Abstract
The elderly have comparatively worse cognitive impairments from traumatic brain injury (TBI) relative to younger adults, but the molecular mechanisms that underlie this exacerbation of cognitive deficits are unknown. Experimental models of TBI have demonstrated that the cyclic AMP-protein kinase A (cAMP-PKA) signaling pathway is downregulated after brain trauma. Since the cAMPPKA signaling pathway is a key mediator of long-term memory formation, we investigated whether the TBI-induced decrease in cAMP levels is exacerbated in aged animals. Aged (19 months) and young adult (3 months) male Fischer 344 rats received sham surgery or mild (1.4–1.6 atmospheres, atm) or moderate (1.7–2.1 atm) parasagittal fluid-percussion brain injury. At various time points after surgery, the ipsilateral parietal cortex, hippocampus, and thalamus were assayed for cAMP levels. Mild TBI lowered cAMP levels in the hippocampus of aged, but not young adult animals. Moderate TBI lowered cAMP levels in the hippocampus and parietal cortex of both age groups. In the thalamus, cAMP levels were significantly lowered after moderate, but not mild TBI. To determine if the TBI-induced decreases in cAMP had physiological consequences in aged animals, hippocampal long-term potentiation (LTP) in the Schaffer collateral pathway of the CA1 region was assessed. LTP was significantly decreased in both young adult and aged animals after mild and moderate TBI as compared to sham surgery animals. Rolipram rescued the LTP deficits after mild TBI for young adult animals and caused a partial recovery for aged animals. However, rolipram did not rescue LTP deficits after moderate TBI in either young adult or aged animals. These results indicate that exacerbation of cognitive impairments in aged animals with TBI may be due to decreased cAMP levels and deficits in hippocampal LTP.
Keywords: cAMP, fluid-percussion, long-term potentiation, phosphodiesterase, rolipram, traumatic brain injury
The incidence of traumatic brain injury (TBI) is characterized by a tri-modal age distribution, with the highest incidence occurring in young children under the age of 5, young adults between 15–24 years of age, and in the elderly greater than 65 years (Faul et al., 2010). The numbers of elderly in the US are projected to increase by 42% between 2010 and 2050, with nearly one in five people age 65 or older by 2030 (Vincent and Velkoff, 2010). Concurrently, the incidence in TBI has doubled in the past 18 years (Ramanathan et al., 2012). Age is one of the most important predictors of outcome after TBI since the ability to withstand brain injury diminishes with age. This is reflected in the fact that the highest rates of TBI-associated hospitalizations and death occur in the elderly even though the elderly are ranked as the third highest age group for TBI incidence (Susman et al., 2002, Stocchetti et al., 2012). Aged adults are more likely to remain severely disabled or vegetative after TBI as compared to young adults, and 91% of severe TBI patients older than 56 years suffer significant disability (Wilson et al., 1988, Stocchetti et al., 2012). Even a mild TBI that produces only a temporary cognitive impairment in a young adult can result in a significant, prolonged cognitive disability in an aged adult (Susman et al., 2002). TBI is an epigenetic risk factor for Alzheimer’s and Parkinson’s diseases, compounding recovery from TBI (Bazarian et al., 2009). This makes TBI a significant health problem in the elderly; one that is likely to grow as our population ages.
The progressive loss in the ability to handle stress and injury in aged adults has been recapitulated in experimental models of brain injury. Aged animals as compared to young adult animals exhibit more severe impairments in water maze performance and motor ability after TBI (Hamm et al., 1992, Maughan et al., 2000, Onyszchuk et al., 2008, Itoh et al., 2012). Even middle-aged rats in comparison to young adult rats have larger cortical lesions and severely impaired water maze performance after bilateral cortical contusion (Hoane et al., 2004). There have been some mechanistic studies to determine why injury-induced impairments are exacerbated in older animals. Previous studies have not detected any age-related differences in blood pressure, blood glucose levels, and weight loss, suggesting that the worsened pathology in aged animals after TBI is more likely caused by changes in biochemical signaling cascades rather than systemic complications due to age (Hamm et al., 1991, Hamm et al., 1992, Gilmer et al., 2010). Accordingly, higher toxic levels of calcium accumulation in hippocampal neurons occur after TBI and intracellular calcium levels return to basal levels more slowly in older animals (Osteen et al., 2001). Aged animals also have higher levels of pro-inflammatory cytokines such as interleukin-1β, tumor necrosis factor-α, and interleukin-6, increased oxidative damage, and decreased expression of neuroprotective genes after brain trauma (Sandhir et al., 2004, Shah et al., 2006, Shao et al., 2006, Onyszchuk et al., 2008, Anderson et al., 2009, Gilmer et al., 2010, Itoh et al., 2012). These studies indicate that several aspects of secondary injury mechanisms in TBI are aggravated with age.
There are significant age-related changes in cyclic AMP (cAMP)-mediated signaling in the non-injured brain and this may underlie some of the impairments seen in hippocampal synaptic plasticity and learning in the aged animal after TBI. cAMP is an important second messenger in activating several intracellular signaling pathways, including protein kinase A (PKA) which phosphorylates a key transcription factor required for long-term memory formation, cAMP response-element binding protein (CREB) (Waltereit and Weller, 2003). PKA activity and basal phosphorylation levels of CREB are lower the hippocampi of aged rats as compared to young adult rats (Mons et al., 2004, Reis et al., 2005). Furthermore, CRE-binding activity is lower in memory-impaired aged animals (Karege et al., 2001a). This may be due to depression of adenylyl cyclase activity or levels, as norepinephrine signaling through the β-adrenergic receptor is unable to fully stimulate adenylyl cyclase activity in the aged rat hippocampus (Bickford-Wimer et al., 1987, Parfitt and Bickford-Wimer, 1990, Mons et al., 2004).
These impairments in the cAMP-PKA pathway probably result in impaired synaptic plasticity. Aged animals have deficits in hippocampal long-term potentiation (LTP) that are reversed by phosphodiesterase inhibitors such as rolipram, which raises cAMP levels (Bach et al., 1999). Correspondingly, aged rats have hippocampal-dependent memory deficits that are ameliorated by rolipram administration (Bach et al., 1999). Furthermore, in an Alzheimer mouse model, transgenic mice expressing mutant β-APP and presenilin-1 proteins show improvements in hippocampal long-term potentiation (LTP) and hippocampal-dependent learning with rolipram treatment (Gong et al., 2004). In our previous study, we found that cAMP levels are significantly decreased in young adult animals after TBI and that rolipram could rescue the decrease in cAMP levels (Atkins et al., 2007). Thus, we hypothesized that aged animals have a worse functional outcome after TBI due to a decrease in cAMP levels and that these deficits can be rescued with rolipram treatment.
EXPERIMENTAL PROCEDURES
Fluid-percussion brain injury surgery
All experimental procedures were in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Miami Institutional Animal Care and Use Committee. Animals were screened daily by in-house veterinarian technicians for health problems such as cataracts, jaundice, and tumors; only healthy animals were included in the study. Food and water intake were monitored and available ad libitum. Animals were singly housed and maintained on a 12-h light/dark schedule. Male Fischer 344 rats (3 months, 128 total or 19 months, 161 total, NIA contract colony, Charles Rivers Laboratories, Wilmington, MA, USA) were anesthetized with 3% isoflurane, 70% N2O, and 30% O2. The animals received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to midline) and a beveled plastic 18 gauge syringe hub was secured to the craniotomy using cyanoacrylate and dental cement. After 18–24 h of recovery, the animal was anesthetized with 3% isoflurane, 70% N2O, and 30% O2, intubated endotracheally and mechanically ventilated (Stoelting, Wood Dale, IL, USA) with 1% isoflurane, 70% N2O, and 30% O2. To facilitate mechanical ventilation, pancuronium bromide (1 mg/kg) was administered through the tail artery. A mild (1.5±0.1 atmospheres, atm) or moderate fluid-percussion pulse (1.9±0.2 atm) was delivered to the right parietal cortex (Sanders et al., 1999, 2001). These injury severity settings produce a graded level of cognitive deficits and pathological changes in the brain, similar to clinical studies of injury severity (Sanders et al., 1999, 2001). Sham-operated rats underwent all surgical manipulations except the fluid-percussion pulse. Rectal and temporalis muscle thermistors were used to maintain core and brain temperatures at 36.8–37.3 °C using feedback warming lamps. Blood gases (pO2 and pCO2), blood pH, and mean arterial blood pressure (MABP) were monitored 15 min before TBI and up to 1 h after TBI. All efforts were made to minimize the number of animals used, and reduce discomfort and prevent infection by administering penicillin/benzathine (20,000 IU/kg, intramuscular) once prior to the surgery, and buprenorphine was given immediately after the surgery (0.01 mg/kg, subcutaneously).
cAMP assays
cAMP levels were measured using a direct cAMP ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA) according to the manufacturer's protocol for the nonacetylated method. The young (3 months) or aged (19 months) animals received either sham surgery or mild or moderate TBI followed by recovery for 15 min (n=7/group), 60 min (n=7/group), 1 day (n=7/group), or 3 days (n=7/group except for moderate TBI aged animals n=3). Sham animals were allowed to recover for 15 min after sham surgery (n=8/group for young adult and aged) or 60 min after sham surgery (n=8 young adult and n=7 aged). No significant differences were found in cAMP levels between sham groups at 15 min versus 60 min recovery from surgery in either age group, so sham groups were pooled (not shown). The right (injured) parietal cortex, hippocampus, and thalamus were rapidly dissected at 4 °C and frozen on liquid nitrogen. The tissue was briefly sonicated on ice (14 s, setting 1.9, Branson sonifier 450, Danbury, CT, USA) in 20 volumes of 100 mM HCl and 500 µM 3-isobutyl-1-methylxanthine. Each sample was assayed in duplicate. Total protein was assayed using the Coomassie Plus assay kit (Thermo Fisher Scientific, Waltham, MA, USA) and used for normalization.
Western blot analysis
To assess for changes in basal CREB phosphorylation after sham surgery in young adult versus aged animals, the right parietal cortex (n=12/each age group: 15 min recovery n=5; 60 min recovery n=3; 1 day recovery n=4) and hippocampus (n=8/each age group: 15 min recovery n=5; 60 min recovery n=3) were dissected at 4 °C in saline and frozen on liquid nitrogen within 2 min of decapitation. The tissue was homogenized with a Dounce homogenizer (35 strokes, 4 °C) in 1 ml (cortex) or 750 µl (hippocampus) of lysis buffer: 15 mM Tris pH 7.6, 0.25 M sucrose, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1.25 µg/ml pepstatin A, 10 µg/ml leupeptin, 25 µg/ml aprotinin, 0.5 mM PMSF, 0.1 mM Na3VO4, 50 mM NaF, 2 mM Na4P2O7, and 1× phosphatase inhibitor cocktail set II (EMD Millipore, Billerica, MA, USA). Homogenates were boiled with 1× sample buffer for 7–9 min at 95 °C. Samples were assayed for total protein using the Coomassie Plus assay kit (Thermo Fisher Scientific). Equal amounts of protein (30 µg/lane) were electrophoresed (12.5% SDS-PAGE) and western blotted using antibodies against phospho-CREB Ser133 (1:1000, Cell Signaling Technology, 9191, Danvers, MA, USA), total CREB (1:1000, Cell Signaling Technology, 9192), and β-actin (1:5000, Sigma, AC-15, St. Louis, MO, USA). Epitopes were visualized with HRP-conjugated secondary antibodies (1:1000–1:5000, Cell Signaling Technology) using the Phototope HRP Western blot detection system (Cell Signaling Technology) and developed on film (Phenix X-ray film BX; Phenix Research Products, Candler, NC, USA). Films were developed so that densities were in a linear range and they were densitized using ImageJ 1.38× (NIH). Changes in phosphorylated CREB were normalized to total CREB and β-actin levels to determine if there was a net phosphorylation change in CREB.
Hippocampal slice preparation and electrophysiology
Slice preparation
Young adult and aged animals received sham surgery or mild or moderate TBI followed by recovery for 2 weeks (n=9–11 group). Rats were decapitated under 3% isoflurane, 70% N2O, and 30% O2 anesthesia, the brain was quickly removed, and the ipsilateral hippocampus was dissected in sucrose-based artificial cerebrospinal fluid (aCSF): 110 mM sucrose, 60 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 28 mM NaHCO3, 7 mM MgCl2, 0.5 mM CaCl2, and 5 mM d-glucose, equilibrated with 95% O2/5% CO2 at 4 °C. The ipsilateral hippocampus was rapidly sectioned into transverse slices 400 µm thick using a vibratome (Vibratome 3000, St. Louis, MO, USA) at 4 °C. Slices were recovered in aCSF (125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 10 mM d-glucose, 2 mM CaCl2, and 1 mM MgCl2, saturated with 95% O2/5% CO2) for 1 h at room temperature. After equilibration, slices were transferred to a submerged recording chamber and perfused at a rate of 2.5–3 ml/min with aCSF and maintained at 31 °C.
Field recordings
Hippocampal field potential recordings were obtained using borosilicate glass recording electrodes filled with 2M NaCl (1–3 MΩ) placed in stratum radiatum of area CA1. A platinum-iridium cluster stimulating electrode (tip diameter 25 µm; FHC, Bowdoin, ME, USA) was placed in the Schaffer collateral pathway. The field excitatory postsynaptic potentials (fEPSPs) were filtered (1 kHz) with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA) and digitized at 20 kHz with an A/D converter (NI PCI 6221, National Instruments, Austin, TX, USA). For input/output (I/O) curves, current intensities that elicited threshold and maximum fEPSPs were determined by gradually increasing the current intensity until the fEPSP slope plateaued. Paired-pulse facilitation (PPF) was tested by delivering paired-pulse stimuli at intervals of 50, 100, 150, and 200 ms. For baseline recordings and PPF, the stimulus intensity was adjusted to give fEPSP slopes of about 30–40% of the maximum fEPSPs. No significant differences in baseline fEPSP slopes were observed between age groups or sham versus mild or moderate TBI animals. Baseline responses were recorded at 0.033 Hz and were established for at least 20 min prior to tetanization. High-frequency stimulation (100 Hz, 1 s long, single train) was used to induce LTP at the same stimulation intensity used for baseline recordings. Data acquisition and analysis were performed using the WinLTP program (Anderson and Collingridge, 2007).
Rolipram (Sigma) was dissolved in dimethyl sulfoxide (DMSO) at 10 mM, and then diluted in aCSF for a final concentration of 1 µM. Hippocampal slices were pretreated with the drug or vehicle 10 min prior to the high-frequency stimulation and for 30 min after the tetanization. This allowed the drug to equilibrate within the hippocampal slices prior to the tetanization since previous studies have found that rolipram is required to be present during the tetanization to facilitate LTP expression (Barad et al., 1998, Navakkode et al., 2004). Neither rolipram nor vehicle alone (DMSO 0.1%) altered basal synaptic transmission.
Statistical analysis
Data represent mean±SD. Basal changes in cAMP and CREB phosphorylation were analyzed by Student’s t-test. Trauma-induced changes in cAMP levels were analyzed with two-way analysis of variance (ANOVA) with the factors time after surgery × age and post-hoc Bonferroni’s correction. Hippocampal electrophysiological recordings were analyzed with a repeated measures two-way ANOVA; for I/O curves the factors were animal group (Sham+Young Adult, TBI+Young Adult, Sham+Aged, TBI+Aged) × current intensity, for PPF the factors were animal group (Sham+Vehicle, Sham+Rolipram, TBI+Vehicle, TBI+Rolipram) × inter-stimulus interval, and for LTP the factors were animal group (Sham+Vehicle, Sham+Rolipram, TBI+Vehicle, TBI+Rolipram) × time, and post-hoc Bonferroni’s correction. Hippocampal fEPSP slopes at 60 min post-tetanus were analyzed with a three-way ANOVA with the factors surgery × drug × age followed by two-way ANOVAs for significant interactions and Bonferroni’s post-hoc correction. Significance was set at P<0.05. * represents P<0.05, ** represents P<0.01, and *** represents P<0.001.
RESULTS
Basal cAMP levels and CREB phosphorylation in aged animals
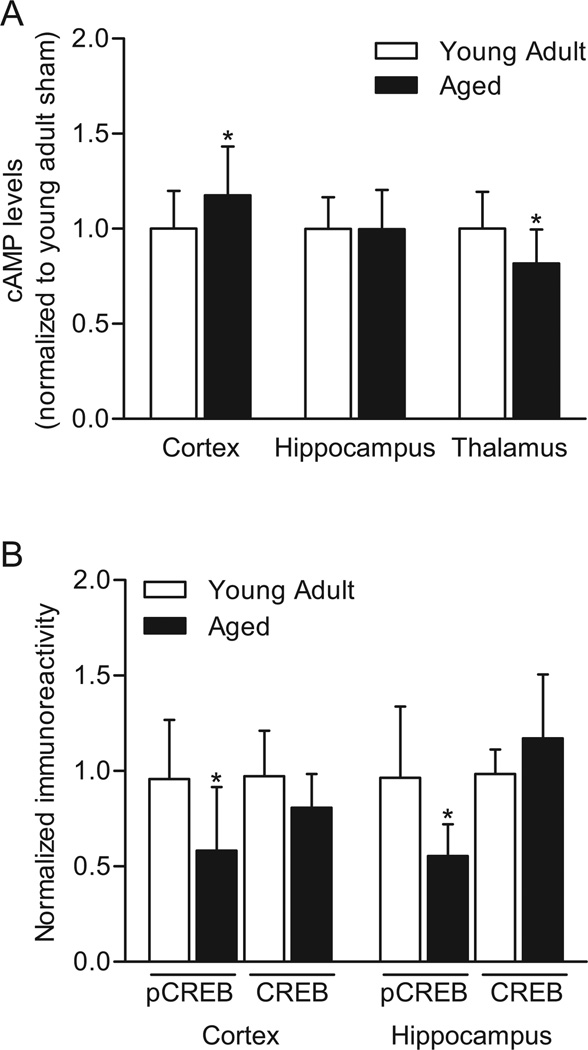
The effects of age on basal cAMP levels and CREB phosphorylation were assessed by comparing aged (19 months) to young adult (3 months) Fischer 344 rats. The right parietal cortex, hippocampus and thalamus were assayed by ELISA for cAMP levels and by western blotting for CREB Ser133 phosphorylation and total CREB levels (Fig. 1). Basal cAMP levels were significantly higher in the parietal cortex of aged animals as compared to young adult animals, unchanged in the hippocampus, and significantly lower in the thalamus. In accordance with previous results, phosphorylated CREB levels were significantly lower in both the parietal cortex and hippocampus in aged animals as compared to young adult animals (Foster et al., 2001, Hattiangady et al., 2005, Kudo et al., 2005). There were no significant differences in total CREB levels in young adult versus aged animals in these brain regions.
Fig. 1.
Basal changes in cAMP signaling in aged animals (19 months) as compared to young adult animals (3 months). (A) cAMP levels in the right parietal cortex, hippocampus, and thalamus from aged animals (n=8 at 15 min and n=7 at 60 min after sham surgery) were analyzed relative to cAMP levels from young adult animals (n=8 at 15 min and n=8 at 60 min after sham surgery). (B) Basal levels of phosphorylated CREB and total CREB in aged animals were analyzed relative to young adult animals in the right parietal cortex (n=12/group) and hippocampus (n=8/group). *P<0.05 for aged versus young adult.
Effects of TBI on attrition in aged animals
No attrition was observed in sham animals or mild TBI animals for either age group. The attrition rate for moderate TBI in young adult animals was 27% (14 of 52 moderate TBI) and for aged animals was 62% (53 of 86 moderate TBI for all experiments). There were no significant differences in mean survival time between age groups and of the animals that died after moderate TBI, both young adult and aged animals died within 15 min of the brain trauma. Blood pO2, pCO2 or pH levels before and after TBI for young adult and aged animals were not significantly different and these parameters were within normal physiological ranges (Table 1). Significant decreases in MABP were observed for both young adult and aged animals at 15 min after moderate TBI and for aged animals after mild and moderate TBI, but were still maintained within normal physiological ranges. Necropsies were done on a subset of animals, which indicated multi-focal areas of hemorrhage in the brains and vascular congestion in the lungs.
Table 1.
Physiological Parameters
| Treatment | Weight | ATM | MABP | Blood pO2 | Blood pCO2 | Blood pH | Head temperature |
Rectal temperature |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15’ prior | 15’ post | 15’ prior | 15’ post | 15’ prior | 15’ post | 15’ prior | 15’ post | 15’ prior | 15’ post | 15’ prior | 15’ post | |||||
| cAMP assay | Young adult (82) | Sham (16) | 275.7±34.9 | NA | 141.8±17.4 | 137.4±23.7 | 145.1±23.7 | 134.2±5.6 | 37.6±3.1 | 38.3±2.5 | 7.48±0.04 | 7.47±0.04 | 36.7±0.2 | 36.7±0.1 | 37.0±0.1 | 37.0±0.1 |
| Mild TBI 15’ (7) | 302.3±46.5 | 1.51±0.07 | 144.0±13.2 | 134.6±12.7 | 142.3±8.2 | 128.0±6.7 | 36.4±2.1 | 38.2±2.0 | 7.48±0.04 | 7.46±0.02 | 36.6±0.2 | 36.7±0.1 | 37.0±0.0 | 37.0±0.1 | ||
| Mod TBI 15’ (7) | 304.6±34.1 | 2.04±0.10 | 141.7±14.4 | 123.8±11.9 | 142.6±26.0 | 121.9±25.6 | 36.9±2.5 | 36.9±2.7 | 7.49±0.03 | 7.47±0.02 | 36.7±0.2 | 36.7±0.2 | 36.8±0.2 | 36.9±0.1 | ||
| Mild TBI 1h (7) | 342.7±23.0 | 1.51±0.07 | 146.8±6.6 | 136.0±9.8 | 121.3±9.0 | 127.4±14.7 | 37.8±3.0 | 37.2±1.6 | 7.48±0.03 | 7.48±0.03 | 36.7±0.1 | 36.7±0.1 | 37.0±0.1 | 37.0±0.1 | ||
| Mod TBI 1h (7) | 285.8±16.7 | 1.90±0.12 | 153.2±8.0 | 139.8±10.8 | 138.2±16.4 | 131.0±25.1 | 40.6±4.1 | 37.1±2.8 | 7.46±0.04 | 7.49±0.02 | 36.7±0.2 | 36.7±0.1 | 37.0±0.0 | 37.0±0.1 | ||
| Mild TBI 1d (7) | 338.3±29.8 | 1.60±0.06 | 137.8±15.0 | 127.0±15.2 | 136.6±29.8 | 146.1±30.6 | 36.9±1.1 | 36.4±1.3 | 7.49±0.02 | 7.48±0.03 | 36.7±0.2 | 36.7±0.2 | 37.0±0.1 | 37.0±0.1 | ||
| Mod TBI 1d (7) | 317.0±31.9 | 1.89±0.07 | 142.6±9.4 | 134.7±6.5 | 145.6±16.0 | 132.6±8.8 | 36.7±1.8 | 37.4±2.4 | 7.51±0.02 | 7.49±0.04 | 36.8±0.1 | 36.7±0.1 | 37.0±0.0 | 37.0±0.1 | ||
| Mild TBI 3d (7) | 325.0±9.1 | 1.59±0.04 | 140.2±16.6 | 126.8±7.9 | 139.4±25.1 | 136.1±23.0 | 36.4±1.8 | 38.1±2.7 | 7.50±0.02 | 7.48±0.02 | 36.8±0.1 | 36.7±0.1 | 37.0±0.0 | 36.9±0.1 | ||
| Mod TBI 3d (7) | 313.1±50.3 | 1.89±0.04 | 144.1±11.4 | 128.1±9.2 | 120.6±15.1 | 125.7±20.7 | 36.7±1.5 | 38.6±2.2 | 7.50±0.02 | 7.48±0.03 | 36.8±0.2 | 36.7±0.1 | 37.0±0.0 | 37.0±0.0 | ||
| Aged (109) | Sham (15) | 477.5±22.0 | NA | 147.3±6.3 | 141.3±16.7 | 135.7±22.1 | 124.7±10.1 | 41.0±2.6 | 40.3±2.8 | 7.45±0.02 | 7.46±0.03 | 36.7±0.2 | 36.8±0.1 | 37.0±0.1 | 37.0±0.1 | |
| Mild TBI 15’ (7) | 468.1±34.9 | 1.51±0.07 | 143.6±6.1 | 137.6±9.7 | 134.3±31.8 | 133.6±24.4 | 40.8±3.4 | 37.8±2.8 | 7.45±0.02 | 7.46±0.02 | 36.7±0.2 | 36.7±0.2 | 37.0±0.1 | 37.0±0.0 | ||
| Mod TBI 15’ (7) | 430.4±51.4 | 1.93±0.10 | 144.2±13.6 | 131.5±11.5 | 143.7±30.7 | 162.9±37.0* | 40.7±3.2 | 37.1±2.2 | 7.46±0.03 | 7.47±0.02 | 36.7±0.2 | 36.6±0.1 | 37.0±0.0 | 37.0±0.0 | ||
| Mild TBI 1h (7) | 467.6±17.4 | 1.50±0.08 | 141.3±11.8 | 136.1±13.6 | 128.0±17.6 | 126.5±20.6 | 40.4±3.2 | 38.4±2.0 | 7.45±0.02 | 7.46±0.02 | 36.8±0.1 | 36.7±0.1 | 37.0±0.1 | 37.0±0.0 | ||
| Mod TBI 1h (7) | 480.0±31.8 | 1.81±0.09 | 147.9±7.6 | 129.2±15.9 | 115.1±12.7 | 136.0±27.9 | 39.5±2.7 | 37.7±1.9 | 7.46±0.02 | 7.47±0.03 | 36.7±0.2 | 36.7±0.1 | 36.9±0.2 | 36.9±0.1 | ||
| Mild TBI 1d (7) | 449.3±11.4 | 1.59±0.04 | 148.1±13.9 | 133.3±23.2 | 120.1±23.1 | 124.4±17.3 | 38.7±3.1 | 35.7±1.3* | 7.46±0.02 | 7.47±0.02 | 36.8±0.1 | 36.7±0.2 | 37.0±0.0 | 37.0±0.0 | ||
| Mod TBI 1d (7) | 461.0±33.5 | 1.86±0.08 | 149.7±5.3 | 113.0±11.8*** | 125.1±24.7 | 113.5±20.4 | 37.3±2.0 | 37.6±3.2 | 7.48±0.02 | 7.45±0.03 | 36.6±0.1 | 36.8±0.2 | 37.0±0.0 | 36.8±0.2 | ||
| Mild TBI 3d (7) | 462.6±21.3 | 1.61±0.07 | 139.0±13.9 | 127.0±19.9 | 134.8±31.8 | 158.6±35.2 | 39.5±2.5 | 39.2±2.1 | 7.45±0.03 | 7.43±0.04 | 36.7±0.1 | 36.7±0.2 | 37.0±0.0 | 36.9±0.2 | ||
| Mod TBI 3d (3) | 438.7±51.1 | 1.83±0.06 | 141.3±8.7 | 120.9±11.8 | 135.0±19.5 | 128.7±38.0 | 39.5±1.8 | 37.3±0.9 | 7.44±0.05 | 7.45±0.03 | 36.7±0.2 | 36.7±0.2 | 36.9±0.2 | 37.0±0.0 | ||
| Electrophysiology | Young adult (34) | Sham 2wk (11) | 302.5±24.6 | NA | 141.2±11.7 | 140.2±9.3 | 142.3±24.3 | 130.3±20.0 | 37.5±3.0 | 37.8±1.6 | 7.50±0.03 | 7.50±0.02 | 36.7±0.1 | 36.7±0.2 | 37.0±0.0 | 36.9±0.2 |
| Mild TBI 2wk (9) | 290.2±36.9 | 1.58±0.07 | 147.2±11.8 | 135.1±9.4 | 153.8±21.3 | 141.0±15.2 | 37.9±3.2 | 37.6±1.3 | 7.49±0.03 | 7.48±0.02 | 36.6±0.2 | 36.6±0.2 | 37.0±0.0 | 36.9±0.2 | ||
| Mod TBI 2wk (10) | 310.1±15.2 | 1.93±0.08 | 140.1±13.4 | 125.9±12.9* | 145.8±22.7 | 130.7±24.0 | 39.4±3.6 | 36.6±1.1 | 7.48±0.03 | 7.50±0.02 | 36.7±0.2 | 36.8±0.2 | 37.0±0.0 | 37.0±0.1 | ||
| Aged (40) | Sham 2wk (10) | 480.0±22.0 | NA | 141.0±8.9 | 146.2±8.7 | 136.7±34.3 | 128.7±28.9 | 39.1±3.2 | 38.0±1.7 | 7.47±0.03 | 7.48±0.02 | 36.7±0.1 | 36.6±0.2 | 37.0±0.0 | 36.9±0.1 | |
| Mild TBI 2wk (10) | 468.0±28.4 | 1.62±0.06 | 141.3±9.8 | 132.3±10.6* | 139.4±35.6 | 141.0±15.2 | 40.4±2.5 | 37.1±2.1 | 7.45±0.03 | 7.47±0.03 | 36.6±0.1 | 36.7±0.1 | 37.0±0.2 | 37.0±0.0 | ||
| Mod TBI 2wk (9) | 477.0±14.3 | 1.84±0.05 | 137.0±7.6 | 123.6±10.6*** | 131.6±30.0 | 133.4±33.6 | 38.9±3.1 | 38.2±2.5 | 7.47±0.02 | 7.46±0.03 | 36.6±0.1 | 36.8±0.1 | 37.0±0.0 | 37.0±0.1 | ||
N values in parentheses. ATM, atmospheres pressure; MABP, mean arterial blood pressure; Mod, Moderate; NA, not applicable
P<0.05,
P<0.001 versus age-matched sham animals
Attrition after moderate TBI: cAMP assay young adults (10); cAMP assay aged (42); electrophysiology young adults (4); electrophysiology aged (11)
Effects of mild TBI on cAMP levels
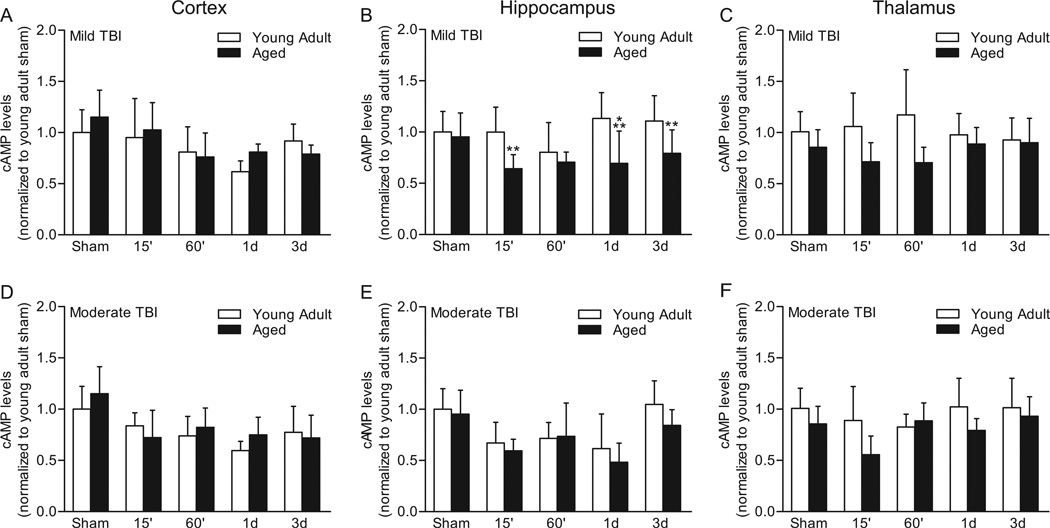
We next investigated whether mild TBI, which has been previously characterized in this model to result in modest pathology and minor behavioral impairments, would differentially decrease cAMP levels in aged versus young adult animals (Fig. 2) (Sanders et al., 1999, 2001). For cAMP levels in the parietal cortex, no significant interaction of time after surgery and age was found (F(4,77)=1.64, P=0.174). There was no main effect of age (F(1,77)=1.107, P=0.296), but there was a main effect of time after surgery (F(4,77)=9.338, P<0.001). Post-hoc analysis of the main effect of time after surgery revealed that mild TBI significantly lowered cAMP levels in the parietal cortex at 60 min (P<0.001), 1 d (P<0.001), and 3 d (P<0.05) as compared to sham injury. In the ipsilateral hippocampus, an interaction of age and time after surgery (F(4,77)=3.51, P=0.011) was detected. A significant difference in cAMP levels was detected between young adult and aged animals at 15 min (P=0.003), 1 d (P<0.001), and 3 d (P=0.009) after mild TBI. There was a main effect of age (F(1,77)=23.96, P<0.001) and time after surgery (F(4,77)=3.76, P=0.008). cAMP levels were significantly lower in the ipsilateral hippocampus of aged animals, but not young adult animals, as compared to age-matched sham animals at 15 min (P=0.007), 60 min (P=0.046), and 1 d (P=0.033) after mild TBI. In the ipsilateral thalamus, the interaction between age and time after surgery was not significant (F(4,77)=2.16, P=0.082), although there was a trend. A main effect of age was observed (F(1,77)=17.84, P<0.001), and there was no main effect of time after surgery (F(4,77)=0.11, P=0.978). These results indicate that mild TBI significantly lowers cAMP levels in the parietal cortex of both young adult and aged animals between 60 min to 3 d post-injury. In contrast, mild TBI significantly lowers hippocampal cAMP levels only in aged animals and only between 15 min to 1 d post-injury.
Fig. 2.
cAMP levels in the ipsilateral parietal cortex (A, D), hippocampus (B, E), and thalamus (C, F) after TBI. Mild TBI (A, B, C) significantly lowered cAMP levels in the ipsilateral parietal cortex at 60 min, 1 d, and 3 d, but there were no significant differences between age groups. In the ipsilateral hippocampus, cAMP levels were significantly different between young adult and aged animals at 15 min, 1 d, and 3 d post-injury. In the thalamus, no significant interaction or effect of mild TBI was observed, although a significant effect of age was observed. Moderate TBI (D, E, F) significantly lowered cAMP levels in the ipsilateral parietal cortex at all time points tested (15 min, 60 min, 1 d, and 3d) and in the hippocampus at 15 min, 60 min and 1 d, but no significant interaction of age and time after surgery was found for either brain region. In the thalamus, no significant change in cAMP levels was observed, although there was a trend for significant decrease at 15 min post-injury. **P<0.01, ***P<0.001 for sham versus TBI, n=16 for sham young adult groups, n=15 for sham aged groups, n=7/for all TBI groups, except for moderate TBI aged animals at 3 days, n=3.
Effects of moderate TBI on cAMP levels
In the ipsilateral parietal cortex after moderate TBI, no significant interaction of age and time after surgery was found (F(4,75)=1.51, P=0.209) and there was no main effect of age (F(1,75)=0.98, P=0.327). A main effect of time after surgery was detected (F(4,75)=13.76, P<0.001). Post-hoc analysis of the main effect of time after surgery indicated that cAMP levels were significantly lowered at 15 min (P<0.001), 60 min (P<0.001), 1 d (P<0.001) and 3 d (P<0.001) after moderate TBI as compared to sham. For the ipsilateral hippocampus, no significant interaction of age and time after surgery was found (F(4,75)=0.63, P=0.645). There was no main effect of age (F(1,75)=2.44, P=0.123). The main effect of time after surgery was significant (F(4,75)=15.15, P<0.001), with cAMP levels significantly lowered at 15 min (P<0.001), 60 min (P<0.001), and 1 d (P<0.001) after moderate TBI as compared to sham levels. In the thalamus, no significant interaction of time after surgery and age was found (F(4,73)=1.66, P=0.168). There was a main effect of age (F(1,73)=9.26, P=0.003) and time after surgery (F(4,73)=2.67, P=0.039). However, post-hoc analysis of the main effect of time after surgery did not reveal any significant change in cAMP levels in TBI animals as compared to sham animals. Although there was a trend for a significant decrease at 15 min post-injury, this was not significant (P=0.058). These results indicate that moderate TBI significantly lowers cAMP levels in the parietal cortex of both young adult and aged animals between 15 min to 3 d post-injury. Unlike mild TBI, moderate TBI significantly lowered hippocampal cAMP levels in both young adult and aged animals between 15 min to 1 d post-injury.
Rescue of hippocampal LTP impairments caused by mild TBI with rolipram
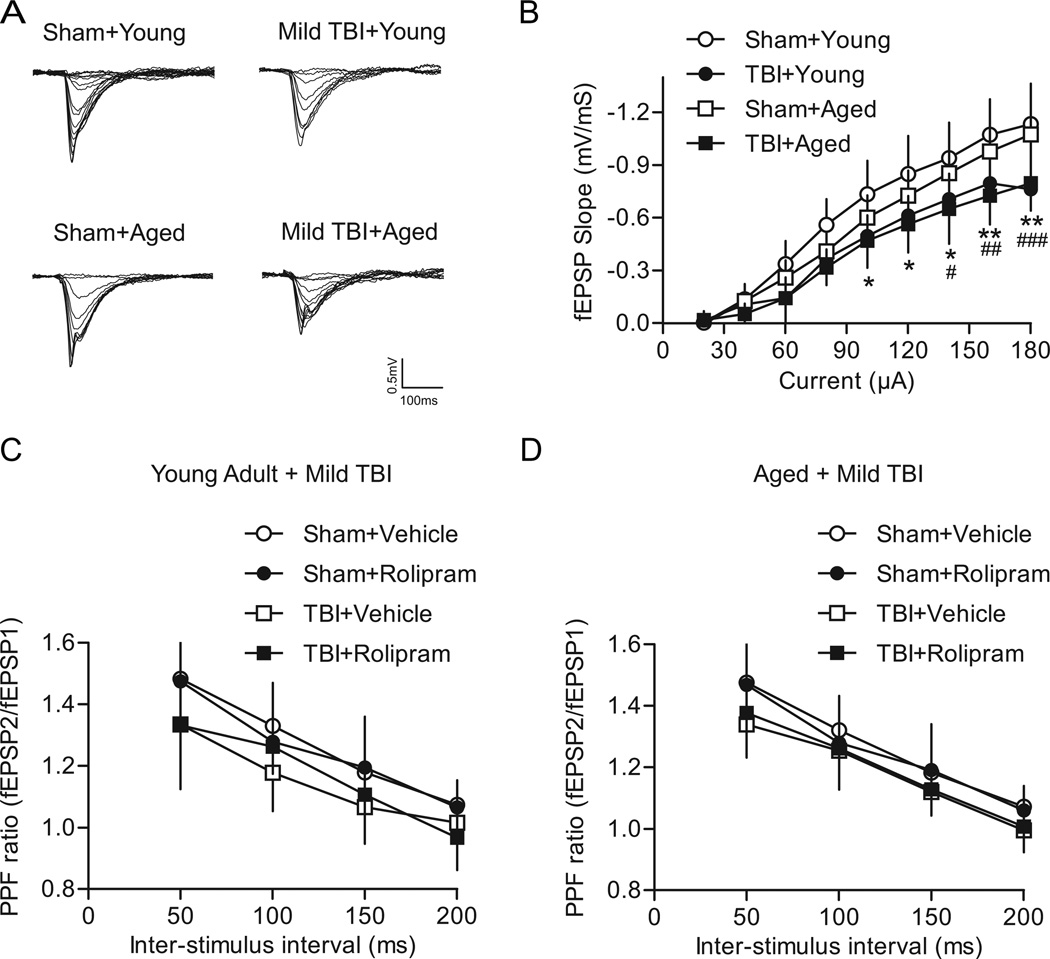
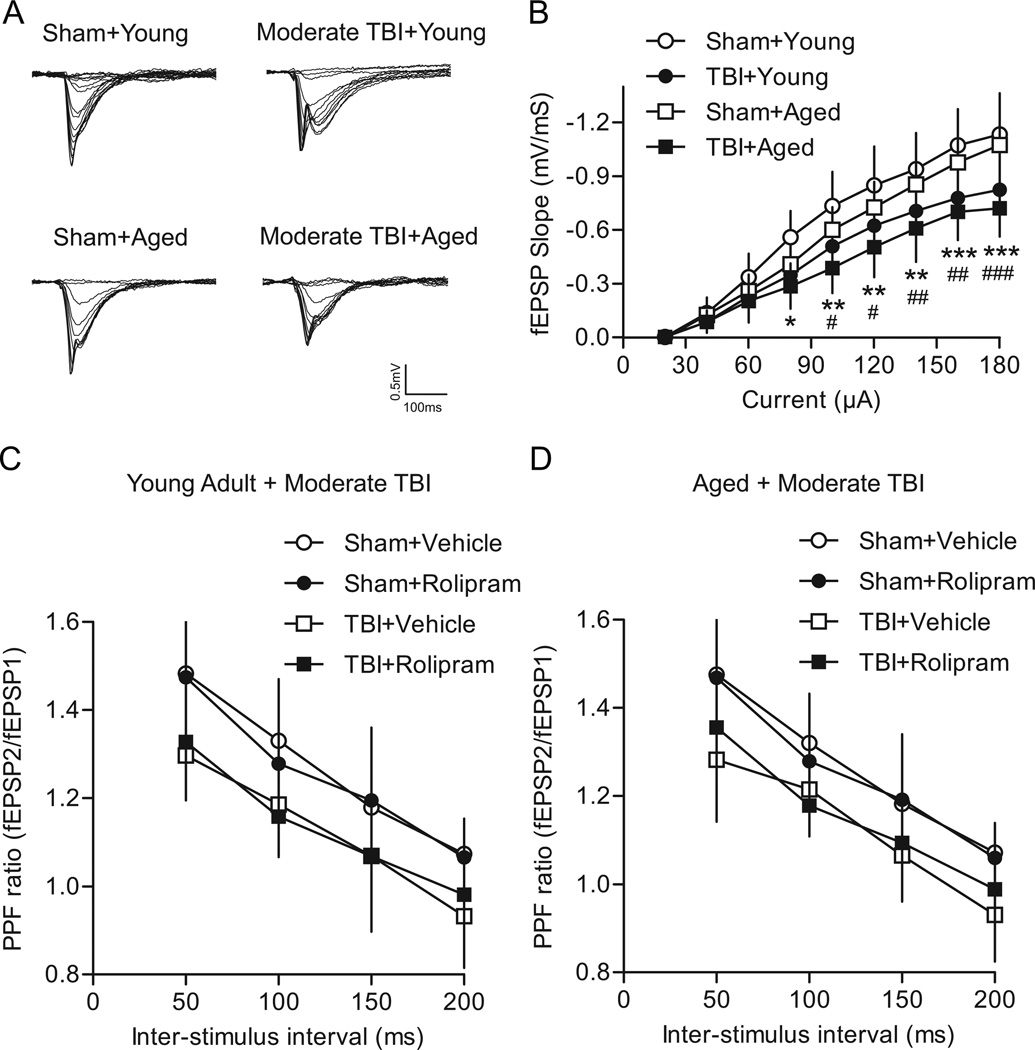
The effect of mild TBI on basal synaptic transmission and synaptic plasticity in area CA1 of the hippocampus was assessed at 2 weeks post-injury (Fig. 3). For I/O curves of the Schaffer collateral-CA1 synapse, there was a significant interaction of animal group versus current intensity (F(24,248)=3.07, P<0.001). As expected, a main effect of current intensity was found (F(8,248)=398.76, P<0.001) and a main effect of animal group (F(3,248)=6.68, P=0.001) was detected. Mild TBI caused a downward shift in the I/O relationship of young adult mild TBI animals as compared to young adult sham animals at current intensities of 100 µA (P=0.013), 120 µA (P=0.015), 140 µA (P=0.017), 160 µA (P=0.003), and 180 µA (P=0.004). Likewise, a similar depression in the I/O relationship was observed for aged mild TBI animals as compared to aged sham animals at current intensities of 140 µA (P=0.036), 160 µA (P=0.005), and 180 µA (P=0.001). There were no significant differences in the I/O curves from young adult sham versus aged sham animals, and between young adult mild TBI versus aged mild TBI animals. These results indicate that mild TBI depresses the I/O relationship for both young adult and aged animals.
Fig. 3.
Effects of mild TBI on basal synaptic transmission in area CA1 of the hippocampus. (A) Superimposed traces of fEPSPs evoked by different current intensities. (B) Summary of I/O curves of fEPSP slopes for young adult sham (n=10), young adult mild TBI (n=7), aged sham (n=9) and aged mild TBI hippocampal slices (n=9). The I/O curve was shifted significantly downward in hippocampal slices from young adult and aged mild TBI animals as compared to respective age-matched sham controls. *P<0.05, **P<0.01 for young adult sham versus young adult mild TBI animals, #P<0.05, ##P<0.01, ###P<0.001 for aged sham versus aged mild TBI animals. (C) The PPF ratio for young adult animals after mild TBI as compared to sham surgery. Rolipram (1 µM), when given for 10 min to equilibrate the slices to the drug, had no significant effect on the PPF ratio. Sham+vehicle n=9, sham+rolipram n=7, mild TBI+vehicle n=6, mild TBI+rolipram n=7. (D) The PPF ratio in aged animals after mild TBI. No significant effect of rolipram treatment was found. Sham+vehicle n=9, sham+rolipram n=7, mild TBI+vehicle n=8, mild TBI+rolipram n=8.
Presynaptic functioning after mild TBI was evaluated by measuring paired-pulse facilitation (PPF). To determine if rolipram would affect presynaptic functioning, rolipram (1 µM) or vehicle (0.1% DMSO) was pre-equilibrated in the hippocampal slices for 10 min and then PPF was assessed again in the presence of rolipram or vehicle. There was no significant interaction of animal group and inter-stimulus interval for young adult animals (F(9,75)=0.86, P=0.567; Fig. 3C) or aged animals (F(9,84)=0.53, P=0.848; Fig. 3D). As expected, PPF significantly decreased with increasing inter-stimulus intervals for both aged groups (young adult F(3,75)=95.21, P<0.001; aged F(3,84)=140.46, P<0.001). For young adult animals, there was also a main effect of animal group (F(3,75)=3.08, P=0.046), but the post-hoc analysis did not reveal any significant differences between animal groups. In aged animals, there was no significant main effect of animal group (F(3,84)=2.49, P=0.081). These results indicate that mild TBI did not alter some presynaptic mechanisms for either age group. In addition, rolipram had no significant effect on PPF for either young adult or aged animals.
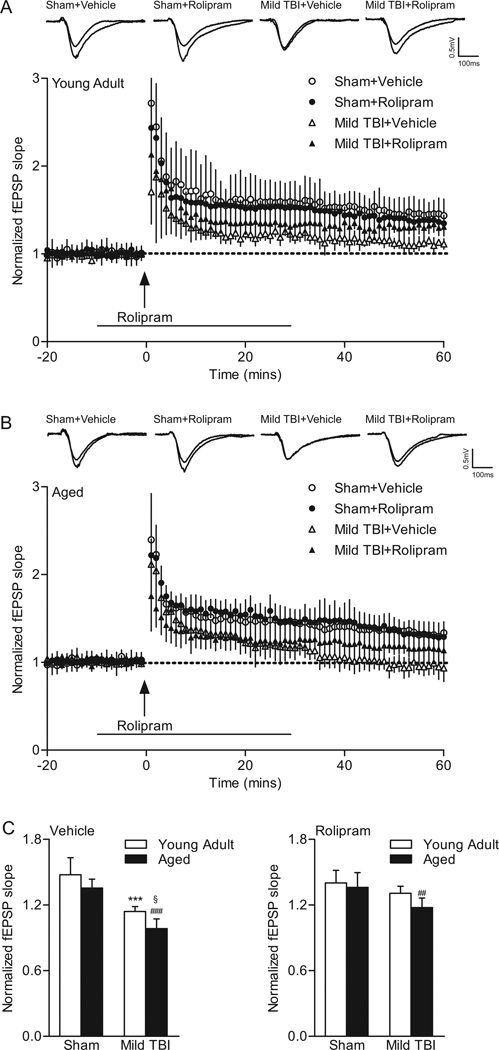
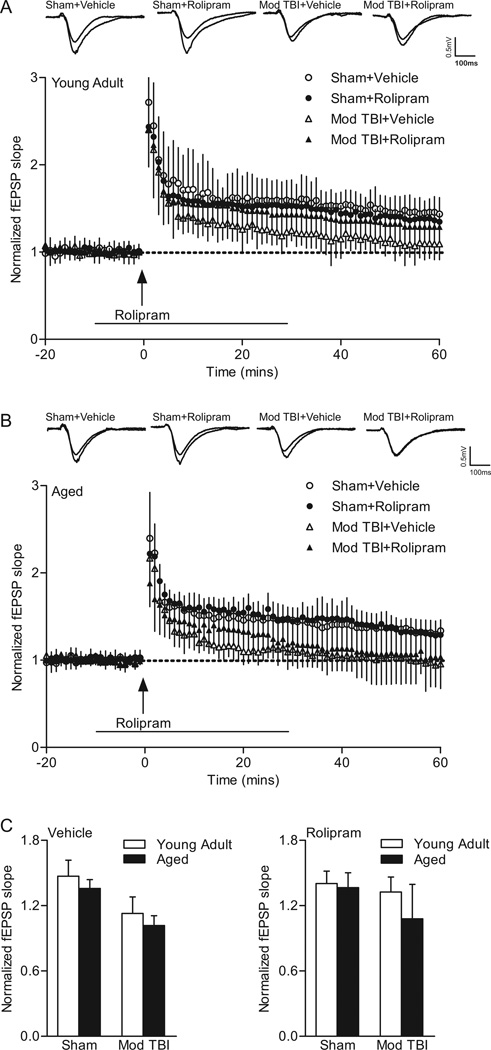
To test the effects of mild TBI on hippocampal LTP, baseline recordings were made for at least 20 min, and then hippocampal slices were stimulated with one-train 100 Hz tetanic stimulation for 1 sec in duration (Fig. 4). To determine if rolipram would affect LTP after mild TBI, rolipram (1 µM) or vehicle (0.1% DMSO) was delivered beginning 10 min prior to tetanic stimulation and for 30 min after the tetanic stimulation. In young adult animals, a repeated measures two-way ANOVA of fEPSP responses from 0–60 min post-tetanus indicated no significant interaction of animal group and time (F(177,1888)=0.96, P=0.626). Significant main effects of animal group (F(3,1888)=13.90, P<0.001) and time (F(59,1888)=47.48, P<0.001) were found. Post-hoc analysis of LTP in young adult animals indicated a significant difference between sham vehicle-treated animals as compared to mild TBI vehicle-treated (P<0.001) and mild TBI rolipram-treated (P=0.008) animals. No significant differences in LTP were observed between young adult sham animals treated with vehicle versus rolipram or for young adult mild TBI animals treated with vehicle versus rolipram. For aged animals, a repeated measures two-way ANOVA of fEPSPs from 0–60 min post-tetanus revealed a significant interaction between animal group and time (F(177,1829)=3.04, P<0.001). There were significant main effects of animal group (F(3,1829)=20.37, P<0.001) and time (F(59,1829)=84.50, P<0.001). Post-hoc analysis indicated that there was a significant difference in LTP in aged sham vehicle-treated animals as compared to both aged mild TBI vehicle-treated (P<0.001) and rolipram-treated (P<0.001) animals. LTP from 0–60 min post-tetanus was not significantly different between aged sham animals treated with vehicle versus rolipram. These results indicate that mild TBI significantly decreased LTP in both young adult and aged animals.
Fig. 4.
The phosphodiesterase IV inhibitor rolipram rescued LTP deficits caused by mild TBI in young adult animals, but not in aged animals. fEPSPs slopes were normalized to the 20 min baseline period from (A) young adult (sham+vehicle n=10, sham+rolipram n=10, mild TBI+vehicle n=8, mild TBI+rolipram n=8) and (B) aged animals (sham+vehicle n=9, sham+rolipram n=8, mild TBI+vehicle n=8, mild TBI+rolipram n=10). Rolipram (1 µM) was given for 10 min prior and for 30 min (line) after tetanic stimulation (arrow). Insets: fEPSP responses at 5 min before and 45 min after tetanization. (C) Average fEPSP slopes at 60 min post-tetanus. Mild TBI significantly reduced the mean fEPSP slope elicited by the tetanization in slices from both young adult (***P<0.001 for young adult sham+vehicle versus young adult TBI+vehicle slices) and aged animals (###P<0.001 for aged sham+vehicle versus aged TBI+vehicle slices, §P=0.024 for mild TBI young adult versus mild TBI aged animals). Rolipram completely rescued the deficits in LTP due to mild TBI in young adult animals and had a partial, non-significant rescue in aged animals (##P=0.003 for aged sham+rolipram versus aged TBI+rolipram animals).
To further explore the effects of mild TBI and rolipram on LTP expression, we analyzed the fEPSP slopes at 60 min post-tetanus with a three-way ANOVA with the factors surgery, drug, and age (Fig. 4C). There was a significant interaction between surgery and drug (F(1,63)=18.39, P<0.001) but not between age and drug (F(1,63)=1.15, P=0.288) or age and surgery (F(1,63)=1.58, P=0.213). A two-way ANOVA of surgery and drug confirmed the significant interaction (F(3,63)=6.90, P<0.001), and indicated a main effect of both surgery (F(3,63)=41.68, P<0.001) and drug (F(1,63)=8.49, P=0.005). Mild TBI resulted in a significant decrease in the potentiated response at 60 min post-tetanus for both age groups (young adult P<0.001, aged P<0.001) as compared to sham age-matched vehicle controls. In addition, mild TBI reduced the potentiated response at 60 min post-tetanus in vehicle-treated aged animals as compared to vehicle-treated young adult animals (P=0.024). Rolipram treatment rescued the deficits in LTP in young adult animals (mild TBI+rolipram versus sham+rolipram P<0.371). For aged animals, rolipram improved the deficits in LTP, but this was non-significant and the potentiation level was still significantly decreased as compared to age-matched sham surgery levels (P=0.003). These results indicate that rolipram treatment rescued the deficits in LTP at 60 min post-tetanus in young adult, but not significantly in aged animals.
Effects of rolipram on moderate TBI-induced LTP impairments in young and aged animals
Like mild TBI, moderate TBI resulted in a significant downward shift in the I/O curve for both age groups, indicative of decreased CA1 excitability after TBI (Fig. 5). A significant interaction of animal group and current intensity was observed (F(24,272)=6.04, P<0.001) as well as significant main effects of animal group (F(3,272)=7.16, P<0.001) and current intensity (F(8,272)=543.45, P<0.001). In young adult moderate TBI animals, there was a decrease in fEPSP slopes at current intensities of 80 µA (P=0.019), 100 µA (P=0.009), 120 µA (P=0.010), 140 µA (P=0.006), 160 µA (P<0.001), and 180 µA (P<0.001) as compared to young adult sham controls. Similarly, in aged moderate TBI animals, the fEPSP slopes at current intensities of 100 µA (P=0.024), 120 µA (P=0.018), 140 µA (P=0.007), 160 µA (P=0.002), and 180 µA (P<0.001) were significantly decreased as compared to aged sham controls. No significant differences in I/O curves were observed for young adult sham versus aged sham animals or for young adult moderate TBI versus aged moderate TBI animals.
Fig. 5.
Effects of moderate TBI on basal synaptic transmission. (A) Superimposed traces of fEPSPs evoked by different intensities. (B) Summary of I/O curves for young adult sham (n=10), young adult moderate TBI (n=10), aged sham (n=9), and aged moderate TBI (n=8) animals. The I/O curve was shifted significantly downward in moderate TBI animals as compared to age-matched sham controls. *P<0.05, **P<0.01, ***P<0.001 young adult moderate TBI versus young adult sham animals; #P<0.05, ##P<0.01, ###P<0.001 aged moderate TBI versus aged sham animals. Effects of moderate TBI on PPF were examined in young adult (C) and aged (D) animals. PPF was significantly decreased after moderate TBI in both vehicle and rolipram-treated young adult animals, and in aged vehicle-treated animals as compared to vehicle-treated age-matched sham controls. Young adult: sham+vehicle n=9, sham+rolipram n=7, moderate TBI+vehicle n=7, moderate TBI+rolipram n=7. Aged: sham+vehicle n=9, sham+rolipram n=7, moderate TBI+vehicle n=8, moderate TBI+rolipram n=8.
To determine if presynaptic functioning was affected after moderate TBI, PPF was assessed (Fig. 5C, D). There was no significant interaction between animal group and inter-stimulus intervals on PPF ratios for either young adult (F(9,78)=0.43, P=0.916) or aged animals (F(9,84)=0.88, P=0.551). There was an expected main effect of inter-stimulus interval for both young adult (F(3,78)=87.44, P<0.001) and aged animals (F(3,84)=142.42, P<0.001). A main effect of animal group was also observed for young adult (F(3,78)=5.79, P=0.004) and aged animals (F(3,84)=5.15, P=0.006). Moderate TBI caused a significant decrease in PPF in young adult animals treated with either vehicle (P=0.017) or rolipram (P=0.035) as compared to vehicle-treated young adult sham animals. In aged animals, moderate TBI also caused a significant decrease in PPF for vehicle-treated animals (P=0.016) as compared to vehicle-treated aged sham animals. Although there was a trend for a significant decrease in PPF in rolipram-treated aged moderate TBI animals, this was not significant (P=0.094). No significant differences in PPF were observed between vehicle-treated sham animals versus rolipram-treated sham animals, or between vehicle-treated moderate TBI animals versus rolipram-treated moderate TBI animals for either age group. These results indicate that moderate TBI caused a decrease in PPF in both age groups, and this was not rescued by rolipram treatment.
The effect of moderate TBI on hippocampal LTP was analyzed by repeated measures two-way ANOVA of fEPSP slopes between 0–60 min post-tetanus (Fig. 6). In young adult animals, no significant interaction between animal group and time (F(177, 2124)=0.57, P=1.00) was found. A main effect of animal group (F(3,2124)=9.01, P<0.001) and time (F(59,2124)=84.34, P<0.001) was detected. Post-hoc analysis of the main effect of animal groups indicated that moderate TBI caused a significant decrease in LTP in vehicle-treated young adult TBI animals as compared to vehicle-treated young adult sham animals (P<0.001). No significant effect of rolipram was found in sham or moderate TBI young adult animals, although in TBI animals, there was a trend (P=0.056). In aged animals, there was a significant interaction of animal group and time (F(177,1711)=1.33, P=0.003). There were main effects of animal group (F(3,1711)=12.06, P<0.001) and time (F(59,1711)=71.24, P<0.001). Post-hoc analysis indicated that LTP was significantly decreased in aged moderate TBI animals as compared to aged sham animals for both vehicle (P<0.001) and rolipram treatment (P=0.012). Rolipram treatment had no significant effect on LTP in aged sham animals and aged moderate TBI animals. These results indicate that moderate TBI induced LTP deficits in both young adult and aged animals and this was partially, but non-significantly rescued in young adult animals, but not in aged animals.
Fig. 6.
Rolipram did not rescue the LTP deficits caused by moderate (mod) TBI in young adult or aged animals. fEPSPs slopes were normalized to the 20 min baseline period from (A) young adult (sham+vehicle n=10, sham+rolipram n=10, moderate TBI+vehicle n=10, moderate TBI+rolipram n=10) and (B) aged animals (sham+vehicle n=9, sham+rolipram n=8, moderate TBI+vehicle n=8, moderate TBI+rolipram n=8). Rolipram (1 µM) was delivered for 10 min prior and 30 min (line) after tetanic stimulation (arrow). Insets: fEPSP responses from 5 min before and 45 min after tetanization. (C) Average fEPSP slopes at 60 min post-tetanization. Moderate TBI significantly reduced the potentiation in fEPSP slope elicited by tetanization (arrow) in both young adult and aged animals as compared to age-matched sham controls. There is a trend for reversal of the deficits in LTP by rolipram in young adult animals, but not in aged moderate TBI animals.
To further analyze the effects of rolipram on hippocampal LTP expression, a three-way ANOVA of fEPSP slopes at 60 min post-tetanus (surgery × drug × age) was conducted. This revealed a significant interaction between surgery and drug (F(1,63)=4.36, P=0.041), but not between age and drug (F(1,63)=0.16, P=0.694) or age and surgery (F(1,63)=1.86, P=0.178). When surgery and drug were analyzed with a two-way ANOVA, no significant interaction between surgery and drug (F(3,63)=2.33, P=0.083) or main effect of drug (F(1,63)=1.71, P=0.196) was detected. However, a main effect of surgery (F(3,63)=19.38, P<0.001) was observed. Post-hoc analysis of the main effect of surgery indicated that moderate TBI resulted in a significant decrease in potentiation as compared to age-matched sham controls (young adult P<0.001, aged P<0.001). The decrease in LTP at 60 min post-tetanus was not rescued by rolipram treatment in either age group, although there was a non-significant trend for a partial rescue in young adult animals.
DISCUSSION
In this study, we investigated whether TBI exacerbates changes in cAMP in aged animals. We first determined if, in non-injured aged animals, the reported decrease in basal phospho-CREB correlated with decreased basal cAMP (Karege et al., 2001a, Hattiangady et al., 2005). Surprisingly, we observed a small, but significant increase in cAMP in the aged parietal cortex and no change in the hippocampus (Davare and Hell, 2003). The increase in cAMP in the parietal cortex may be indicative of pathology as it has been reported that cAMP is increased in cortical blood vessels in Alzheimer’s disease patients (Martinez et al., 2001). Alternatively, this may reflect an age-related decrease in phosphodiesterase activity specifically in the cortex (Tohda et al., 1996). However, a caveat of this study is that the use of sham animals rather than naïve animals may have altered basal cAMP, in particular since anesthesia alone can alter signaling mechanisms in aged animals (Mawhinney et al., 2012). Several studies have reported significantly decreased adenylyl cyclase expression and activity in the cortex and hippocampus of aged animals, corresponding to our findings of decreased basal CREB phosphorylation in aged animals (Karege et al., 2001b, Mons et al., 2004, Reis et al., 2005). However, given the lack of age-related changes in basal cAMP in the hippocampus, these results suggest that the decrease in basal phospho-CREB in the aged hippocampus may also be due to increased phosphatase activity, which has been previously reported (Foster et al., 2001, Hsu et al., 2002, Monti et al., 2005).
Next, we evaluated whether TBI would reduce cAMP levels to a greater extent in aged animals as compared to young adult animals (Atkins et al., 2007). In general our findings supported the hypothesis that aging results in an exacerbation of cAMP changes after mild TBI in the hippocampus, but not in the cortex. For moderate TBI, cAMP levels decreased in both age groups. These results are consistent with previous results demonstrating that TBI results in a greater decline in mitochondria functioning and increased neuronal loss in aged animals as compared to young adult animals (Hoane et al., 2004, Onyszchuk et al., 2008, Gilmer et al., 2010).
TBI in the elderly is an under-recognized health problem, resulting in mortality rates comparable to those of Alzheimer’s disease, diabetes and influenza (Susman et al., 2002, Stocchetti et al., 2012). In this study, we found a similar effect of injury severity on mortality rates in aged animals. There was no mortality resulting from mild TBI in either age group, and in moderate TBI, there was a high attrition rate in aged animals resulting primarily from lung edema. Further studies assessing the mechanisms of pulmonary complications after TBI may shed light on why age is such a strong predictor of mortality after TBI.
An interesting observation from these studies was that there was no differential effect of TBI severity on young adult versus aged animals for I/O curves; both mild and moderate TBI depressed I/O curves to a similar degree in both age groups (Reeves et al., 2000, Witgen et al., 2005, Norris and Scheff, 2009). As previously reported, we did not observe age-related changes in basal synaptic transmission or LTP in sham animals (Barnes et al., 1997, Bach et al., 1999). Both mild and moderate TBI caused deficits in LTP, and this was rescued by rolipram treatment in young adult mild TBI animals and only partially in aged mild TBI animals. Rolipram did not rescue LTP deficits after moderate TBI in either age group (Barad et al., 1998, Navakkode et al., 2004). These results are consistent with the model that age-dependent deficits in the cAMP-PKA-CREB pathway may underlie, in part, the worse outcome in aged animals after mild TBI (Hamm et al., 1992, Maughan et al., 2000, Itoh et al., 2012). We speculate that a cAMP-PKA independent compensatory mechanism may exist in aged sham animals to maintain earlyphase LTP, but that this capacity is significantly impaired after mild TBI and completely lost after moderate TBI (Shankar et al., 1998, Hsu et al., 2002). Although NMDA receptor-dependent LTP is diminished in aged animals, L-type voltage-gated calcium channel-dependent LTP is increased (Shankar et al., 1998, Watabe and O'Dell, 2003, Boric et al., 2008). Further studies are needed to determine if voltage-gated calcium channel-dependent LTP is altered after TBI.
An important caveat to these studies is that hippocampal LTP was assessed at 2 weeks post-injury and it is unknown whether cAMP levels are still altered in aged animals at this time point after trauma. In previous studies, the age-related deficits in hippocampal LTP were attributed to a reduced ability to raise cAMP levels during tetanization, rather than decreased basal cAMP (Mons et al., 2004, Kudo et al., 2005, Monti et al., 2005, Reis et al., 2005, Porte et al., 2008). Thus, it is possible that although basal cAMP levels have returned to non-injured levels 2 weeks after brain trauma, the ability to stimulate cAMP may be more affected in aged animals after TBI as compared to young adult animals. Further studies are needed to definitively determine if rolipram rescued the deficits in LTP through cAMP-PKA-CREB regulation or through other pathways affected by phosphodiesterase IV (Lin et al., 2003).
Although rolipram partially rescued the LTP deficits in mild TBI aged animals, no recovery was observed after moderate TBI. It is unlikely that this was due to a change in basal cAMP levels because mild and moderate TBI reduced cAMP levels to comparable levels in aged animals. However, a higher dose of rolipram could have possibly rescued the LTP deficits after moderate TBI (Barad et al., 1998). Alternatively, other pathomechanisms may have also contributed to the deficits in LTP unaffected by rolipram. For example, the pro-inflammatory cytokine interleukin 1-β increases more significantly after moderate TBI as compared to mild TBI, and is known to contribute to age-related LTP impairments (Murray and Lynch, 1998, Sandhir et al., 2004, Godbout and Johnson, 2006, Shah et al., 2006). Oxidative damage is greater in aged animals after TBI, and oxidation of either α-Ca2+/calmodulin-dependent protein kinase II or protein kinase C, key mediators of LTP, may be altered in aged animals after TBI (Zhang et al., 2009, Bodhinathan et al., 2010). Dysfunction of energy homeostasis and impairments in mitochondrial metabolism are also exacerbated by age after TBI (Gilmer et al., 2010). These studies suggest that although rolipram partially rescued hippocampal LTP deficits in aged animals after mild TBI, treatments targeting other pathomechanisms should be considered for improving outcome after TBI in aged individuals. Systemic, chronic rolipram treatment rescues age-related decreases in neurotransmitter systems, such as choline acetyltransferase activity and [3H]MK-801 binding, suggesting that rolipram may improve circuitry in multiple pathways beyond cAMP in the aged brain (Asanuma et al., 1993, Kato et al., 1997, Kato et al., 1998).
CONCLUSION
We have evidence to show that mild TBI lowers cAMP levels in the hippocampus more in aged animals as compared to young adult animals. Deficits in hippocampal LTP induced by mild TBI were rescued completely by rolipram in young adult animals, but only partially in aged animals. Rolipram did not significantly rescue deficits in LTP after moderate TBI in either age group, although there was a trend for a partial rescue in young adult animals. These studies indicate that therapies utilizing combinatorial approaches including phosphodiesterase inhibitors may be a potential therapeutic avenue for the treatment of cognitive deficits in the elderly after mild TBI.
RESEARCH HIGHLIGHTS.
-
►
Mild TBI decreased cAMP in the hippocampi of aged, but not young adult animals
-
►
Moderate TBI decreased cAMP in aged and young adult rats
-
►
A phosphodiesterase inhibitor rolipram rescued LTP deficits in young adult animals
-
►
Rolipram partially rescued LTP in aged animals after mild but not moderate TBI
Acknowledgements
The authors thank Dayaris Morffi for technical assistance and Drs. W. Dalton Dietrich, H.M. Bramlett, I. Hentall, and Kaming Lo for critical reading of the manuscript. We thank the University of Miami Biostatistics Collaboration and Consulting Core for statistical support. This work was supported by NIH grants AG033266, NS069721, NS056072 and The Miami Project to Cure Paralysis.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- atm
atmospheres
- cAMP
cyclic AMP
- CREB
cAMP response-element binding protein
- fEPSP
field excitatory postsynaptic potential
- FPI
fluid-percussion brain injury
- I/O
input/output
- LTP
long-term potentiation
- MABP
mean arterial blood pressure
- PKA
protein kinase A
- PPF
paired-pulse facilitation
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009;26:1557–1566. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods. 2007;162:346–356. doi: 10.1016/j.jneumeth.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Ogawa N, Kondo Y, Hirata H, Mori A. Effects of repeated administration of rolipram, a cAMP-specific phosphodiesterase inhibitor, on acetylcholinergic indices in the aged rat brain. Arch Gerontol Geriatr. 1993;16:191–198. doi: 10.1016/0167-4943(93)90009-7. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–452. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24:439–451. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer PC, Parfitt K, Hoffer BJ, Freedman R. Desipramine and noradrenergic neurotransmission in aging: failure to respond in aged laboratory animals. Neuropharm. 1987;26:597–605. doi: 10.1016/0028-3908(87)90153-5. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric K, Munoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J Neurosci. 2008;28:8034–8039. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Hell JW. Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1.2 during aging. Proc Natl Acad Sci U S A. 2003;100:16018–16023. doi: 10.1073/pnas.2236970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths. Atlanta: GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer LK, Ansari MA, Roberts KN, Scheff SW. Age-related mitochondrial changes after traumatic brain injury. J Neurotrauma. 2010;27:939–950. doi: 10.1089/neu.2009.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm RJ, Jenkins LW, Lyeth BG, White-Gbadebo DM, Hayes RL. The effect of age on outcome following traumatic brain injury in rats. J Neurosurg. 1991;75:916–921. doi: 10.3171/jns.1991.75.6.0916. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, White-Gbadebo DM, Lyeth BG, Jenkins LW, Hayes RL. The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurg. 1992;31:1072–1077. doi: 10.1227/00006123-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Lasley LA, Akstulewicz SL. Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav Brain Res. 2004;153:189–197. doi: 10.1016/j.bbr.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Liang YC, Wu HM, Chen YL, Lo SW, Ho WC. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 2002;12:787–802. doi: 10.1002/hipo.10032. [DOI] [PubMed] [Google Scholar]
- Itoh T, Imano M, Nishida S, Tsubaki M, Mizuguchi N, Hashimoto S, Ito A, Satou T. Increased apoptotic neuronal cell death and cognitive impairment at early phase after traumatic brain injury in aged rats. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0394-5. In press. [DOI] [PubMed] [Google Scholar]
- Karege F, Lambercy C, Schwald M, Steimer T, Cisse M. Differential changes of cAMP-dependent protein kinase activity and 3H-cAMP binding sites in rat hippocampus during maturation and aging. Neurosci Lett. 2001a;315:89–92. doi: 10.1016/s0304-3940(01)02358-8. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Lambercy C, Murama JJ, Cisse M, Malafosse A. A non-radioactive assay for the cAMP-dependent protein kinase activity in rat brain homogenates and age-related changes in hippocampus and cortex. Brain Res. 2001b;903:86–93. doi: 10.1016/s0006-8993(01)02409-x. [DOI] [PubMed] [Google Scholar]
- Kato H, Araki T, Chen T, Itoyama Y, Kogure K. Effect of rolipram on age-related changes in cyclic AMP-selective phosphodiesterase in the rat brain: an autoradiographic study. Methods Find Exp Clin Pharmacol. 1998;20:403–408. doi: 10.1358/mf.1998.20.5.485701. [DOI] [PubMed] [Google Scholar]
- Kato H, Araki T, Chen T, Liu XH, Hiranuma T, Murase K, Itoyama Y, Kogure K. Effects of chronic treatment with a cyclic AMP-selective phosphodiesterase inhibitor, rolipram, on excitatory amino acid neurotransmission systems in young and aged rat brains. J Neural Transm. 1997;104:269–280. doi: 10.1007/BF01273187. [DOI] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054:30–37. doi: 10.1016/j.brainres.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem. 2003;87:1076–1085. doi: 10.1046/j.1471-4159.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- Martinez M, Hernandez AI, Hernanz A. Increased cAMP immunostaining in cerebral vessels in Alzheimer's disease. Brain Res. 2001;922:148–152. doi: 10.1016/s0006-8993(01)03009-8. [DOI] [PubMed] [Google Scholar]
- Maughan PH, Scholten KJ, Schmidt RH. Recovery of water maze performance in aged versus young rats after brain injury with the impact acceleration model. J Neurotrauma. 2000;17:1141–1153. doi: 10.1089/neu.2000.17.1141. [DOI] [PubMed] [Google Scholar]
- Mawhinney LJ, de Rivero Vaccari JP, Alonso OF, Jimenez CA, Furones C, Moreno WJ, Lewis MC, Dietrich WD, Bramlett HM. Isoflurane/nitrous oxide anesthesia induces increases in NMDA receptor subunit NR2B protein expression in the aged rat brain. Brain Res. 2012;1431:23–34. doi: 10.1016/j.brainres.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N, Segu L, Nogues X, Buhot MC. Effects of age and spatial learning on adenylyl cyclase mRNA expression in the mouse hippocampus. Neurobiol Aging. 2004;25:1095–1106. doi: 10.1016/j.neurobiolaging.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15:1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. J Neurosci. 2004;24:7740–7744. doi: 10.1523/JNEUROSCI.1796-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Scheff SW. Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J Neurotrauma. 2009;26:2269–2278. doi: 10.1089/neu.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Parfitt KD, Bickford-Wimer P. Age-related subsensitivity of cerebellar Purkinje neurons to locally applied beta 1-selective adrenergic agonist. Neurobiol Aging. 1990;11:591–596. doi: 10.1016/0197-4580(90)90022-r. [DOI] [PubMed] [Google Scholar]
- Porte Y, Buhot MC, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol Aging. 2008;29:1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Ramanathan DM, McWilliams N, Schatz P, Hillary FG. Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma. 2012;29:1371–1378. doi: 10.1089/neu.2011.2197. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Kao CQ, Phillips LL, Bullock MR, Povlishock JT. Presynaptic excitability changes following traumatic brain injury in the rat. J Neurosci Res. 2000;60:370–379. doi: 10.1002/(SICI)1097-4547(20000501)60:3<370::AID-JNR12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Reis GF, Lee MB, Huang AS, Parfitt KD. Adenylate cyclase-mediated forms of neuronal plasticity in hippocampal area CA1 are reduced with aging. J Neurophysiol. 2005;93:3381–3389. doi: 10.1152/jn.00827.2003. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Dietrich WD, Green EJ. Cognitive function following traumatic brain injury: Effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J Neurotrauma. 1999;16:915–925. doi: 10.1089/neu.1999.16.915. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Dietrich WD, Green EJ. Behavioral, electrophysiological, and histopathological consequences of mild fluid-percussion injury in the rat. Brain Res. 2001;904:141–144. doi: 10.1016/s0006-8993(01)02424-6. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Shah SA, Prough DS, Garcia JM, DeWitt DS, Hellmich HL. Molecular correlates of age-specific responses to traumatic brain injury in mice. Exp Gerontol. 2006;41:1201–1205. doi: 10.1016/j.exger.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Shao C, Roberts KN, Markesbery WR, Scheff SW, Lovell MA. Oxidative stress in head trauma in aging. Free Radic Biol Med. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Paterno R, Citerio G, Beretta L, Colombo A. Traumatic brain injury in an aging population. J Neurotrauma. 2012;29:1119–1125. doi: 10.1089/neu.2011.1995. [DOI] [PubMed] [Google Scholar]
- Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Tohda M, Murayama T, Nogiri S, Nomura Y. Influence of aging on rolipram-sensitive phosphodiesterase activity and [3H]rolipram binding in the rat brain. Biol Pharm Bull. 1996;19:300–302. doi: 10.1248/bpb.19.300. [DOI] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. Curr Popul Rep. 2010:1125–1138. [Google Scholar]
- Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- Watabe AM, O'Dell TJ. Age-related changes in theta frequency stimulation-induced long-term potentiation. Neurobiol Aging. 2003;24:267–272. doi: 10.1016/s0197-4580(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Wilson JA, Pentland B, Currie CT, Miller JD. The functional effects of head injury in elderly. Brain Inj. 1988;1:183–188. doi: 10.3109/02699058709034456. [DOI] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Zhang GR, Liu M, Cao H, Kong L, Wang X, O'Brien JA, Wu SC, Cook RG, Geller AI. Improved spatial learning in aged rats by genetic activation of protein kinase C in small groups of hippocampal neurons. Hippocampus. 2009;19:413–423. doi: 10.1002/hipo.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]