stress have been demonstrated (27).
The molecular mechanisms by which MTX reduces cardiovascular risk in RA may be mediated by its inhibition of dihydrofolate reductase and the release of adenosine. Reiss et al. have described the effect of MTX on reverse cholesterol transport and inhibition of foam cell formation in human THP-1 macrophages, suggesting that MTX may retard the development of atherosclerotic plaque by increasing cholesterol efflux from macrophages (28). In MTX users, adenosine is produced, with subsequent upregulation of ATP-binding cassette transporter A1 and cholesterol 27-hydroxylase, two proteins that facilitate the export of cholesterol from macrophages (29,30). These potential effects on atherogenesis presumably are transient and thus require ongoing MTX use for these protective effects to be maintained. Taken together, these findings have served as the rationale for the cardiovascular inflammation reduction trial (CIRT), a randomized controlled trial testing if low-dose MTX reduces the risk of myocardial infarction, stroke or cardiovascular death (31). The strength of the protective relationship between MTX and survival, however, suggests that MTX may be associated with reductions in mortality not only from cardiovascular disease, but also from other conditions (8,9,32).
While we included adjustment for measures of disease activity, socioeconomic factors, and access to subspecialty care, other factors that were not considered but are closely linked to MTX use may provide the true causal links. Furthermore, the association may not be MTX-specific; any medication that effectively suppresses the disease process in RA may improve survival in RA. Though this is not true for prednisone (32–34); observational studies suggest that this may be true for the TNF inhibitors (35–37).
The following limitations in this study warrant mention. The database had incomplete data on smoking habits, therefore we were not able to include this important covariable in our analyses. Furthermore, all data was ascertained by self-report without physician confirmation of medication use. Because data on MTX dose was not available, we were unable to assess the relationship between MTX dosage and mortality in this cohort. Finally, our cohort was fairly homogeneous, with the vast majority being Caucasian, thus our results may not be generalizable to other ethnic groups. Several methods of propensity score adjustment are available. We chose covariate adjustment rather than matching or stratification, because the marked temporal trends in MTX propensity makes the latter methods impractical, as these would group patients from quite different eras together. The fact that MTX propensities early in the study were very small makes the use of inverse-probability-of-treatment-weighting strategy prone to strong biases (38). Simulation studies have shown that covariate adjustment performs similarly to matching and stratification for propensity adjustment (39), and so we decided to use covariate adjustment by the propensity scores in this study. We have, however, performed a sensitivity analysis using inverse probability of treatment weighting models to see how this method affected the results. Although there was some attenuation in the association of MTX with overall survival, it was still protective and strongly statistically significant.
In summary, we report the protective relationship between use of MTX and mortality in a large cohort of RA patients followed prospectively for 25 years. These findings have implications for the use of MTX in the treatment of RA. Our results support the ongoing use of MTX as a cornerstone of RA treatment, with a survival benefit independent of its effects on pain and functional limitations. For patients in whom MTX monotherapy does not achieve complete control, add-on therapy may be more appropriate than switching to other medications, as MTX may still carry a survival benefit.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (AR43584). Drs. Dasgupta and Ward are supported by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. The authors thank Dr. Frederick Wolfe for generously sharing data used in these analyses and V. Bharathi Lingala for her assistance with data management.
Footnotes
Dr. Wasko has served as site Principal Investigator for Centocor trials and as consultant to UCB and Centocor. The remaining authors have no potential conflict of interest with regard to the work enclosed herein. No commercial interests have provided support in any fashion for the preparation of this manuscript.
Contributor Information
Mary Chester M. Wasko, West Penn Allegheny Health System, Allegheny Singer Research Institute, Pittsburgh, PA.
Abhijit Dasgupta, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda MD.
Helen Hubert, Consulting Epidemiologist Menlo Park, CA.
James F. Fries, Stanford University, Palo Alto, CA.
Michael M. Ward, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda MD.
References
- 1.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11(3):229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 2007;46(2):350–7. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- 4.Sacks JJ, Helmick CG, Langmaid G. Deaths from arthritis and other rheumatic conditions, United States, 1979–1998. J Rheumatol. 2004;31(9):1823–8. [PubMed] [Google Scholar]
- 5.Gonzalez A, Maradit KH, Crowson CS, Nicola PJ, Davis JM, III, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 6.Ward MM. Recent improvements in survival in patients with rheumatoid arthritis: better outcomes or different study designs? Arthritis Rheum. 2001;44(6):1467–9. doi: 10.1002/1529-0131(200106)44:6<1467::AID-ART243>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 8.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 9.Krause D, Schleusser B, Herborn G, Rau R. Response to methotrexate treatment is associated with reduced mortality in patients with severe rheumatoid arthritis. Arthritis Rheum. 2000;43(1):14–21. doi: 10.1002/1529-0131(200001)43:1<14::AID-ANR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford) 2005;44(5):677–80. doi: 10.1093/rheumatology/keh610. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 13.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–93. [PubMed] [Google Scholar]
- 14.Harrell FE. Regression Modeling Strategies. 1. New York: Springer-Verlag New York, LLC; 2001. [Google Scholar]
- 15.Breiman L. Random forests. Machine Learning. 2001;45(1):5–32. [Google Scholar]
- 16.Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med. 2010;29(3):337–46. doi: 10.1002/sim.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat. 2008;2:841–60. [Google Scholar]
- 18.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 19.Landewe RBM, van den Borne BEEM, Breedveld FC, Dijkmans BAC. Methotrexate effects in patients with rheumatoid arthritis with cardiovascular comorbidity. Lancet. 2000;355:1616–7. doi: 10.1016/S0140-6736(00)02222-4. [DOI] [PubMed] [Google Scholar]
- 20.van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8(5):R151. doi: 10.1186/ar2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naranjo A, Sokka T, Descalzo MA, Calvo-Alen J, Horslev-Petersen K, Luukkainen RK, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10(2):R30. doi: 10.1186/ar2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon DH, Avorn J, Katz JN, Weinblatt ME, Setoguchi S, Levin R, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(12):3790–8. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 23.Micha R, Imamura F, von Wyler BM, Solomon DH, Hernan MA, Ridker PM, et al. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am J Cardiol. 2011 doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49(2):295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 25.Turiel M, Tomasoni L, Sitia S, Cicala S, Gianturco L, Ricci C, et al. Effects of long-term disease-modifying antirheumatic drugs on endothelial function in patients with early rheumatoid arthritis. Cardiovasc Ther. 2010;28(5):e53–e64. doi: 10.1111/j.1755-5922.2009.00119.x. [DOI] [PubMed] [Google Scholar]
- 26.Myasoedova E, Crowson CS, Nicola PJ, Maradit-Kremers H, Davis JM, III, Roger VL, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38(8):1601–6. doi: 10.3899/jrheum.100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Barbary AM, Hussein MS, Rageh EM, Hamouda HE, Wagih AA, Ismail RG. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol. 2011;38(2):229–35. doi: 10.3899/jrheum.100582. [DOI] [PubMed] [Google Scholar]
- 28.Reiss AB, Carsons SE, Anwar K, Rao S, Edelman SD, Zhang H, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008;58(12):3675–83. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coomes E, Chan ES, Reiss AB. Methotrexate in atherogenesis and cholesterol metabolism. Cholesterol. 2011;2011:503028. doi: 10.1155/2011/503028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen DY, Chih HM, Lan JL, Chang HY, Chen WW, Chiang EP. Blood lipid profiles and peripheral blood mononuclear cell cholesterol metabolism gene expression in patients with and without methotrexate treatment. BMC Med. 2011;9:4. doi: 10.1186/1741-7015-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7 (Suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 32.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford) 2011;50(1):101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caplan L, Wolfe F, Russell AS, Michaud K. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J Rheumatol. 2007;34(4):696–705. [PubMed] [Google Scholar]
- 34.Sihvonen S, Korpela M, Mustonen J, Huhtala H, Karstila K, Pasternack A. Mortality in patients with rheumatoid arthritis treated with low-dose oral glucocorticoids. A population-based cohort study. J Rheumatol. 2006;33(9):1740–6. [PubMed] [Google Scholar]
- 35.Carmona L, Descalzo MA, Perez-Pampin E, Ruiz-Montesinos D, Erra A, Cobo T, et al. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66(7):880–5. doi: 10.1136/ard.2006.067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsson LT, Turesson C, Nilsson JA, Petersson IF, Lindqvist E, Saxne T, et al. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66(5):670–5. doi: 10.1136/ard.2006.062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lunt M, Watson KD, Dixon WG, Symmons DP, Hyrich KL. No evidence of association between anti-tumor necrosis factor treatment and mortality in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2010;62(11):3145–53. doi: 10.1002/art.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–70. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 39.Austin PC, Grootendorst P, Normand SL, Anderson GM. Conditioning on the propensity score can result in biased estimation of common measures of treatment effect: a Monte Carlo study. Stat Med. 2007;26(4):754–68. doi: 10.1002/sim.2618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


