Abstract
Intervertebral disc degeneration (IDD) is the leading cause of debilitating spinal disorders such as chronic lower back pain. Aging is the greatest risk factor for IDD. Previously, we demonstrated IDD in a murine model of a progeroid syndrome caused by reduced expression of a key DNA repair enzyme. This led us to hypothesize that DNA damage promotes IDD. To test our hypothesis, we chronically exposed adult wild-type (Wt) and DNA repair-deficient Ercc1−/Δ mice to the cancer therapeutic agent mechlorethamine (MEC) or ionization radiation (IR) to induce DNA damage and measured the impact on disc structure. Proteoglycan, a major structural matrix constituent of the disc, was reduced 3-5x in the discs of MEC- and IR-exposed animals compared to untreated controls. Expression of the protease ADAMTS4 and aggrecan proteolytic fragments were significantly increased. Additionally, new PG synthesis was reduced 2-3x in MEC- and IR-treated discs compared to untreated controls. Both cellular senescence and apoptosis were increased in discs of treated animals. The effects were more severe in the DNA repair-deficient Ercc1−/Δ mice than in Wt littermates. Local irradiation of the vertebra in Wt mice elicited a similar reduction in PG. These data demonstrate that genotoxic stress drives degenerative changes associated with IDD.
Keywords: Intervertebral disc, aging, DNA damage, genotoxic stress, matrix proteoglycan
1. Introduction
Aging is a major etiologic factor of intervertebral disc degeneration (IDD), a condition responsible for many common spine-related disorders associated with enormous economic loss [1]. With aging, there is a progressive loss of ECM proteoglycans (PG) in the discs with a concomitant accumulation of degraded matrix molecules [2]. Loss of disc matrix PGs inevitably leads to loss of hydration, resulting in altered biomechanics and pathologic outcomes such as spine stiffness, spinal stenosis, and disabling chronic back pain [2; 3]. In particular the nucleus pulposus (NP) of the disc becomes more fibrous as the PG content diminishes, leading to annulus cracks and fissures [4]. Ossification and thinning of the cartilaginous endplate, microfractures in the adjacent subchondral bone, and bone sclerosis, are also found with increasing age [5]. This likely contributes to a reduction in nutrient supply to the disc, accumulation of cellular waste products and an increasingly acidic environment (pH 6.3-6.6) that severely compromises cell function or causes cell senescence and death [6]. The driving force behind these disc degenerative changes during the aging process is still poorly understood.
Aging is thought to arise, at least in part, as a consequence of time-dependent accumulation of stochastic damage to cellular macromolecules [7; 8]. This impairs tissue homeostasis and leads to impaired ability of the tissue to respond to stress [9]. Accumulation of damaged proteins, DNA and mitochondria, telomere shortening, attrition of quality control mechanisms (autophagy, DNA repair, etc.), and the loss in number or function of multipotent stem cells are types of damage implicated in aging [10; 11; 12; 13; 14]. There is compelling evidence to implicate DNA damage as a type of stochastic damage that promotes aging [10]. Inherited defects in DNA repair mechanisms lead to a variety of syndromes, the majority of which are characterized by accelerated aging of one or more organ systems [11]. Furthermore, many human progeroid syndromes, or diseases of accelerated aging, are caused by inherited defects in genome maintenance mechanism 14. These progerias, including Cockayne syndrome, Werner syndrome, ataxia telangiectasia and trichothiodystrophy, demonstrate that failure to repair DNA damage promotes rapid aging [11; 15].
Previously we reported spontaneous age-dependent IDD in a murine model (Ercc1−/Δ mice) of a human progeroid syndrome caused by deficiency of the DNA repair endonuclease, ERCC1-XPF [16]. These mice showed aging-related degenerative changes in their discs, including loss of disc height, premature loss of disc (PG), reduced matrix PG synthesis, and enhanced apoptosis and cell senescence. This led us to hypothesize that DNA damage is a driving force behind matrix loss, disc aging and IDD. Herein, we tested this hypothesis by chronically challenging both wild-type (Wt) and DNA repair-deficient Ercc1−/Δ mice with a subtoxic dose of the chemotherapeutic agent mechlorethamine (MEC) or ionization radiation (IR) to induce DNA damage. MEC, a nitrogen mustard alkylating agent, reacts primarily with the N7 position of guanine residues to form monoadducts and 1,3 G-G interstrand crosslinks [17; 18]. A prototype of alkylating agents, MEC has been used extensively as an anticancer chemotherapeutic drug [19; 20]. Both MEC and IR accelerated loss of disc matrix PG and greatly enhanced cellular senescence and apoptosis. The effects were more pronounced in DNA repair-deficient Ercc1−/Δ mice than Wt littermates. These findings provide strong evidence that DNA damage can drive degenerative changes associated with IDD even in a normal host. This provides a mechanism by which smoking could promote IDD and indicates that cancer survivors may be at increased risk for IDD.
2. Materials and Methods
2.1. Exposure to genotoxins
Experiments involving mice were approved by the University of Pittsburgh (Pittsburgh, PA) Institutional Animal Care and Use Committee in accord with the National Institutes of Health guidelines for the humane care of animals. Ercc1−/Δ mice were generated in an f1 hybrid background by crossing heterozygous Ercc1+/− and Ercc1+/Δ mice from two different inbred, C57Bl/6J and FVB/n, backgrounds to obtain genetically identical mice without strain-specific pathology. The mice were genotyped using PCR, as previously described [21]. Six Ercc1−/Δ mice and six of their wild-type (Wt) littermates were chronically exposed to genotoxic stress by subcutaneous administration of a sub-toxic dose of mechlorethamine (MEC) (8 μg/kg once per week for 6 wks). Six Ercc1−/Δ mice and six of their wild-type (Wt) littermates were exposed to total body ionizing radiation (TBI) (0.175 Gy, at a dose rate of 0.054 Gy/min, once per week for 4 weeks using a Shepherd Mark I-68 irradiator with a 137Cs source). Mice were treated beginning at 8 weeks of age and sacrificed 6 weeks following the last exposure. Unexposed age- and sex-matched mice of the same genotype were used as controls.
2.2. Localized irradiation of the spine
Six Wt C57B1/6 mice were irradiated using a 6 MV photon beam obtained from a Varian 23EX linear accelerator. Anesthetized and laid on their side, mice were irradiated with a focused beam from above (20 cm long and 3 cm wide field size) 1.5 cm wide spinal region and the entire length of the spine and tail. During irradiation, a 1 cm thick bolus was placed on the mice which ensured delivery of uniform dose across the entire irradiated region. Using a source to surface distance (SSD) of 100 cm, mice were exposed once to 0, 6 or 10 Gy. Three mice were irradiated at each IR dose when they were 22 weeks old and sacrificed 10 weeks later.
2.3. Isolation of nucleus pulposus, annulus fibrosus and complete intervertebral discs
The spines were isolated from euthanized mice and dissected with the aid of a 5× magnifier. Entire intervertebral discs (IVDs) were removed en bloc from the surrounding vertebral bodies by creating an incision along the endplates. To harvest nucleus pulposus (NP) tissue, an axial cut was made on the disc side of the endplate to expose the disc center, followed by gentle aspiration of the NP tissue using a P-10 pipette tip under a dissecting microscope (20-40× magnification, Nikon SMZ645). After removing the NP tissue a second axial cut was made on the disc side of the opposite endplate in order to isolate annulus fibrosus (AF) tissue.
2.4. Histological staining
Isolated spines were decalcified and embedded in paraffin (Tissue Tek processor and Leica embedder). 7 μm sections were stained with either hematoxylin and eosin (H&E) or safranin O and fast green dyes (Fisher Scientific) by standard procedures and photographed under 40× to 200× magnification (Nikon Eclipse Ts100).
2.5. 1,9-dimethylmethylene blue (DMMB) colorometric assay for sulfated glycosaminoglycans
NP and AF tissue isolated separately from six lumbar IVDs of each mouse were pooled and digested using papain at 60oC for two hours. Glycosaminoglycans (GAG) content was measured in duplicate by the DMMB procedure [22] using chondroitin-6-sulfate (Sigma C-8529) as a standard. The DNA concentration of each sample was measured using the PicoGreen assay (Molecular Probes) and used to normalize the GAG values. Average values from six exposed mice and six unexposed controls were calculated and reported ± standard error of the mean.
2.6. Quantitation of matrix synthesis
Disc organ cultures of isolated functional spine units (FSU), each consisting of vertebra, disc, vertebra, were established as previously described [23]. Four thoracic FSUs (2 FSU per well for duplicate) were cultured in complete growth medium [F-12/D-MEM containing 10% fetal calf serum (FCS), 1% PS (10000 units/ml penicillin, 10 mg/ml streptomycin), and 25 μg/ml L-ascorbic acid] for two days to equilibrate after the trauma of surgical dissection, followed by three day dual labeling incubation with 35S-sulfate (20 μCi/ml). Proteoglycan synthesis was measured by 35S-sulfate incorporation as described previously [24]. The rate of proteoglycan synthesis was calculated as the fmoles of sulfate incorporated per μg DNA. Average values from six samples (3 mice each analyzed in duplicate) and six unexposed controls are shown ± one standard error.
2.7. Immunohistochemistry
Paraffin embedded sections were used to probe for ADAMTS-generated neoeptitope NVTEGE (anti-NITEGE neoepitope antibody, Ab1320[25]), aggrecan (rabbit polyclonal IgG anti-aggrecan, amino acids 1177-1326 of mouse aggrecan epitope, Millipore, ab1031), and ADAMTS4 (rabbit polyclonal IgG anti-aggrecan, Millipore, ab19165). Immunohistochemistry was performed as described previously [26]. A negative control without addition of the primary antibodies was also performed. The anti-NITEGE neoepitope antibody cross-reacts with the NVTEGE neoepitope generated from mouse aggrecan.
2.8. Measuring apoptosis and cell senescence
Frozen sections of discs were treated with TUNEL reagents (Roche) to detect apoptotic cells as described by the manufacturer and counterstained with the Hoechst nuclear stain to detect total cells in the specimens. Immunohistochemistry was used to localize the senescence marker p16INK4a in disc tissue using tonsil tissue as a positive control. Briefly, slides containing 4μm frozen sections of discs were fixed in cold acetone for 10 minutes, washed 3x in phosphate-buffered saline (PBS), and incubated in Vector M.O.M (Vector Lab) for 1 hr to eliminate background binding of mouse primary monoclonal antibodies on mouse tissues. Sections were incubated overnight at 4°C with mouse monoclonal primary antibody against p16INK4a (Santa Cruz Biotech; 1:50 dilution). After washing with PBS, sections were incubated with biotinylated horse anti-mouse serum (1:200; Vector Lab) for 30 min at room temperature, followed by biotin detection for 30 min using ABC/horse radish peroxidase (ABC Elite; Vector Lab) and AEC chromogen substrate (Skytes) for 10 min at RT. Sections were counterstained with aqueous hematoxylin and blue Scott’s T H2O. At least three random fields in three sections from each tissue sample were imaged at 200X to quantify the percent p16INK4a- and TUNEL-positive cells. Average values from nine fields (3 field/NP × 3 mice) were calculated with one standard error.
2.9. Statistical analysis
Values represent the average of six trials ± standard error (SE), with 95% confidence intervals calculated to determine statistical significance. The confidence intervals were calculated based on the t-distribution because of the small sample size.
3. Results
3.1. Mice exposed to IR or MEC displayed loss of matrix proteoglycan in their intervertebral discs
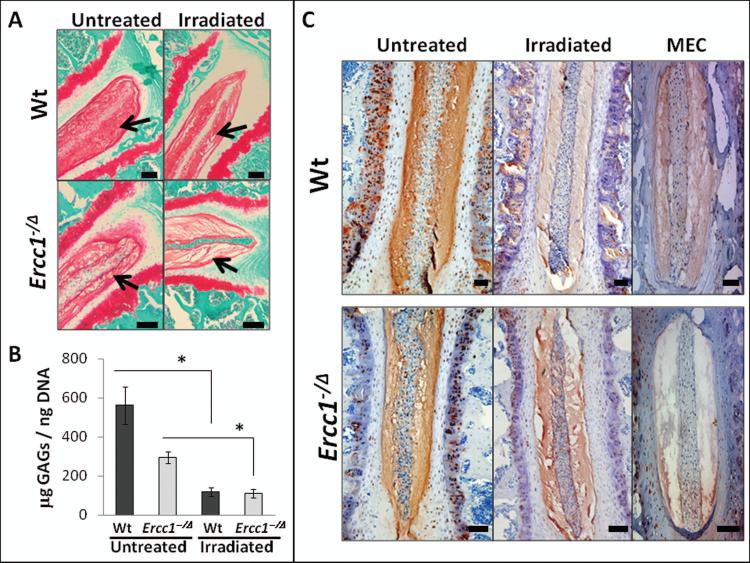
Mice chronically exposed to MEC were previously reported to exhibit substantial reduction in intervertebral disc PG and endplate cellularity by histological staining [16]. Six weeks following chronic, low-dose total body IR, the discs of Wt and Ercc1−Δ mice were isolated. Sections were stained with safranin O/fast green to detect sulfated PGs (Fig. 1A). PG levels were reduced in Wt mice treated with IR compared to untreated animals. Staining was also reduced in Ercc1−/Δ mice compared to Wt littermates, and further reduced if the Ercc1−/Δ mice were irradiated (Fig. 1A). To quantify the differences, the DMMB assay, which measures the sulfated glycosaminoglycans (GAG) component of PG was used (Fig. 1B). The NP GAG content of IR-exposed Wt mice (119 ± 23 μg GAG/ng DNA) was significantly reduced compared to unexposed Wt mice (562 ± 92 μg GAG/ng DNA). Ercc1−Δ mice showed more pronounced effects, with their NP GAG being 112 ± 19 μg GAG/ng DNA from IR-treatment and 296 ± 25 μg GAG/ng DNA from untreated control. These data are consistent with our previous finding that chronic exposure of mice to the genotoxin MEC reduces disc PG [16]. To extend this, sections of discs from mice chronically exposed to total body irradiation (TBI) or MEC were stained for aggrecan, the major extracellular matrix proteoglycan constituent responsible for the osmotic turgidity of intervertebral discs [27]. Aggrecan staining was substantially reduced in Wt mice exposed to IR or MEC compared to untreated controls (Fig. 1C). Similar, albeit more dramatic effects were seen in DNA repair-deficient Ercc1−/Δ mice.
Figure 1. Genotoxic stress-induced loss of intervertebral disc proteoglycan matrix.
A, Safranin O and fast green staining of disc sections of IR-treated Ercc1−/Δ mice and their Wt littermates. Red, proteoglycan staining. Arrows indicate the NP region of the disc. The black bars represent 100 μm B, Quantitation of NP GAG. GAG levels from nucleus puloposus tissues of IR-treated and untreated mice were measured using 1,9-dimethylmethylene assay against GAG standards (chondroitin-6-sulfate) and normalized to total DNA content. * = p < 0.05 from six independent assays. C, Coronal sections of mouse discs were immunostained for aggrecan (brown) and counterstained with aqueous hematoxylin and blue Scott’s tap water. Decreased immunodetection of nucleus pulposus aggrecan was observed in Wt and Ercc1−/Δ mice treated with either IR or MEC. The bar represents 100 μm.
3.2. Chronic exposure to genotoxic stress reduced proteoglycan synthesis
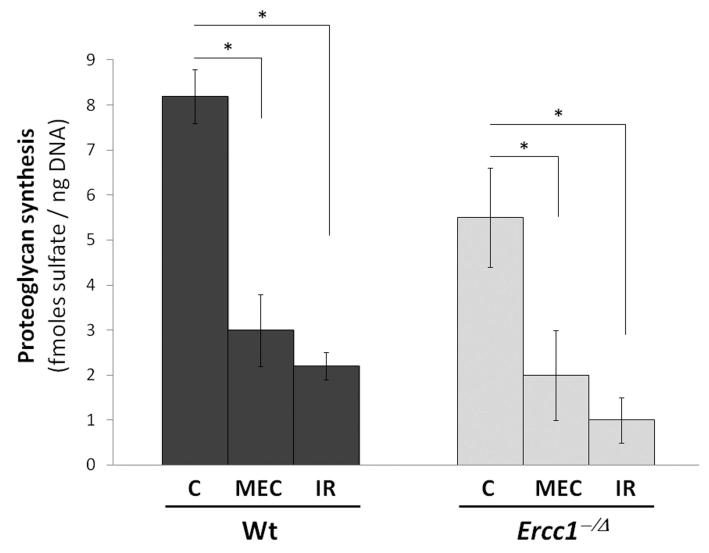
To determine the mechanism by which PG content is lost after exposure to genotoxic stress, we quantitated synthesis of new PG by discs isolated from exposed mice ex vivo, by measuring the level of 35S-sulfate incorporated. MEC-exposed Wt mice incorporated 3.0 ± 0.8 fmoles sulfate/ng DNA, a nearly 3x reduction compared to that of unexposed Wt control mice (8.2 ± 0.6 fmoles sulfate/ng DNA) (Fig. 2). MEC-exposed Ercc1−Δ mice incorporated 2.0 ± 1 fmoles sulfate/ng DNA, also a nearly 3x reduction compared to unexposed Ercc1−Δ mice (5.5 ± 1.1 fmoles sulfate/ng DNA) (Fig. 2). Similar effects were seen with IR treatment. IR-exposed Wt mice incorporated 2.2 ± 0.3 fmoles sulfate/ng DNA, while IR-exposed Ercc1−Δ mice incorporated 1.0 ± 0.5 fmoles sulfate/ng DNA.
Figure 2. Chronic exposure to IR and MEC decreased new matrix protein synthesis in intervertebral discs.
Proteoglycan synthesis as measured by 35S-sulfate incorporation in intervertebral discs of unexposed mice (C) and mice exposed to mechlorethamine (MEC) or ionizing radiation (IR) to induce nuclear DNA damage. * = p < 0.05 from six independent assays (3 mice each analyzed in duplicate).
3.3. Chronic exposure to genotoxic stress induced senescence and apoptosis of disc cells
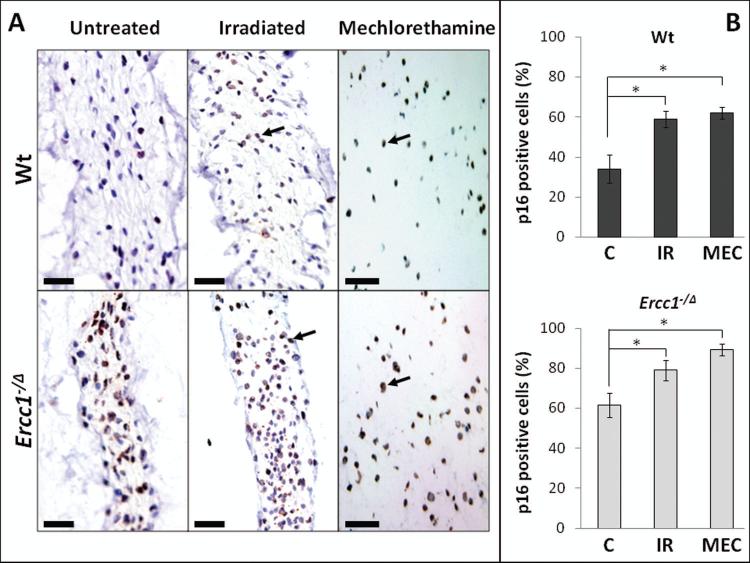
A decline in PG synthesis could be due to the loss of functional cells through senescence or apoptosis [28]. Hence we measured senescent cells by immunodetection of the cellular senescence marker, p16INK4a (Fig. 3A). Thirty-four percent of NP cells from untreated Wt mice stained positively for p16INK4 compared to 62% of untreated Ercc1−/Δ mice. After IR treatment, a significantly greater number of p16INK4 positive cells were detected in the nucleus pulposus of Wt (58%) and Ercc1−/Δ (78%) mice (Fig. 3B). Similarly, MEC treatment increased the number of p16INK4 immunopositive nucleus pulposus cells in Wt (61%) and Ercc1−/Δ (89%) mice significantly (Fig. 3).
Figure 3. Effects of IR and MEC exposure on cellular senesecence in mouse intervetebral discs.
A, immunohistochemical detection of p16INK4a, a senescence marker, to distinguish senescent (brown, arrow) from non-senescent (blue) cells. B, Quantitation of the percent p16INK4a immunopositive cells in the nucleus pulposus of unexposed mice (C) and mice exposed to mechlorethamine (MEC) or ionizing radiation (IR). Top graph, Wt mice. Bottom graph, Ercc1−/Δ mice. * = p < 0.05 from nine random fields.
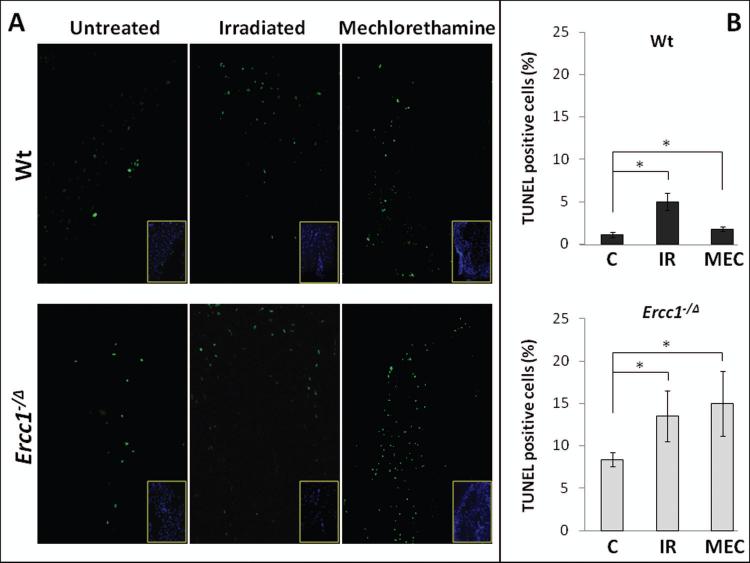
There was also a significant increase in the number of apoptotic disc cells following exposure to MEC and IR, primarily in the annulus fibrosis region (Fig. 4). In untreated Wt mice, 1.1% of disc cells were TUNEL positive compared to 8.4% in untreated Ercc1−/Δ mice. MEC treatment increased the percent of TUNEL positive cells in the disc to 2% (Wt mice) and 15% (Ercc1−/Δ mice) (Fig. 4). Similarly, IR exposure increased the number of TUNEL positive disc cells in Wt (5%) and Ercc1−/Δ (13.5%) mice (Fig. 4).
Figure 4. Enhanced apoptosis in intervertebral discs of IR- and MEC-exposed mice.
A, TUNEL assay to identify apoptotic cells (green) in disc tissue. Insets, nuclear DAPI stain (blue) to reveal tissue cellularity. B, Quantitation of the percent TUNEL positive cells in the annulus fibrosus of unexposed mice (C) and mice exposed to mechlorethamine (MEC) or ionizing radiation (IR). Top graph, Wt mice. Bottom graph, Ercc1−/Δ mice. * = p < 0.05 from nine random fields.
3.4. Chronic exposure to genotoxic stress induced disc aggrecanase expression and proteolysis of aggrecan
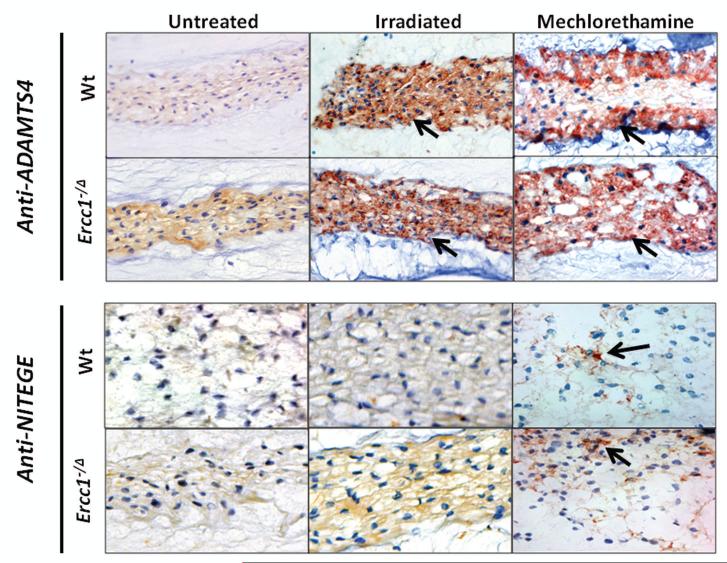
A second mechanism by which genotoxins could cause loss of ECM proteins is via increased protein degradation. ADAMTS4 is one of the most efficient proteolytic enzymes of the PG aggrecan. In untreated mice, ADAMTS4 expression was slightly greater in the discs of DNA repair-deficient Ercc1−/Δ mice compared to their Wt littermates (Fig. 5). Mice of either genotype showed a dramatic increase in ADAMTS4 expression in their intervetebral discs after chronic exposure to IR or MEC. ADAMTS4 staining was intense throughout the nucleus pulposus of Wt and Ercc1−/Δ animals treated with either genotoxin (Fig. 5). Interestingly, no consistent increase in the expression of other matrix metalloproteinases, e.g., ADAMTS-5 or MMP-2, was detected by immunohistochemistry (data not shown).
Fig. 5. Immunohistochemical detection of aggrecanase and aggrecan degradation products.
Sagittal disc sections were analyzed using an anti-ADAMTS4 antibody (top) and antibodies recognizing the C-terminal neo-epitope generated by aggrecanase cleavage in the interglobular domain. ADAMTS4 protein expression (reddish brown, arrow) was dramatically up-regulated throughout the nucleus puloposus of both Wt and Ercc1−/Δ mice following treatment of IR or MEC. MEC, but not IR exposure, also induced aggrecanase-mediated proteolysis of aggrecan (reddish brown, arrows), as evident by increased immunodetection of the aggrecan NVTEGE neo-epitope.
Immunohistochemical detection of NVTEGE neo-epitopes [25; 29] was also performed to measure proteolytic cleavage of the interglobular domain of aggrecan (Fig. 5). The ADAMTS-generated aggrecan G1 fragments terminating in NVTEGE−392 were detected at high level in discs of MEC-exposed mice, especially the Ercc1−/Δ mice, relative to untreated mice. Curiously, mice exposed to IR showed no increase in NVTEGE−392 neo-epitope in their discs. It is possible that ADAMTS4 expressed under IR induction is in the inactive pro-form. Alternatively, the activity of ADAMTS4 could be suppressed by an endogenous inhibitor. Moreover, no significant increase in the MMP-generated aggrecan G1 fragment terminating in VDIPEN−360 was detected following either MEC or IR exposure (data not shown).
3.5. Acute local IR exposure caused a decrease in disc matrix
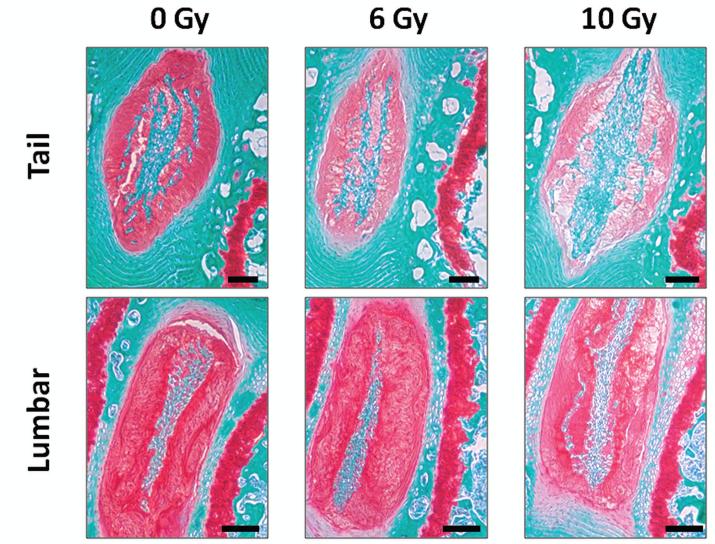
The deleterious effects of MEC and IR treatment on disc health could be due to direct damage to the tissue or a systemic response to genotoxic stress. To test the former, we exposed Wt mice to local IR using a Varian 23EX linear accelerator to irradiate only the tail and the spinal region of mice. The rest of the animal was shielded from exposure (see Method). Mice were exposed to single high doses of IR (6 or 10 Gy) then sacrificed 10 weeks later for analysis. Local IR exposure decreased disc matrix proteoglycan content in the tail and spine of mice in a dose-dependent fashion (Fig. 6). The effect was similar to that of TBI (Fig. 1).
Figure 6. Local ionizing radiation exposure induced loss of intervertebral disc proteoglycan matrix.
Safranin O and fast green staining of sections of discs isolated from the lumbar and tail region of IR-exposed Wt mice. Red, proteoglycan staining. The black bars represent 100 μm.
4. Discussion
The progeroid, DNA repair-deficient Ercc1−/Δ mice spontaneously develop a number of progressive age-associated degenerative changes of the intervertebral disc, suggesting that DNA damage contributes to this process [16]. Here we tested this hypothesis rigorously by exposing both Wt and Ercc1−/Δ mice to two different genotoxic agents, the chemotherapeutic agent MEC and IR. We discovered that both lead to accelerated disc aging, including loss of disc matrix proteoglycan and functional cells, even in normal mice exposed to sub-toxic doses. The effect was exaggerated in the DNA repair-deficient Ercc1−/Δ mice relative to Wt mice, substantiating that nuclear DNA damage is detrimental to disc matrix homeostasis and promotes disc aging and degeneration. However, it remains to be determined whether MEC or IR treatment affects disc health directly by increasing DNA damage within disc cells, or the observed effects are primarily systemic. Damage to neighboring organs and tissues could promote degenerative changes in the discs via secondary processes, for example by inducing cell senescence and senescence associated secretory phenotype (reference Baker/Kirkwood and a second paper by Campisi).
The findings in this study reveal important mechanistic insights into how genotoxic stress induces IDD, and significantly extended our previous study, which only qualitatively described disc histological changes following MEC treatment [16]. Our results are consitent with the fact that tobacco smoking, a source of many potent genotoxins that covalently modify DNA [30], is a major risk factor for disc degeneration [31; 32]. Given these observations, it is plausible that DNA damage could be an underlying cause of increased IDD in smokers, as reported by Battié and coworkers [31].
It is not clear why exposure to MEC leads to ADAMTS-mediated, but not MMP-mediated aggrecanolysis. This could be due to differential regulation of MMP and ADAMTS gene expression under genotoxic stress, or that ADAMTSs, particularly ADAMTS-5, are more efficient enzymes than MMPs at cleaving aggrecan [33]. Extensive research in osteoarthritis suggests that ADAMTS-mediated aggrecanolysis is destructive to cartilage function, whereas MMP-mediated aggrecanolysis is not. This conclusion is based on biochemical and animal studies demonstrating that ADAMTSs are responsible for cleaving within the interglobular domain (IGD) of aggrecan resulting in the loss of GAG, while MMPs cleave within the IGD in a different pool of aggrecan, which does not release GAG [34]. Whether or not genotoxic stress induces similar mechanisms of proteolytic action by MMPs and ADAMTSs on disc aggrecan remains to be further investigated.
Crosslinks are an extremely toxic class of DNA damage because they covalently join both strands of DNA, preventing strand unwinding, an essential step during replication and transcription. Repair of DNA interstrand crosslinks requires the ERCC1-XPF endonuclease [35; 36], making MEC particularly toxic to ERCC1-deficient cells and mice. Discs of ERCC1-XPF-deficient mice chronically exposed to MEC underwent considerable disc degenerative changes, showing loss of functional cells and PG synthesis, and up-regulation of ECM catabolism. The fact that the discs of DNA repair-deficient mice are more severely affected by MEC than Wt mice, emphasizes the contribution of DNA damage to IDD. Administration of MEC into lungs of rats triggers an inflammatory response, including increased expression of COX-2, iNOS, and mediators of extracellular matrix turnover, such as connective tissue growth factor and MMP-9 [37]. Topical exposure to MEC leads to blistering and ulceration, and skin matrix breakdown is associated with induction of several collagenases [38]. Together these studies demonstrate that genotoxic agents cause up-regulation of matrix catabolism in a variety of tissues.
Ionizing radiation liberates electrons from atoms or molecules, producing ions that can be chemically reactive [39]. For example, IR may produce free radicals, such as reactive oxygen species, which can damage DNA. This is in addition to the direct, physical DNA damage caused by ionizing or breaking DNA molecules, leading to single- and double-strand breaks [40]. IR is ubiquitous in the environment, and comes from naturally occurring radioactive materials and cosmic rays. Common man-made sources are artificially produced radioisotopes, X-ray tubes and particle accelerators. Because of its action, IR has long been linked to aging and certain age-related diseases [41]. IR is known to induce a number of processes implicated in biological aging including oxidative stress, chromosomal damage, apoptosis, stem cell exhaustion and inflammation [41]. IR-exposed articular chondrocytes undergo senescence [42], consistent with our finding of IR induced cellular senescence in intervertebral discs (Fig. 3). In our study, IR exposure also perturbed disc matrix homeostasis, especially in DNA repair-deficient Ercc1−/Δ mice. The effects were generally less severe than those seen by MEC, but it is difficult to compare the extent of DNA damage induced by the two genotoxic agents. Our results are consistent with a previous study that demonstrated that IR has a deleterious effect on extracellular matrix, including decreased aggrecan expression, in articular cartilage [43; 44], and provide support for the contributive role of IR exposure in the development of intervertebral disc degeneration [45]. Importantly, we demonstrate that local irradiation of the vertebrae is sufficient to induce PG loss, indicating a direct role for DNA damage on disc cells.
5. Conclusion
Our findings provide novel insights into the mechanisms involved in IDD. Exposure to genotoxic agents, cause decreased disc extracellular matrix in healthy adult mice, an outcome that is attributable to both decreased matrix synthesis and increased degradation. The use of a genetic model with a DNA repair defect (Ercc1−/Δ mice) was important in our study because it revealed that disc matrix loss was more dramatic in these mice compared to Wt mice, demonstrating that DNA damage drives the loss of disc homeostasis. This may explain why smokers are at increased risk of IDD [46; 47].
Genotoxic agents are integral to cancer therapy[48]. Advances in such treatments have resulted in substantial improvements in overall survival [49]. Long-term cancer survivors are, however, at higher risk than the normal population of developing cancer (secondary), cardiac and thyroid dysfunction, osteoporosis, pulmonary disease, and other chronic conditions [50]. Damage to the organ systems caused by chemotherapy and radiation therapy is clearly involved in long-term health effects [51]. Our findings suggest that cancer survivors may be at increased risk of IDD and its sequelae.
Highlights.
Genotoxic stress accelerates disc cellular senescence and matrix proteoglycan loss
Harmful effects of genotoxin on discs more pronounced in DNA repair-deficient mice
Genotoxic stress can promote intervertebral disc aging and degeneration.
Acknowledgements
We thank Dr. Yong Yang for his technical assistance on the local irradiation of the mouse tail and spine. This work was supported by NIH grant AG033046 and the 2010 ORS Collaborative Exchange Award (Nam Vo), NIH ES016114 and the University of Pittsburgh Claude D. Pepper Center (P30AG024827) (Laura Niedernhofer), NIH AR051456 (Paul Robbins), and the Albert B. Ferguson, Jr. M.D. Orthopaedic Fund of the Pittsburgh Foundation. This project used the UPCI Animal Facility and was supported in part by award P30CA047904.
Footnotes
Conflict of interest The authors declared that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine (Phila Pa 1976) 1995;20:11–9. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- [2].Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–74. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- [3].Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- [4].Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- [5].Edelson JG, Nathan H. Stages in the natural history of the vertebral end-plates. Spine (Phila Pa 1976) 1988;13:21–6. doi: 10.1097/00007632-198801000-00006. [DOI] [PubMed] [Google Scholar]
- [6].Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- [7].Holliday R. Aging is no longer an unsolved problem in biology. Ann N Y Acad Sci. 2006;1067:1–9. doi: 10.1196/annals.1354.002. [DOI] [PubMed] [Google Scholar]
- [8].Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–8. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- [9].Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- [10].Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176–80. doi: 10.1016/j.biocel.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- [12].Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–6. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–50. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- [14].Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, Tilstra JS, Feldman CH, Robbins PD, Niedernhofer LJ, Huard J. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 3:608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008 doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- [16].Vo N, Seo HY, Robinson A, Sowa G, Bentley D, Taylor L, Studer R, Usas A, Huard J, Alber S, Watkins SC, Lee J, Coehlo P, Wang D, Loppini M, Robbins PD, Niedernhofer LJ, Kang J. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 2010;28:1600–7. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rojsitthisak P, Jongaroonngamsang N, Romero RM, Haworth IS. HPLC-UV, MALDI-TOF-MS and ESI-MS/MS analysis of the mechlorethamine DNA crosslink at a cytosine-cytosine mismatch pair. PLoS One. 2011;6:e20745. doi: 10.1371/journal.pone.0020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rink SM, Hopkins PB. A mechlorethamine-induced DNA interstrand cross-link bends duplex DNA. Biochemistry. 1995;34:1439–45. doi: 10.1021/bi00004a039. [DOI] [PubMed] [Google Scholar]
- [19].Seam P, Janik JE, Longo DL, Devita VT., Jr. Role of chemotherapy in Hodgkin’s lymphoma. Cancer J. 2009;15:150–4. doi: 10.1097/PPO.0b013e3181a27018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Whittaker SJ, Foss FM. Efficacy and tolerability of currently available therapies for the mycosis fungoides and Sezary syndrome variants of cutaneous T-cell lymphoma. Cancer Treat Rev. 2007;33:146–60. doi: 10.1016/j.ctrv.2006.08.006. [DOI] [PubMed] [Google Scholar]
- [21].Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, Hasty P, Hoeijmakers JH, Niedernhofer LJ. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–92. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- [23].Wang D, Vo NV, Sowa GA, Hartman RA, Ngo K, Choe SR, Witt WT, Dong Q, Lee JY, Niedernhofer LJ, Kang JD. Bupivacaine decreases cell viability and matrix protein synthesis in an intervertebral disc organ model system. Spine J. 2011;11:139–46. doi: 10.1016/j.spinee.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–56. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [25].Lee ER, Lamplugh L, Davoli MA, Beauchemin A, Chan K, Mort JS, Leblond CP. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0-21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev Dyn. 2001;222:52–70. doi: 10.1002/dvdy.1168. [DOI] [PubMed] [Google Scholar]
- [26].Roughley PJ, Lamplugh L, Lee ER, Matsumoto K, Yamaguchi Y. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine (Phila Pa 1976) 36:E914–20. doi: 10.1097/BRS.0b013e3181f1e84f. [DOI] [PubMed] [Google Scholar]
- [27].Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- [28].Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee ER, Lamplugh L, Leblond CP, Mordier S, Magny MC, Mort JS. Immunolocalization of the cleavage of the aggrecan core protein at the Asn341-Phe342 bond, as an indicator of the location of the metalloproteinases active in the lysis of the rat growth plate. Anat Rec. 1998;252:117–32. doi: 10.1002/(SICI)1097-0185(199809)252:1<117::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [30].Ramos KS, Moorthy B. Bioactivation of polycyclic aromatic hydrocarbon carcinogens within the vascular wall: implications for human atherogenesis. Drug Metab Rev. 2005;37:595–610. doi: 10.1080/03602530500251253. [DOI] [PubMed] [Google Scholar]
- [31].Battie MC, Videman T, Gibbons LE, Fisher LD, Manninen H, Gill K. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine (Phila Pa 1976) 1995;20:2601–12. [PubMed] [Google Scholar]
- [32].Wang D, Nasto AL, Roughley P, Leme SA, Houghton M, Usas A, Sowa G, Lee J, Niedernhofer L, Shapiro S, Kang J, Vo N. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis and Cartilage. 2012 doi: 10.1016/j.joca.2012.04.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Durigova M, Nagase H, Mort JS, Roughley PJ. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 30:145–53. doi: 10.1016/j.matbio.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sandy JD. A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. OsteoArthritis and Cartilage. 2006;14:95–100. doi: 10.1016/j.joca.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [35].Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst) 2011;10:781–91. doi: 10.1016/j.dnarep.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rahn JJ, Adair GM, Nairn RS. Multiple roles of ERCC1-XPF in mammalian interstrand crosslink repair. Environ Mol Mutagen. 2010;51:567–81. doi: 10.1002/em.20583. [DOI] [PubMed] [Google Scholar]
- [37].Sunil VR, Patel KJ, Shen J, Reimer D, Gow AJ, Laskin JD, Laskin DL. Functional and inflammatory alterations in the lung following exposure of rats to nitrogen mustard. Toxicol Appl Pharmacol. 2010;250:10–8. doi: 10.1016/j.taap.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wormser U, Brodsky B, Reich R. Topical treatment with povidone iodine reduces nitrogen mustard-induced skin collagenolytic activity. Arch Toxicol. 2002;76:119–21. doi: 10.1007/s00204-001-0307-5. [DOI] [PubMed] [Google Scholar]
- [39].Hall AJGEJ. Radiobiology for the Radiologist. 6th ed Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- [40].Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- [41].Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging (Albany NY) 2009;1:887–902. doi: 10.18632/aging.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, Kim SJ, Kim JI, Hwang SG. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2009;285:1283–95. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rock K, Grandoch M, Majora M, Krutmann J, Fischer JW. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): new insights into mechanisms of matrix remodeling. J Biol Chem. 2011;286:18268–76. doi: 10.1074/jbc.M110.201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ailland J, Kampen WU, Schunke M, Trentmann J, Kurz B. Beta irradiation decreases collagen type II synthesis and increases nitric oxide production and cell death in articular chondrocytes. Ann Rheum Dis. 2003;62:1054–60. doi: 10.1136/ard.62.11.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Samartzis D, Cheung KM. Ionizing radiation exposure and the development of intervertebral disc degeneration in humans: myth or reality. Spine J. 11:979–82. doi: 10.1016/j.spinee.2011.07.019. [DOI] [PubMed] [Google Scholar]
- [46].Dong Wang LAN, Roughley Peter, Dong Qing, Leme Adriana S, Houghton McGarry, Usas Arvydas, Sowa Gwendolyn, Studer Rebecca, Lee Joon, Niedernhofer Laura, Kang James, Shapiro Steven, Vo Nam. Chronic exposure to tobacco smoke increases proteolysis of aggrecan and depletes matrix proteoglycan in intervertebral disc. Journal of Orthopedic Research. 2011 Submitted. [Google Scholar]
- [47].Battie MC, Videman T, Gill K, Moneta GB, Nyman R, Kaprio J, Koskenvuo M. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976) 1991;16:1015–21. [PubMed] [Google Scholar]
- [48].Dy GK, Adjei AA. Systemic cancer therapy: evolution over the last 60 years. Cancer. 2008;113:1857–87. doi: 10.1002/cncr.23651. [DOI] [PubMed] [Google Scholar]
- [49].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [50].Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- [51].Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, Skinner R, Stevens MC, Hawkins MM. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–9. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]