Abstract
Rationale
We test methods to advance (shift earlier) circadian rhythms without producing misalignment between rhythms and sleep. We previously tested 1) a gradually advancing sleep/dark schedule plus morning bright light and afternoon/evening melatonin; and 2) the same sleep schedule with only morning bright light. Now we report on the same sleep schedule with only afternoon/evening melatonin.
Objectives
To examine phase advances, sleepiness and performance in response to melatonin compared to placebo.
Methods
Twelve adults (5 female) aged 20–45 years (mean ± SD = 28.3 ± 7.3 years) completed this within-subjects placebo-controlled counterbalanced study. Participants slept on fixed 8-hour sleep schedules for 9 baseline days. Then, sleep/dark was advanced by 1 h/day for 3 consecutive days of treatment. Participants took 3 mg of melatonin or placebo 11 hours before baseline sleep midpoint (the optimal time to produce phase advances) on the first treatment day and 1 hour earlier each subsequent day. We measured the dim light melatonin onset (DLMO) before and after treatment. Participants rated subjective symptoms throughout the study. They completed the Psychomotor Vigilance Task (PVT) and rated sleepiness from 1 h before pill ingestion until bedtime each treatment day.
Results
Melatonin produced significantly larger advances (1.3 ± 0.7 h) compared to placebo (0.7 ± 0.7 h); however, in the hours between melatonin ingestion and bed, melatonin caused sleepiness and performance decrements.
Conclusions
Adding afternoon/evening melatonin to the gradually advancing sleep schedule increased the phase advance, but given the side effects, like sleepiness, it is better to use morning bright light and perhaps a lower dose of melatonin.
Keywords: circadian rhythms, melatonin, human, dim light melatonin onset, phase shift, phase advance, sleepiness, psychomotor vigilance task, circadian misalignment, jet lag
Introduction
Despite an intrinsic drive promoting sleep and wakefulness, external factors often cause humans to be awake and asleep at inappropriate times with respect to the individual’s circadian system; for example, after traveling across several time zones or when working early morning shifts or night shifts. Misalignment between the circadian clock and the sleep/wake schedule (“circadian misalignment”) is associated with sleep difficulties, decrements in daytime functioning and mood, and gastrointestinal distress (Akerstedt 1988; Akerstedt and Wright 2009; Boulos et al. 1995; Drake et al. 2004; Graeber 1982; Haimov and Arendt 1999; Knutsson 2003; Waterhouse et al. 2000). Chronic circadian misalignment increases the risk for long-term health consequences, including cardiovascular (Costa 1996; Kitamura et al. 2002; Knutsson et al. 1986; Koller 1983; Lo et al. 2008; Ohira et al. 2000; Yamasaki et al. 1998), metabolic (Karlsson et al. 2001; Morikawa et al. 2007), reproductive (Costa 1996; Iglesias et al. 1980), and gastrointestinal dysfunction (Costa 1996; Knutsson and Boggild 2010; Koller 1983), as well as cancer (Blask 2009; Erren et al. 2010; Hansen 2006; Rafnsson et al. 2001; Reynolds et al. 2002; Viswanathan et al. 2007) and cognitive deficits (Cho 2001). Recent findings suggest that for every one hour increase in “social jet lag” (the difference between mid-sleep time on free and work days), risk of obesity increases 3-fold (Roenneberg et al. 2012). Even short-term circadian misalignment in healthy participants impairs metabolic responses that are risk factors for cardiovascular disease and type II diabetes mellitus (Hampton et al. 1996; Scheer et al. 2009).
The circadian system is capable of shifting to adjust to a new light/dark (LD) cycle, such as after jet travel; however, the shift is gradual, not instantaneous. Also, because most humans have an endogenous period that is slightly longer than 24 h (Burgess and Eastman 2008; Czeisler et al. 1999; Eastman et al. 2012; Smith et al. 2009a; Wever 1979), we have an innate tendency to delay (drift later). Therefore, advancing the system (shifting it earlier) is more difficult and typically takes longer than delaying. After flying east, circadian rhythms need to advance to align with sleep and wake in the new time zone. Phase advancing would also benefit shift workers who have early morning shifts or who want to sleep before a night shift, and extreme night owls or patients with delayed sleep phase disorder (DSPD) who struggle to wake up for work or school.
When given exogenously in pill form, melatonin can phase shift the circadian system. Similar to bright light, exogenous melatonin can advance and delay the clock, and the response can be predicted by a phase response curve (PRC) (Burgess et al. 2008; Burgess et al. 2010; Lewy et al. 1998). Maximum advances are produced when 0.5 or 3.0 mg of melatonin is taken in the afternoon/evening, 10 to 11 h before sleep midpoint (Burgess et al. 2010). Other studies using doses ranging from 0.05 to 8 mg have also shown that melatonin can produce circadian phase advances when taken in the afternoon or evening hours (Attenburrow et al. 1995; Deacon and Arendt 1995; Deacon et al. 1994; Krauchi et al. 1997; Mallo et al. 1988; Mundey et al. 2005; Nagtegaal et al. 1998; Paul et al. 2010; Rajaratnam et al. 2003; Samel et al. 1991; Sharkey and Eastman 2002; Wirz-Justice et al. 2004; Yang et al. 2001). Most of these previous studies, however, did not advance sleep/dark, which can restrict how much the circadian system advances. Others (Paul et al. 2010; Rajaratnam et al. 2003) did not test a practical sleep schedule to be used in the real world; participants were kept in bed for 14 or 16 hours after ingesting the pill. Our laboratory uses a gradually shifting sleep/dark schedule to phase shift rhythms (Burgess et al. 2003; Eastman et al. 2005; Eastman and Miescke 1990; Revell et al. 2006; Smith and Eastman 2009; Smith et al. 2009b), and it may also reduce the degree of circadian misalignment during treatment. Our goal is to shift circadian rhythms at the same rate as sleep in order to keep the two aligned. While using a sleep schedule that was advanced by 1 h/day for 3 days, we previously tested the combination of morning bright light and afternoon/evening melatonin (Revell et al. 2006) and morning bright light alone (Burgess et al. 2003) to produce a phase advance. Here we report on using our gradually advancing sleep/dark paradigm with afternoon/evening melatonin alone.
The primary aim of this study was to examine the phase advancing effects of a gradual advance of sleep/dark (1 hour/day) plus 3.0 mg afternoon/evening melatonin compared to placebo. Secondary aims included: (1) to examine whether self-reported symptoms often associated with circadian misalignment were elevated during treatment; and (2) to examine subjective sleepiness and objective performance in the hours between ingesting melatonin and bedtime in comparison to placebo.
Materials and methods
Participants
Fifteen healthy young adults (6 female) were enrolled in a 5-week experimental protocol. Two participants (1 female) were dropped from the study due to non-compliance with the study protocol. One male participant discontinued for personal reasons. Therefore, 12 participants aged 20 to 45 years (mean ± SD = 28.3 ± 7.3 years) completed the study. Data were collected during all months of the year, except December and January at 41 degrees northern latitude.
Participants were free of medical and psychiatric disorders, as assessed by in-person interviews, the Minnesota Multiphasic Personality Inventory-2 (MMPI-2), and part of a health questionnaire (Tasto et al. 1978). Participants reported not taking any prescription medications, except for 3 women who were taking oral contraceptives. Participants reported no more than moderate alcohol (2 or fewer drinks per day) and caffeine (< 300 mg per day) intake, and were non-smokers. Body mass index (BMI) for all participants was < 30 (mean ± SD = 23.6 ± 3.1), and they weighed between 51 and 86 kg (mean ± SD = 70.2 ± 11.3 kg). One female began the study while in the follicular phase of her menstrual cycle and one female was in the luteal phase. The 3 female participants on oral contraceptives started the study while in the pseudo-luteal phase.
Inclusion criteria included habitual sleep duration between 6.5 and 9 h per night, habitual bedtimes between 23:00 and 02:00, and habitual wake times between 07:00 and 10:00. Participants reported no sleep problems over the proximal month of enrollment as assessed by a Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. 1989) score of 5 or less, and no problems with excessive daytime sleepiness as assessed by an Epworth Sleepiness Scale (Johns 1991) score of less than 10. Morningness-eveningness was measured using the Horne-Östberg questionnaire (Horne and Ostberg 1976); 8 participants were intermediate types, 3 were moderate morning types, 1 was moderate evening type (mean = 52.6, SD=7.2). Participants reported not working night shifts or crossing more than 3 times zones in the month before beginning the study.
The Rush University Medical Center Institutional Review Board approved the study protocol, and therefore, the study was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. Each participant gave informed consent and received monetary compensation for participating.
Study Design
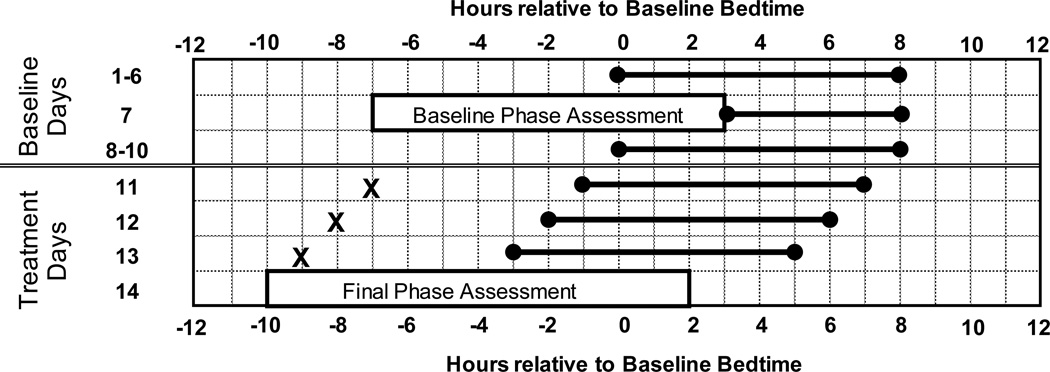
Participants completed a 35-day within-subjects protocol with two conditions (3.0 mg melatonin and placebo) counterbalanced for order. For each condition, participants completed a 2-week protocol (see Figure 1) with a 7-day wash-out period in between. Each participant was given a fixed sleep schedule to follow at home during baseline providing 8 h of time in bed. The sleep schedule assigned to each participant was based on their reported average bedtime and wake time before starting the study. Assigned average baseline bedtime was 00:23 (SD=1.0 h), and bedtimes ranged from 23:00 to 02:00. Thus, average wake time was 8:23, and ranged from 07:00 to 10:00. Participants were instructed to go to bed and wake up within ± 30 minutes of their assigned baseline bedtime and wake-up time during the 7-day wash-out period. Each morning during baseline, participants were required to go outside for at least 10 minutes during the second hour after waking for daylight exposure. The fixed sleep schedule and morning light was designed to stabilize circadian phase and ensure that participants were not sleep deprived before the phase advancing treatment in the laboratory. Participants slept at home on days 1–6 and 8–10; on the other days they slept in private temperature-controlled bedrooms in the laboratory. Participants lived in the laboratory from days 11 through 14. Bedroom lights were controlled by staff members in a separate control room. During waking hours the intensity in the bedrooms and adjoining hallway and bathroom was < 60 lux.
Fig 1.
Two-week protocol plotted relative to baseline bedtime, which was assigned based on the individual’s habitual sleep schedule. Participants completed this protocol twice – once with melatonin and once with placebo – in a counterbalanced order. The protocol has two parts, baseline days (days 1–10) and treatment days (days 11–13). Days 11–13 are referred to as treatment days 1–3 in most of the text and figures. Horizontal lines with dots on the ends illustrate sleep/dark times, which were advanced 1 hour/day during treatment days. During phase assessments, saliva was sampled every 30 minutes in dim light to determine the dim light melatonin onset (DLMO). The phase assessments were timed according to the assigned baseline sleep schedule. Melatonin or placebo pills (illustrated by an X) were given 11 h before baseline sleep midpoint (7 hours before baseline bedtime) on the first treatment day, and then 1 hour earlier each treatment day.
Participants were permitted to consume caffeine (up to 100 mg) during the first 3 h of wake on baseline days 1–3. A maximum of 2 standard alcoholic drinks were permitted on Fridays and Saturdays (days 1, 2, 8, and 9) of the study. Participants were tested for alcohol using a breathalyzer each time they came to the laboratory. Caffeine and alcohol were permitted during the wash-out period, but participants were asked to consume these substances in moderation. Non-steroidal anti-inflammatory drugs were not permitted during the entire study, as these drugs suppress melatonin (Murphy et al. 1996). Recreational drugs and tobacco were prohibited throughout the study, and all participants were screened for common drugs of abuse when they started the study.
Procedures
Exogenous Melatonin Treatment
Participants were given either 3.0 mg melatonin or matching placebo (Ecological Formulas, Cardiovascular Research Ltd., Concord CA) on each treatment day. Pill administration was double-blind. Participants did not eat or drink anything 2 h before until 30 minutes after ingesting the pills. Participants provided saliva samples 1 and 2 h after pill ingestion to confirm that melatonin pills were correctly administered. These samples were later assayed for melatonin concentration. Six participants received melatonin first and 6 received melatonin second.
On the first treatment day we administered the 3.0 mg dose of melatonin 11 h before the participant’s baseline mid-sleep time to obtain the largest phase advance as predicted by the 3.0 mg PRC (Burgess et al. 2010). The timing of the pills was advanced by 1 hour each day because theoretically the circadian system is advancing each day; timing the dose earlier each day increased the likelihood that exogenous melatonin continued to fall near the optimal time of the melatonin PRC. We did not administer melatonin according to body weight because this is not the way it is sold to consumers. The weights of our participants ranged from 51 to 86 kg; thus, the doses ranged from 0.035 to 0.059 mg/kg.
Dim Light Melatonin Onset (DLMO) Phase Assessments
Endogenous salivary melatonin concentration was measured from approximately 2 mL of saliva collected every 30 minutes using Salivettes (Starstedt, Nümbrecht, Germany). Saliva collection for the baseline phase assessment began 7 h before and ended 3 h after assigned baseline bedtime and for the final phase assessment began 10 h before and ended 2 h after baseline bedtime. Participants remained awake in dim light (< 5 lux), sitting in comfortable recliners. Each sample was immediately centrifuged to extract saliva and then frozen. Saliva samples were later radioimmunoassayed (RIA) for melatonin concentration (Pharmasan Labs, Inc. Osceola, WI, USA). An individual’s samples were analyzed in the same batch. The sensitivity of the assay was between 0.7 and 1 pg/ml. The intra-assay coefficient of variation for low daytime levels of salivary melatonin was 12%. The inter-assay coefficients of variation for low daytime levels of salivary melatonin ranged from 13.2 to 15.9%.
Sleep and Ambient Light Monitoring
Participants completed daily sleep logs on a personal data assistant (PDA; palmOne, Tungsten E2), where they recorded bedtime, estimated sleep onset time, night time awakenings > 5 mins, final wake time, and the time they got out of bed. Participants wore activity monitors (Actiwatch-L, Philips Respironics, Inc., Bend OR, USA) on their dominant wrist, which may distinguish sleep from quiet waking better than when worn on the non-dominant wrist. Participants also wore an Actiwatch with photosensor, but without the wrist band, around their necks as a medallion (Actiwatch-L, Philips Respironics, Inc., Bend OR, USA) to measure compliance to the 10-minute outdoor morning light requirement. Wrist activity data were collected in 30-second epochs using medium sensitivity. When sleeping at home, participants called the laboratory’s time-stamped voice mail system at bedtime and wake-up time. Activity and light data with the daily logs were reviewed with participants every 2 to 3 days at the laboratory to verify compliance.
Subjective Symptoms
Within 30 minutes of scheduled bedtime on baseline and treatment days, participants completed the Columbia Jet Lag Scale (Spitzer et al. 1999), which asked them to rate how they felt throughout the entire day. Also throughout the study, participants completed the Stanford Sleepiness Scale (SSS) (Hoddes et al. 1973), and the “How Are You Feeling Right Now” (HAYFRN) questionnaire (both prompted by pre-programmed alarms) on the PDA four times per day: 0.5 h and 5.5 h after waking and 6 h and 1 h before bed. The SSS is a 7-point Likert Scale with the following anchored descriptions: 1=feeling active and vital; alert, wide awake; 2= functioning at high level, but not at peak; able to concentrate; 3= relaxed; awake; not at full alertness; responsive; 4= a little foggy; not at peak; let down; 5= fogginess; beginning to lose interest in remaining awake; slowed down; 6= sleepiness; prefer to be lying down; fighting sleep; woozy; and 7=almost in reverie; sleep onset soon; must struggle to remain awake. The HAYFRN questionnaire included 6 questions to assess current symptoms (physical fatigue, mental fatigue, sadness, anxiety, irritability, and gastrointestinal distress) on a scale from 1 (“very little”) to 10 (“very much”).
On treatment days, participants also rated their sleepiness using the SSS (Hoddes et al. 1973) and the Karolinska Sleepiness Scales (KSS) (Akerstedt and Gillberg 1990) once per hour beginning 1 h before taking the pill until bedtime. The KSS is a 9-point verbally anchored scale with the following anchored steps: 1=extremely alert, 3=alert, 5=neither alert nor sleepy, 7=sleepy, but no difficulty remaining awake, and 9=extremely sleepy, fighting sleep. The points in between had a scale value, but no verbal cue.
Psychomotor Vigilance Task (PVT)
The Psychomotor Vigilance Task (PVT) is a test of sustained attention, and performance on this test shows minimal learning effects in adults (Dinges et al. 1997). Participants completed the PVT on the PDA on treatment days every hour beginning 1 h before taking the pill until bedtime. Participants completed the PVT during baseline and twice each treatment day before these hourly tests to overcome any potential practice effects. The Palm-based vigilance task (PalmPVT©), developed by the Walter Reed Army Institute of Research1, is modeled after the original laboratory-based PVT (Dinges and Powell 1985). The general pattern of responses on the PalmPVT tracks that of the original laboratory-based PVT during baseline periods, and when wakefulness is extended to 28 h (Lamond et al. 2005), 40 h (Thorne et al. 2005), and 62 h (Lamond et al. 2008). Participants responded to a visual stimulus (bulls-eye) as quickly as possible by pressing a designated button on the device with the dominant thumb, after which their reaction time in milliseconds appeared on the screen. PDAs were initialized at the laboratory using the PalmPVT© (version 2.0.1) software. Test settings included the following: a 5-minute testing session, immediate feedback of reaction time in milliseconds after each stimulus was presented, and a random inter-stimulus time interval ranging from 1 to 5 seconds. The following three outcome variables derived from the PalmPVT assessed slowing and variability in performance: median reaction time (RT), mean optimal RT (mean of the 10% fastest RTs), and lapses (number of responses > 500 msec).
Data and Statistical Analysis
Dim Light Melatonin Onset (DLMO)
The raw melatonin curves were smoothed using a locally weighted least squares (LOWESS) curve generated using Prism software (GraphPad, Inc., San Diego CA, USA). The threshold for each phase assessment was the minimum of the fitted curve plus 25% of the distance from the fitted minimum to the fitted maximum. Then, the 2 thresholds within each condition were averaged. The DLMOs were the clock times at which the smoothed curves crossed this averaged threshold. The average (± SD) DLMO threshold across all participants and across both conditions was 10.37 (± 2.27) pg/mL. The phase shift is the baseline DLMO minus the final DLMO; by convention, phase advances are positive numbers and phase delays are negative numbers.
A 2 (condition: melatonin vs. placebo)-by-2 (time: baseline vs. final phase assessment) repeated measures ANOVA was computed to determine whether the DLMO shifted more with melatonin than with placebo.
Actigraphically estimated sleep
Wrist activity data were analyzed using the Actiware 5 software (version 5.59, Respironics, Inc., Bend Oregon, USA) to estimate sleep/wake (medium threshold). Each sleep episode was inspected within a rest interval beginning with the participants’ reported bedtime and ending with their reported wake up time on their daily sleep log. The following variables were derived: Sleep Onset Time, Wake Time, and Total Sleep Time.
Subjective Symptoms: Baseline vs. Treatment Days
Subjective symptoms were analyzed from the SSS and HAYFRN questionnaires, and from the Columbia Jet Lag Scale. Subjective ratings on days 2–6 were averaged to define baseline.
To examine how the ratings on the SSS and HAYFRN questionnaires changed across the 4 times of day, and to determine whether these changes differed between baseline and treatment days, we computed 3 (condition: baseline vs. placebo vs. melatonin)-by-4 (time of day: after wake vs. morning/afternoon vs. afternoon/evening vs. before bed) repeated measures ANOVAs. SSS and HAYFRN fatigue ratings from the first time of day (30 minutes after wake) were examined in more detail because this time of day is likely the most vulnerable to decrement when sleep and wake are advanced. To examine whether these subjective ratings 30 minutes after waking differed between baseline and the 3 treatment days, and whether these ratings differed between melatonin and placebo conditions, we computed 2 (condition: melatonin vs. placebo)-by-4 (day: baseline, treatment day 1, 2, and 3) repeated measures ANOVAs. The same analysis was used to examine the daily Columbia Jet Lag Scale ratings among baseline and treatment days, and between melatonin and placebo conditions. When the assumption of sphericity was violated, Greenhouse-Geisser corrections were used, though the original degrees of freedom are reported here.
Psychomotor Vigilance and Subjective Sleepiness: Acute effects of Melatonin vs. Placebo
PVT Median RTs, PVT Optimal RTs, PVT Lapses, SSS ratings, and KSS ratings were collected 1 hour before the pill (“pre-pill”), at pill time, at hourly intervals after the pill, and at bedtime (5.75 h after the pill). Because these measures typically show high degrees of between-subject variability (Van Dongen et al. 2004), each participant’s pre-pill values were subtracted from values at each subsequent time point. To examine the acute effects of 3.0 mg melatonin on the PVT and SSS, we computed 2 (condition: melatonin vs. placebo)-by-7 (time: 0, 1, 2, 3, 4, 5, and 5.75 h after pill) repeated measures ANOVAs. When the assumption of sphericity was violated, Greenhouse-Geisser corrections were used, though the original degrees of freedom are reported here.
Results
Dim Light Melatonin Onset (DLMO) Phase Shifts
Complete melatonin data were available for 11 participants. The baseline DLMO occurred 3.0 ± 0.9 h before baseline bedtime, and melatonin was administered 4.0 ± 0.9 h before the baseline DLMO on Treatment Day 1.
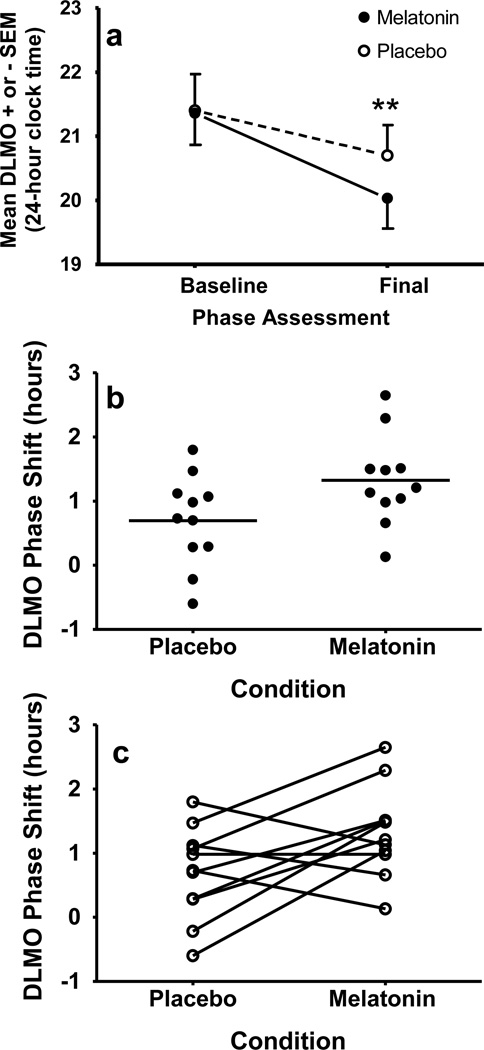
Baseline and final DLMOs, and DLMO phase shifts for each condition are illustrated in Figure 2 and Table 1. The baseline DLMO did not differ between the melatonin and placebo conditions (Table 1 and Fig 2a), which shows that circadian phase changes after one condition did not carry over to the other condition. The DLMO advanced in both conditions [time effect: F(1,10)=37.86, p < .001], but the DLMO advanced more with melatonin than with placebo [condition-by-time interaction: F(1,10)=5.46, p=.042]. Adding melatonin to the gradual advance of the sleep schedule shifted the DLMO an average of 38 minutes (± 54 min) more than placebo. Fig 2, panels b and c, show that there was overlap in the amount of phase shift between the conditions, but no participants delayed in the melatonin condition. Panel c shows that the majority of participants (7 of 11) advanced more in the melatonin condition compared to the placebo condition.
Fig 2.
DLMO phase shifts in response to a gradual advance of sleep (1 hour/day) with melatonin or placebo. a) Average baseline and final DLMOs. ** = p< 0.01, b) Individual phase shifts; mean phase shifts are indicated by horizontal lines. c) Lines are drawn to connect each individual’s phase shifts. In b and c positive numbers indicate phase advances.
Table 1.
Circadian phase and phase shifts marked by the dim light melatonin onset (DLMO). Participants completed melatonin and placebo conditions in counterbalanced order.
| Baseline DLMO | Final DLMO | Phase Advance (h) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Placebo | 21:24 (1:54) | 20:42 (1:36) | 0.7 (0.7) |
| Melatonin | 21:22 (1:36) | 20:02 (1:36) | 1.3*(0.7) |
Significantly different from placebo (p<0.05).
Sleep
Actigraphic estimates of sleep are shown in Table 2. Participants fell asleep soon after lights out and woke up slightly before lights on during baseline and during treatment days. Thus, they fell asleep and woke up about one hour earlier each treatment day, as planned. There were no differences in total sleep time between baseline and treatment days or between the melatonin and placebo conditions. Therefore, we did not covary total sleep time in subsequent analyses.
Table 2.
Scheduled bedtime (lights out) and scheduled wake time (lights on) in 24 hour clock time and scheduled time in bed in the dark (in hours). Sleep onset, wake time and total sleep time were determined by wrist actigraphy.
| Baseline | Treatment | |||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Bedtime (lights out) | 00:23 (1:00) | 23:23 (1:00) | 22:23 (1:00) | 21:23 (1:00) |
| Placebo Sleep Onset | 00:30 (1:00) | 23:36 (1:00) | 22:33 (1:00) | 21:31 (0:54) |
| Melatonin Sleep Onset | 00:30 (1:00) | 23:28 (1:00) | 22:28 (1:00) | 21:30 (1:00) |
| Wake time (lights on) | 08:23 (1:00) | 07:23 (1:00) | 06:23 (1:00) | 05:23 (1:00) |
| Placebo Wake Time | 08:16 (1:00) | 07:18 (1:00) | 06:17 (1:00) | 05:18 (1:00) |
| Melatonin Wake Time | 08:15 (1:00) | 07:18 (1:00) | 06:14 (1:00) | 05:13 (1:00) |
| Time in Bed in the Dark | 8.0 (0) | 8.0 (0) | 8.0 (0) | 8.0 (0) |
| Placebo Total Sleep time | 7.2 (0.4) | 7.3 (0.4) | 7.3 (0.3) | 7.2 (0.5) |
| Melatonin Total Sleep Time | 7.2 (0.4) | 7.3 (0.3) | 7.2 (0.5) | 7.2 (0.4) |
Subjective Symptoms: Baseline vs. Treatment Days
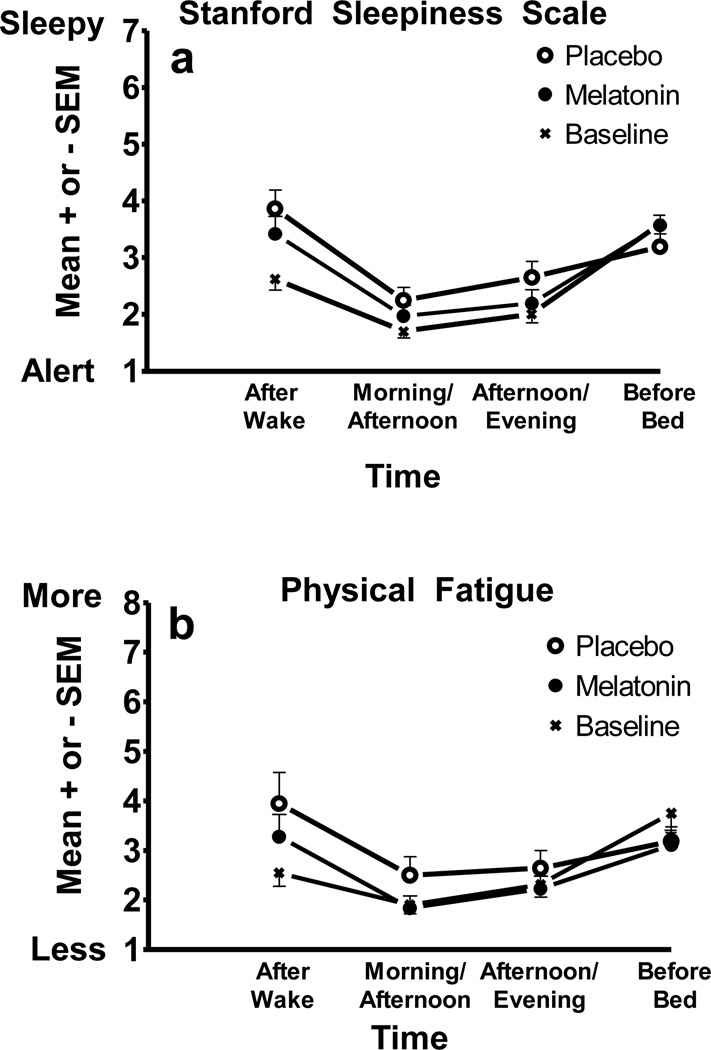
Figure 3 shows the SSS scores (panel a) and the Physical Fatigued scores (panel b) from the HAYFRN questionnaire. Participants were most sleepy and fatigued right after waking and right before bed, and less so in the middle of the day. Significant time-of-day effects emerged for sleepiness [F(3,33)=28.00, p<.001], physical fatigue [F(3,33)=16.21, p<.001], and mental fatigue [F(3,33)=16.76, p<.001]. Polynomial contrasts showed that a quadratic trend explained the pattern of change across the day for sleepiness [F(1,11)=69.62, p<.001], physical fatigue [F(1,11)=25.02, p<.001], and mental fatigue [F(1,11)=28.78, p<.001]. Sadness, anxiety, irritability, and gastrointestinal distress did not change across the day.
Fig 3.
a) Subjective sleepiness from the SSS and b) physical fatigue ratings completed 0.5 h after waking, 5.5 h after waking (Morning/Afternoon), 6 h before bed (Afternoon/Evening), and 1 h before bed. The sleepiness scale maximum is 7. The physical fatigue scale maximum is 10. Scores were averaged for baseline days 2–6 and the 3 treatment days.
SSS ratings differed between baseline and the treatment days [F(2,22)=5.11, p=.015], though this difference was primarily driven by ratings in the morning soon after waking. Simple contrasts showed that 30 minutes after wake, participants reported more sleepiness during treatment days compared to baseline, regardless of whether they took melatonin or placebo in the afternoons (p’s < .001). Fatigue, sadness, anxiety, irritability, and gastrointestinal distress ratings from the HAYFRN questionnaire did not significantly differ between baseline and treatment days.
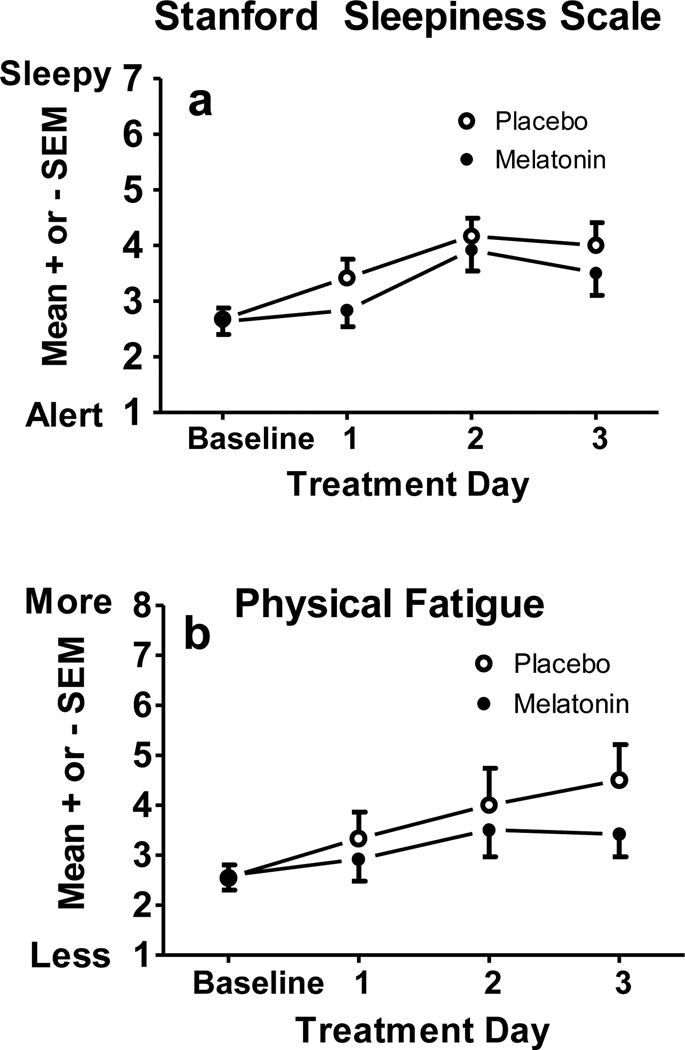
A more detailed look at the sleepiness and fatigue ratings taken 30 minutes after waking are shown in Figure 4. Participants became sleepier and more fatigued after waking as the treatment days progressed. A significant treatment day effect emerged for SSS [F(3,33)=14.15, p < .001], physical fatigue [F(3,33)=5.68, p=.003], and mental fatigue [F(3,33)=3.80, p=.045]. Simple contrasts show that participants were sleepier and more physically fatigued after waking on all treatment days compared to baseline (p’s < .05). Mental fatigue (graph not shown) followed the same pattern. Sleepiness and fatigue ratings were not significantly different between melatonin and placebo conditions, though non-significant trends emerged for SSS [F(1,11)=4.38, p=.06] and physical fatigue [F(1,11)=4.26, p=.06] showing less morning sleepiness and fatigue in the melatonin condition. Ratings of sadness, anxiety, irritability, and gastrointestinal distress did not differ among baseline and treatment days.
Fig 4.
a) Subjective sleepiness from the SSS and b) physical fatigue ratings 0.5 h after waking. The sleepiness scale maximum is 7. The physical fatigue scale maximum is 10. Baseline was the average of days 2–6.
Subjective symptoms after waking did not differ between melatonin and placebo conditions for any of the subjective ratings at baseline, which suggests that elevated ratings of sleepiness and fatigue experienced at the end of one condition did not carry over to the next.
Columbia scale scores did not significantly change from baseline to Treatment Days 1, 2, and 3 [time effect: F(3,33)=1.51, p=.24]. A trend for a difference between conditions [condition effect: F(1,11)=4.58, p=.06], and a time-by-condition interaction [F(3,33)=2.43, p=.08] emerged; Columbia scale scores did not differ between conditions at baseline, but scores were slightly elevated in the melatonin condition compared to the placebo condition.
Psychomotor Vigilance and Subjective Sleepiness: Acute effects of Melatonin vs. Placebo
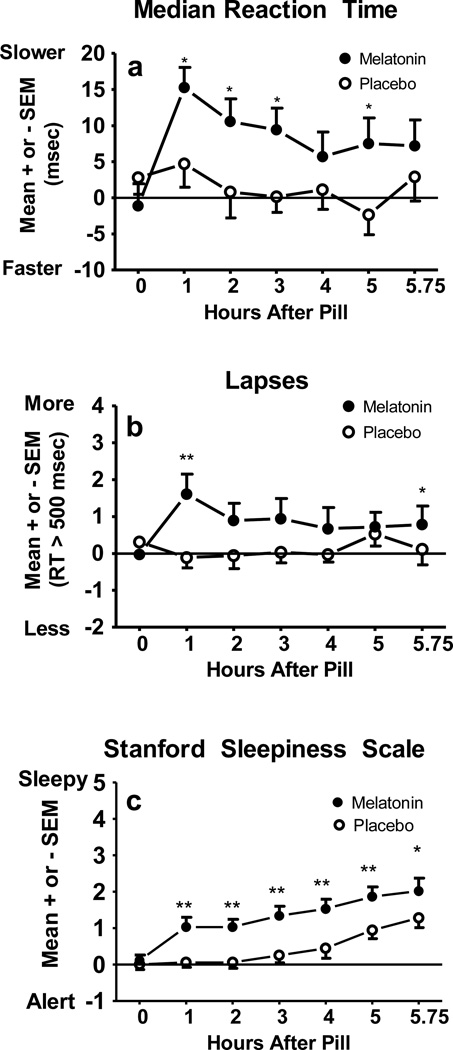
As illustrated in Figure 5 (panels a and b), median RT and number of lapses remained stable after taking the placebo pill and remained close to pre-pill values (horizontal line at Y=0). After ingesting the melatonin pill, however, median RT slowed and lapses increased, especially one hour after ingesting the melatonin pill. Optimal RT (data not illustrated) showed the same pattern as median RT. A significant condition-by-time interaction emerged for median RT [F(6,66)=3.98, p < .01], optimal RT [F(6,66)=2.98, p=.04], and lapses [F(6,66)=2.87, p=.02]. Median RT [condition main effect: F(1,11)=5.42, p=.04] and optimal RT [condition main effect: F(1,11)=9.05, p=.01] remained high for several hours after taking the melatonin pill; post-hoc comparisons between conditions showed differences 1, 2, 3, and 5 h after the pill was taken. A trend for a difference between conditions emerged for lapses [condition main effect: F(1,11)=4.22, p=.06].
Fig 5.
a) Psychomotor Vigilance Task (PVT) median reaction time, b) PVT lapses, and c) subjective sleepiness ratings. The horizontal lines at zero represent the mean values measured 1 hour before pill ingestion. *p<.05, **p<.01.
Figure 5c shows that sleepiness increased from pill time to bedtime in both conditions, but participants rated themselves as more sleepy after taking melatonin compared to after taking placebo. A condition-by-time interaction emerged for the SSS [F(6,66)=3.10, p=.01] and the KSS [F(6,66)=4.64, p=.01]. Figure 5c illustrates that post-hoc comparisons between melatonin and placebo conditions for the SSS were significant at every hourly assessment after the pill was ingested until scheduled bedtime. The KSS ratings showed the same pattern.
Discussion
In our gradually advancing sleep/dark protocol, 3.0 mg of afternoon/evening melatonin produced a slightly larger circadian phase advance than placebo. The 1.3-h phase advance with melatonin, however, was less than the 3-h advance of the sleep schedule; thus, a small amount of circadian misalignment was produced with both melatonin and placebo. This small amount of misalignment did not affect the sleep of these young adults, as measured by actigraphy. They did, however, become sleepier and more fatigued in the mornings as the treatment days progressed, which is likely the result of waking up at an earlier circadian phase than usual. This morning increase in sleepiness and fatigue occurred regardless of whether they took melatonin or placebo during the preceding afternoons/evenings, though this is expected given the small phase differences between the melatonin and placebo conditions. Finally, melatonin had side effects; sleepiness and performance decrements were apparent after the pill was ingested in the afternoon/evening, and these decrements persisted until bedtime. Given the modest phase advance with melatonin and the significant side effects, we cannot recommend taking 3.0 mg of melatonin alone with a gradually advancing sleep schedule in order to phase advance the circadian clock. Instead, we continue to recommend using morning bright light with the gradually advancing sleep schedule, and perhaps adding a smaller dose of afternoon/evening melatonin (Eastman and Burgess 2009; Revell et al. 2006; Revell and Eastman 2005; Revell and Eastman 2012).
The current study is one in a series with the overall goal of testing methods to phase advance the circadian system without producing circadian misalignment. All of these studies advanced sleep/dark 1 h/day for 3 days. Taken collectively, we can make a few general conclusions. First, the average phase advance we observed in the placebo condition of the current study (0.7 h) is similar to that of our previous dim light “placebo” group (0.6 h) (Burgess et al. 2003), which confirms that shifting sleep/dark earlier can phase advance the system. Second, adding 3 mg of melatonin to the gradual shift of sleep/dark in the current study increased the phase advance slightly (1.3 h), but descriptively not quite as much as morning bright light, which produced average advances of 1.5, 1.7 and 2.1 h (Burgess et al. 2003; Revell et al. 2006). The largest advances (averages of 2.5 and 2.6 h) were produced by combining afternoon/evening melatonin and morning bright light (Revell et al. 2006). Thus, morning bright light or morning bright light plus evening/afternoon melatonin is better than melatonin alone to phase advance the system. Finally, these studies provide support for the hypothesis, first proposed by Wirz-Justice and colleagues (2004), that melatonin and light are additive in their phase shifting abilities. The phase advance with 3.0 mg of melatonin alone in the current study (1.3 h) added to the advance with intermittent morning bright light alone (averages of 1.5 and 1.7 h) (Burgess et al. 2003; Revell et al. 2006) roughly adds up to the advance from combining 3.0 mg melatonin and morning intermittent bright light (2.6 h) (Revell et al. 2006).
The relative contribution of shifting the sleep/wake schedule and taking exogenous melatonin to the observed phase advance was roughly equal in the current study. Circadian rhythms advanced by 0.7 h after the gradual sleep/dark schedule advance (the placebo condition) and by 1.3 h when 3.0 mg of melatonin was added. Therefore, melatonin increased the advance by roughly the same amount (1.3 - 0.7 = 0.6). Other studies (Paul et al. 2010; Rajaratnam et al. 2003; Sharkey and Eastman 2002) also show this additive effect, and a descriptive trend emerges such that phase advances are larger after more days of advanced sleep timing and more days of melatonin administration (1.0–1.3 h with 1 day of advanced sleep and 1 day of melatonin administration (Paul et al. 2010); 3.0–3.9 h with 8 days of advanced sleep and 4 days of melatonin administration (Sharkey and Eastman 2002); 5.3 h with 8 days of advanced sleep and 8 days of melatonin administration (Rajaratnam et al. 2003)).
In the current study, the sleep schedule was advanced 3 h, but circadian rhythms did not advance as much (1.3 h with melatonin and 0.7 h with placebo), so a small amount of circadian misalignment was produced. Greater sleepiness and fatigue in the morning as treatment days progressed indicate that this circadian misalignment was felt by the study participants. Sleep, mood, and gastrointestinal problems, however, were not affected. If the advancing sleep schedule continued, then the amount of circadian misalignment would have increased and would likely have consequences for sleep, mood and performance. It remains unclear, however, the degree of circadian misalignment necessary to observe a negative impact on these measures, and it may depend on the measured outcome and whether misalignment is chronic or acute. We continue to suggest that a gradually advancing sleep/wake schedule in combination with afternoon/evening melatonin and morning bright light is the best way to advance the circadian system without producing circadian misalignment.
It is possible that if we had used a smaller dose of melatonin in the current study that the same circadian phase advance would have been produced, but without the side effects of sleepiness and impaired performance seen with the 3.0 mg dose. In our previous study (Revell et al. 2006), sleepiness, measured by the SSS, between taking the 0.5 mg melatonin pill in the afternoon/evening and bedtime did not differ from placebo. Similar to the current study, however, sleepiness (SSS) was elevated after taking 3.0 mg of melatonin compared to placebo, though in this previous study the difference did not reach statistical significance even though the increase in sleepiness was about the same amount (1 point on the SSS). The lack of significance was probably due to the between-subjects design, whereas the current study was a within-subjects design which increased our ability to detect a difference between melatonin and placebo. The finding that melatonin makes humans sleepy is not new (Arendt et al. 1984; Cajochen et al. 1996; Cajochen et al. 1997; Deacon et al. 1994; Dollins et al. 1993; Dollins et al. 1994; Nickelsen et al. 1989; Rogers et al. 2003; Tzischinsky and Lavie 1994; Yang et al. 2001), and accounts for melatonin’s popularity as a sleep aid. Given the dose response relationship for sleepiness (Dollins et al. 1994), it is best to use the lowest possible dose when melatonin is not taken at bedtime.
In the current study, reaction times lengthened and lapses increased on the PVT in the hours between taking the melatonin pill and bedtime, compared to placebo. These decrements were most pronounced in the first hour after taking the melatonin pill, but impairment persisted until bedtime. These data mimic previous studies (Dollins et al. 1993; Dollins et al. 1994; Rogers et al. 2003), and further emphasize the need to caution those who take melatonin in the afternoon or evening not to drive or engage in an activity that requires full alertness, even if relatively low doses are taken. Although one noteworthy study did not find an adverse effect on driving performance after ingesting 5 mg of melatonin (Suhner et al. 1998), a significant increase in subjective sleepiness emerged, which led the authors to similarly conclude that caution should be used when driving after taking melatonin.
There are several reasons to believe that if we had used the lower dose of melatonin in the current study (e.g., 0.5 mg) that the same phase advance would have been produced as with 3.0 mg. One of our previous studies (Revell et al. 2006) showed no difference in phase advance between 0.5 and 3.0 mg afternoon/evening melatonin when combined with the 1 h/day gradually advancing sleep/dark schedule plus intermittent morning bright light. Our melatonin PRC studies (Burgess et al. 2008; Burgess et al. 2010) also showed no difference in the magnitude of advances between the 0.5 and 3.0 mg doses. Finally, another study of ours (Sharkey and Eastman 2002), to be discussed in more detail below, did not find a significant difference in the magnitude of phase advance with 3.0 mg compared to 0.5 mg. It should be noted that Deacon & Arendt (1995) reported a dose response relationship for phase advancing with melatonin. When administered 7 h before bedtime, the phase advance in the DLMO was 0.36 h with 0.05 mg, 0.69 h with 0.5 mg and 1.43 h with 5.0 mg. A statistical difference emerged between the 0.5 and 5.0 mg doses, but not between the 0.05 and 0.5 mg doses. Perhaps this was because the dose difference (in mg) between 0.5 and 5.0 mg of melatonin was much greater than between the 0.05 and 0.5 mg doses. It was also greater than between our 2 doses (0.5 and 3.0 mg). Another possibility for the apparent dose response found by Deacon & Arendt emerges from our melatonin PRC studies. The optimal time to produce advances is earlier for the larger dose, and perhaps 7 h before bed was a good time for the 5 mg dose, but less so for the 0.5 and 0.05 mg doses.
The optimal timing of melatonin to produce phase advances according to our 3.0 and 0.5 mg melatonin PRC studies is 10–11 h before the midpoint of the usual sleep episode (Burgess et al. 2010). Specifically, for 3 mg the optimal time is about 11 h before mid-sleep time (Burgess et al. 2010) or about 5 h before the DLMO (Burgess et al. 2008). We gave the melatonin 11 h before mid-sleep time, which when examined post-hoc was 4 h before the baseline DLMO on the first treatment day. On subsequent days, the time of melatonin administration was 1 hour earlier each day, but the DLMO advanced much less. Therefore, the pill was given earlier and earlier relative to the DLMO. Thus, we were successful in timing the melatonin pill to occur around the peak of the phase advance portion of the PRC.
In another study designed to phase advance circadian rhythms (Sharkey and Eastman 2002), we obtained much larger phase advances than in the current study. Participants ingested 3.0 mg of melatonin, 0.5 mg of melatonin, or placebo 11.5 h before baseline mid-sleep time (7.5 h before baseline bedtime). The DLMO advanced 3.9 h with 3.0 mg melatonin, 3.0 h with 0.5 mg melatonin, and 1.7 h with placebo. The phase advances with melatonin were much larger than in the current study and even larger than in our previous study in which morning bright light was used in combination with melatonin (Revell et al. 2006). There are several reasons that could account for the larger phase advances in the Sharkey and Eastman study (2002). Most important, sleep/dark was abruptly advanced by 7 h, whereas in our other studies, sleep/dark was advanced 3 h and the advance was gradual (1 h/day) to avoid the circadian misalignment caused by large, abrupt shifts of the sleep period. The large abrupt advance of sleep/dark, however, undoubtedly exerted a stronger “pull” on the circadian clock than the smaller, gradual advance. Also, there were 4 days of melatonin administration in the Sharkey and Eastman study and only 3 days in the current study and our more recent study (Revell et al. 2006). Last, final circadian phase was measured after 8 advanced sleep/dark periods, whereas in our other studies it was measured after only three. The acute side effects of sleepiness and reduced vigilance after taking melatonin were avoided in the Sharkey and Eastman study because participants went to bed after taking the melatonin. When placebo was taken before bed instead of melatonin (last 4 of the 8 advanced sleep episodes), however, sleep duration was reduced presumably because there was still a large amount of circadian misalignment (the DLMO advanced 3–4 h whereas the time for sleep advanced 7 h). Future research is necessary to determine the pros and cons of abrupt vs. gradual advance shifts of sleep using more comparable protocols (e.g., same magnitude of advance in sleep, same dose and timing of melatonin, same measures of sleepiness, mood and performance, etc.).
The phase advancing properties of melatonin can be used to attenuate the chronic “social jet lag” that many experience, as well as, to help extreme evening-types (night owls) and people with the delayed sleep phase disorder (DSPD) adopt an earlier sleep schedule. Several studies have shown phase advances in the DLMO and sleep onset time of people with DSPD in response to exogenous melatonin as summarized in a recent meta-analysis by van Geijlswijk and colleagues (2010). A pilot study (Mundey et al. 2005) showed DLMO advances depend on the time of melatonin administration relative to the baseline DLMO, with larger advances in response to the earliest administration times (~6.5 h before the DLMO). These data agree with our PRCs to melatonin (Burgess et al. 2008; Burgess et al. 2010). To our knowledge, there have been no studies in which our method for producing circadian phase advances (gradually advancing the sleep schedule, morning bright light and afternoon/evening melatonin) was used to advance the clocks of people who find it difficult to fall asleep as early as demanded by their work, family, or social obligations. This would require a few days with no morning time commitments in order to start with the natural “delayed” time for sleep before gradually advancing it. Then maintenance would be required, consisting of a fixed sleep/dark period even on weekends, continued melatonin administration and at least occasional morning bright light to prevent the circadian clock from delaying back to its normal delayed circadian phase.
Melatonin is a popular remedy for jet lag, partly because of its sleep-inducing effects and partly because of its phase-shifting effects (Arendt 2009; Revell and Eastman 2012; Sack et al. 2007). Melatonin can hasten the phase advance needed to adjust to a new time zone when flying east, but if it is only taken after landing, then there will still be a large degree of circadian misalignment, and thus jet lag, until the system entrains to the new time zone. To reduce or even eliminate jet lag, we recommend phase advancing circadian rhythms with a gradually advancing sleep schedule plus morning bright light and afternoon/evening melatonin before flying (Crowley and Eastman in press; Eastman and Burgess 2009; Revell and Eastman 2012).
Acknowledgments
Melatonin and matching placebo were provided by Ecological Formulas (a division of Cardiovascular Research Ltd., Concord, CA). We are grateful to Thomas Molina, Jacqueline Muñoz, Jillian Canton, Heather Holly, Elisabeth Beam, Nicole Woodrick, Christina Suh, Carlo Legasto, Jessica Stroup, and Heather Gunn for their assistance with data collection and data management. We thank Louis Fogg, Ph.D. for statistical advice. This work was supported by a grant from the National Institutes of Health (R01 NR007677) awarded to C.I. Eastman. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Nursing Research. The National Institute of Nursing Research and the National Institutes of Health had no involvement in designing the study, data collection, data analysis, and interpretation, writing of the manuscript, nor in the decision to submit the manuscript for publication.
Footnotes
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
WRAIR Test Battery and Palm-PVT task design by D. Thorne, Palm & PC programs by J. Shapiro, contract technical support by D. Redmond, project concept by G. Belenky.
References
- Akerstedt T. Sleepiness as a consequence of shift work. Sleep. 1988;11:17–34. doi: 10.1093/sleep/11.1.17. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Wright K. Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin. 2009;4:257–271. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J. Managing jet lag: Some of the problems and possible new solutions. Sleep Med Rev. 2009;13:249–256. doi: 10.1016/j.smrv.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Arendt J, Borbely AA, Franey C, Wright J. The effects of chronic, small doses of melatonin given in the late afternoon on fatigue in man: a preliminary study. Neurosci Lett. 1984;45:317–321. doi: 10.1016/0304-3940(84)90245-3. [DOI] [PubMed] [Google Scholar]
- Attenburrow MEJ, Dowling BA, Sargent PA, Sharpley AL, Cowen PJ. Melatonin phase advances circadian rhythm. Psychopharmacol. 1995;121:503–505. doi: 10.1007/BF02246501. [DOI] [PubMed] [Google Scholar]
- Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms. 1995;10:167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms. 2008;23:374–376. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586.2:639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clinical Endocrinol Metab. 2010;95:3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Krauchi K, von Arx MA, Mori D, Graw P, Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci Lett. 1996;207:209–213. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Krauchi K, Wirz-Justice A. The acute soporific action of daytime melatonin administration: effects on the EEG during wakefulness and subjective alertness. J Biol Rhythms. 1997;12:636–643. doi: 10.1177/074873049701200619. [DOI] [PubMed] [Google Scholar]
- Cho K. Chronic 'jet lag' produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Costa G. The impact of shift and night work on health. Appl Ergon. 1996;27:9–16. doi: 10.1016/0003-6870(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Eastman CI. Light and Melatonin Treatment for Jet Lag Disorder. In: Kushida CA, editor. Encyclopedia of Sleep. Oxford, UK: Elsevier Inc; (in press) [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- Deacon S, English J, Arendt J. Acute phase-shifting effects of melatonin associated with suppression of core body temperature in humans. Neurosci Lett. 1994;178:32–34. doi: 10.1016/0304-3940(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–655. [Google Scholar]
- Dollins AB, Lynch HJ, Wurtman RJ, Deng MH, Kischka KU, Gleason RE, Lieberman HR. Effect of pharmacological daytime doses of melatonin on human mood and performance. Psychopharmacol. 1993;112:490–496. doi: 10.1007/BF02244899. [DOI] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Nat Acad Sci. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Burgess HJ. How to travel the world without jet lag. Sleep Med Clin. 2009;4:241–255. doi: 10.1016/j.jsmc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: A strategy to prevent or reduce jet lag. Sleep. 2005;28:33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Miescke KJ. Entrainment of circadian rhythms with 26-hr bright light and sleep-wake schedules. Am J Physiol. 1990;259:R1189–R1197. doi: 10.1152/ajpregu.1990.259.6.R1189. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (African Americans) Have Shorter Free-Running Circadian Periods Than Whites (Caucasian Americans) Chronobiol Int. 2012 doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erren TC, Falaturi P, Morfeld P, Knauth P, Reiter RJ, Piekarski C. Shift work and cancer: the evidence and the challenge. Dtsch Arztebl Int. 2010;107:657–662. doi: 10.3238/arztebl.2010.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber RC. Alterations in performance following rapid transmeridian flight. In: Brown FM, Graeber RC, editors. Rhythmic Aspects of Behavior. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1982. pp. 173–212. [Google Scholar]
- Haimov I, Arendt J. The prevention and treatment of jet lag. Sleep Med Rev. 1999;3:229–240. doi: 10.1016/s1087-0792(99)90004-7. [DOI] [PubMed] [Google Scholar]
- Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, Ribeiro D, Arendt J. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- Hansen J. Risk of breast cancer after night- and shift work: current evidence and ongoing studies in Denmark. Cancer Causes Control. 2006;17:531–537. doi: 10.1007/s10552-005-9006-5. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiol. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Iglesias R, Terres A, Chavarria A. Disorders of the menstrual cycle in airline stewardesses. Aviat Space Environ Med. 1980;51:518–520. [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Onishi K, Dohi K, Okinaka T, Ito M, Isaka N, Nakano T. Circadian rhythm of blood pressure is transformed from a dipper to a non-dipper pattern in shift workers with hypertension. J Hum Hypertens. 2002;16:193–197. doi: 10.1038/sj.jhh.1001328. [DOI] [PubMed] [Google Scholar]
- Knutsson A. Health disorders of shift workers. Occup Med. 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986 Jul;:89–91. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- Knutsson A, Boggild H. Gastrointestinal disorders among shift workers. Scand J Work Environ Health. 2010;36:85–95. doi: 10.5271/sjweh.2897. [DOI] [PubMed] [Google Scholar]
- Koller M. Health risks related to shift work. Int Arch Occup Environ Health. 1983;53:59–75. doi: 10.1007/BF00406178. [DOI] [PubMed] [Google Scholar]
- Krauchi K, Cajochen C, Mori D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol. 1997;272:R1178–R1188. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- Lamond N, Dawson D, Roach GD. Fatigue assessment in the field: validation of a hand-held electronic psychomotor vigilance task. Aviat Space Environ Med. 2005;76:486–489. [PubMed] [Google Scholar]
- Lamond N, Jay SM, Dorrian J, Ferguson SA, Roach GD, Dawson D. The sensitivity of a palm-based psychomotor vigilance task to severe sleep loss. Behav Res Methods. 2008;40:347–352. doi: 10.3758/brm.40.1.347. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lo SH, Liau CS, Hwang JS, Wang JD. Dynamic blood pressure changes and recovery under different work shifts in young women. Am J Hypertens. 2008;21:759–764. doi: 10.1038/ajh.2008.186. [DOI] [PubMed] [Google Scholar]
- Mallo C, Zaidan R, Faure A, Brun J, Chazot G, Claustrat B. Effects of a four-day nocturnal melatonin treatment on the 24h plasma melatonin, cortisol and prolactin profiles in humans. Acta Endocrinologica. 1988;119:474–480. doi: 10.1530/acta.0.1190474. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Effect of shift work on body mass index and metabolic parameters. Scand J Work Environ Health. 2007;33:45–50. doi: 10.5271/sjweh.1063. [DOI] [PubMed] [Google Scholar]
- Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Nagtegaal JE, Kerkhof GA, Smits MG, Swart ACW, Van der meer YG. Delayed sleep phase syndrome: A placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res. 1998;7:135–143. doi: 10.1046/j.1365-2869.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- Nickelsen T, Demisch L, Demisch K, Radermacher B, Schoffling K. Influence of subchronic intake of melatonin at various times of the day on fatigue and hormonal levels: A placebo-controlled, double-blind trial. J Pineal Res. 1989;6:325–334. doi: 10.1111/j.1600-079x.1989.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Ohira T, Tanigawa T, Iso H, Odagiri Y, Takamiya T, Shimomitsu T, Hayano J, Shimamoto T. Effects of shift work on 24-hour ambulatory blood pressure and its variability among Japanese workers. Scand J Work Environ Health. 2000;26:421–426. doi: 10.5271/sjweh.563. [DOI] [PubMed] [Google Scholar]
- Paul MA, Miller JC, Gray GW, Love RJ, Lieberman HR, Arendt J. Melatonin treatment for eastward and westward travel preparation. Psychopharmacol. 2010;208:377–386. doi: 10.1007/s00213-009-1737-7. [DOI] [PubMed] [Google Scholar]
- Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes and Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SM, Dijk DJ, Middleton B, Stone BM, Arendt J. Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-hour production of reproductive hormones. J Clin Endocrinol Metab. 2003;88:4303–4309. doi: 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91:54–59. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. How to trick Mother Nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. Jet lag and its prevention. In: Barkoukis TJ, Matheson JK, Ferber R, Doghramji K, editors. Therapy in Sleep Medicine. Elsevier; 2012. pp. 390–401. [Google Scholar]
- Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants (United States) Cancer Causes and Control. 2002;13:317–324. doi: 10.1023/a:1015284014563. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Rogers NL, Kennaway DJ, Dawson D. Neurobehavioural performance effects of daytime melatonin and temazepam administration. J Sleep Res. 2003;12:207–212. doi: 10.1046/j.1365-2869.2003.00360.x. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: Part 1, basic principles, shift work and jet lag disorders. Sleep. 2007;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samel A, Wegmann HM, Vejvoda M, Maab H, Gundel A, Schutz M. Influence of melatonin treatment on human circadian rhythmicity before and after a simulated 9-hr time shift. J Biol Rhythms. 1991;6:235–248. doi: 10.1177/074873049100600304. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Nat Acad Sci. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS One. 2009a;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–725. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Medicine. 2009b;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, Lewy AJ. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatr. 1999;156:1392–1396. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- Suhner A, Schlagenhauf P, Tschopp A, Hauri-Bionda R, Friedrich-Koch A, Steffen R. Impact of melatonin on driving performance. J Travel Med. 1998;5:7–13. doi: 10.1111/j.1708-8305.1998.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. 1978 NIOSH Publication #78–154, NIOSH Publication #78–154. [Google Scholar]
- Thorne DR, Johnson DE, Redmond DP, Sing HC, Belenky G, Shapiro JM. The Walter Reed palm-held psychomotor vigilance test. Behav Res Methods. 2005;37:111–118. doi: 10.3758/bf03206404. [DOI] [PubMed] [Google Scholar]
- Tzischinsky O, Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994;17:638–645. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- van Geijlswijk IM, Korzilius HP, Smits MG. The Use of Exogenous Melatonin in Delayed Sleep Phase Disorder: A Meta-analysis. Sleep. 2010;33:1605–1614. doi: 10.1093/sleep/33.12.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Edwards B, Nevill A, Atkinson G, Reilly T, Davies P, Godfrey R. Do subjective symptoms predict our perception of jet-lag? Ergonomics. 2000;43:1514–1527. doi: 10.1080/001401300750003943. [DOI] [PubMed] [Google Scholar]
- Wever RA. The Circadian System of Man: Results of Experiments Under Temporal Isolation. Springer-Verlag: Springer-Verlag; 1979. [Google Scholar]
- Wirz-Justice A, Krauchi K, Cajochen C, Danilenko KV, Renz C, Weber JM. Evening melatonin and bright light administration induce additive phase shifts in dim light melatonin onset. J Pineal Res. 2004;36:192–194. doi: 10.1111/j.1600-079x.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32:417–423. doi: 10.1161/01.hyp.32.3.417. [DOI] [PubMed] [Google Scholar]
- Yang CM, Spielman AJ, D'Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]