Abstract
AIM: To study the effects of combined early fluid resuscitation and hydrogen inhalation on septic shock-induced lung and intestine injuries.
METHODS: Wistar male rats were randomly divided into four groups: control group (Group A, n = 15); septic shock group (Group B, n = 15); early fluid resuscitation-treated septic shock group (Group C, n = 15); and early fluid resuscitation and inhalation of 2% hydrogen-treated septic shock group (Group D, n = 15). The activity of hydroxyl radicals, myeloperoxidase (MPO), superoxide dismutase (SOD), diamine oxidase (DAO), and the concentration of malonaldehyde (MDA) in the lung and intestinal tissue were assessed according to the corresponding kits. Hematoxylin and eosin staining was carried out to detect the pathology of the lung and intestine. The expression levels of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α in lung and intestine tissue were detected by enzyme-linked immunosorbent assay method. The expression levels of Fas and Bcl2 in lung tissues were determined by immunohistochemistry and Western blotting.
RESULTS: Septic shock elicited a significant increase in the levels of MDA (10.17 ± 1.12 nmol/mg protein vs 2.98 ± 0.64 nmol/mg protein) and MPO (6.79 ± 1.02 U/g wet tissue vs 1.69 ± 0.14 U/g wet tissue) in lung tissues. These effects were not significantly decreased by Group C pretreatment, but were significantly reduced by Group D pretreatment (MDA: 4.45 ± 1.13 nmol/mg protein vs 9.56 ± 1.37 nmol/mg protein; MPO: 2.58 ± 0.21 U/g wet tissue vs 6.02 ± 1.16 U/g wet tissue). The activity of SOD (250.32 ± 8.56 U/mg protein vs 365.78 ± 10.26 U/mg protein) in lung tissues was decreased after septic shock, and was not significantly increased by Group C pretreatment, but was significantly enhanced by Group D pretreatment (331.15 ± 9.64 U/mg protein vs 262.98 ± 5.47 U/mg protein). Histological evidence of lung hemorrhage, neutrophil infiltration and overexpression of IL-6, IL-8, and TNF-α was observed in lung tissues, all of which were attenuated by Group C and further alleviated by Group D pretreatment. Septic shock also elicited a significant increase in the levels of MDA, MPO and DAO (6.54 ± 0.68 kU/L vs 4.32 ± 0.33 kU/L) in intestinal tissues, all of which were further increased by Group C, but significantly reduced by Group D pretreatment. Increased Chiu scoring and overexpression of IL-6, IL-8 and TNF-α were observed in intestinal tissues, all of which were attenuated by Group C and further attenuated by Group D pretreatment.
CONCLUSION: Combined early fluid resuscitation and hydrogen inhalation may protect the lung and intestine of the septic shock rats from the damage induced by oxidative stress and the inflammatory reaction.
Keywords: Early fluid resuscitation, Inhalation of hydrogen gas, Septic shock, Lung, Intestine, Oxidative damage
INTRODUCTION
Septic shock is a common complication of infection, severe trauma, and major operation in patients. Septic shock is an autoimmune injury of the body, and the pathogenesis is very complicated. The immunocyte is activated in patients with septic shock, and the respiratory burst creates massive amounts of reactive oxygen species (ROS) which cannot be cleaned by the antioxidant defense systems[1,2]. Oxidative stress induced by ROS can increase lipid peroxidation, and can also increase the permeability of the alveolar epithelial cells by destroying the cell membrane. Simultaneously, the aggregation of neutrophilic leukocytes in the lung further induces the respiratory burst. The imbalance of antioxidant defense systems against oxidative stress then further damages the alveolar epithelial cells.
In order to ensure the blood supply of major organs, including the heart and brain, in patients with septic shock, blood flow is redistributed. There is a significant decrease in blood flow of the gastrointestinal tract which induces severe ischemia, hypoxia, and reperfusion injury. Inflammatory factors are activated which induce further injury in the gastrointestinal tract, and may consequently produce multiple organ dysfunction syndrome (MODS)[3]. Despite recent advances in antibiotic therapy and intensive care, sepsis is still considered to be one of the most common causes of death in intensive care units. The main therapy for septic shock is hemostasis and fluid resuscitation which can prevent MODS by improving hemodynamics[4]. However, fluid resuscitation is not effective in microcirculation disturbance and hypoxia. So, new and effective therapies for septic shock should be developed to improve the patient outcomes.
Recently, it has been suggested that molecular hydrogen exerts a therapeutic antioxidant activity by selectively reducing hydroxyl radicals (•OH, the most cytotoxic ROS) and effectively protects against organ damage[5-7]. Xie et al[1,8,9] also report that hydrogen inhalation can significantly decrease levels of oxidative product, increase activities of antioxidant enzymes, and reduce levels of high-mobility group box 1 in serum and lung tissue, thus improving the survival rate of mice with sepsis. However, there are no reports combining fluid resuscitation and hydrogen inhalation as treatment for septic shock. Therefore, the present study was designed to investigate the protective effects of combined early fluid resuscitation and 2% hydrogen on the lung and intestine in a lipopolysaccharide (LPS)-induced septic shock rat model.
MATERIALS AND METHODS
Animals and chemicals
Male Wistar rats weighing 180-200 g were used for the study. They were obtained from the Laboratory Animal Center (China Medical University, Shenyang, China). LPS (L-2880 from Escherichia coli serotype 055:B5) was obtained from Sigma Chemical Co. (St. Louis, MO, United States). The •OH, myeloperoxidase (MPO), superoxidase dismutase (SOD), and malonaldehyde (MDA) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α enzyme-linked immunosorbent assay (ELISA) kits, rabbit polyclonal anti-Bcl-2 and rabbit polyclonal anti-Fas were purchased from Wuhan Boster Bio-engineering Limited Company (Wuhan, China).
Experimental design
Sixty male Wistar rats were divided into four groups randomly, 15 rats in each: control group (Group A); septic shock group (Group B); early fluid resuscitation + septic shock group (Group C); and early fluid resuscitation + inhalation of 2% hydrogen + septic shock group (Group D). Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (300 mg/kg) and tracheally ventilated using a Colombus ventilator (HX-300, TaiMeng Technologies, China), with a respiratory frequency of 100 breaths/min and a tidal volume of 10 mL/kg. The inhaled gas used for Group D was a hydrogen-air mixture (2% hydrogen in air), and the inhaled gas for other groups was room air. Left common carotid artery was cannulated to monitor the heart rate and mean arterial pressure (MAP). The femoral vein was cannulated for drug administration and fluid resuscitation. An electric heater was used to maintain body temperature. Half an hour after the rats reached a stable status, except for the rats in Group A, LPS was administered at a dose of 15 mg/kg by slow intravenous injection (not less than 2 min) to establish the septic shock rat model. Then, 0.2 mL saline was used to ensure that the LPS remaining in the syringe was completely injected[10]. Group A rats received intravenous injections of the same volume of saline. Fluid challenge (10 mL/kg per 15 min) was performed when MAP or arterial blood flow decreased to 80% of baseline values. If the percent change in pulse pressure > 13% with a persistent decrease in MAP or arterial blood flow, the fluid challenge was repeated. Norepinephrine was introduced when MAP remained low despite a normal percent change in pulse pressure (< 13%). Norepinephrine concentration was either progressively increased or decreased between 0.5 and 6 μg/kg.min, as needed, to maintain the MAP at ± 10% of baseline value[10]. Vital signs, fluid volume and the amount of norepinephrine were also recorded.
Preparation of lung tissues
The rats were sacrificed 2 h after the septic shock rat model was established. Arterial blood (1 mL) was collected for assessing the arterial blood gases. The heart and lung were removed through a thoracotomy and the dorsal lobe of the right lung was cut into three blocks (0.1 cm × 0.1 cm × 0.1 cm). The excess liquid of the first block was drained by the filter paper, and wet weight (W) was recorded. Then the same block was dried in a drying oven at a temperature of 70 °C for 24 h to measure dry weight (D). The second block was fixed with 4% paraformaldehyde at a temperature of 4 °C, and then, the block was embedded in paraffin and sliced for hematoxylin and eosin (HE) staining and immunohistochemical study. The other lung tissue block was frozen at -70 °C for Western blotting analysis. The right hilum was ligated, and 2 mL of saline solution was used for the left lung lavage, and about 3.0-3.6 mL of bronchial alveolar lavage fluid (BALF) was collected. The BALF was centrifuged at 1500 × g for 4 min, and the supernatant was collected and stored at -20 °C for inflammatory mediator analysis.
Preparation of small intestine tissues
The rats were sacrificed 2 h after the septic shock rat model was established. Arterial blood (1 mL) was collected for assessing the arterial blood gases. Another 4 mL arterial blood was centrifuged at 2000 rpm for 10 min, and the supernatant serum was collected and stored at -20 °C. A laparotomy was performed, two small intestinal segments were resected from 10 cm distal to the ligament of Treitz, and the intestinal segments were gently washed using saline rinse cooled to 4 °C. These two small intestine segments (cut to 1 cm × 1 cm) were fixed with 4% paraformaldehyde and 2.5% glutaraldehyde, respectively. The other small intestine segments used for assessing the oxidative stress were frozen at -70 °C.
Determination of oxidative damage
Small intestinal tissues and lung tissues were homogenated, centrifuged at 12 000 × g for 20 min, and the supernatant serum was collected. The OH, MDA, SOD, and MPO activities were measured according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Measurement of serum diamine oxidase activity
The frozen serum was thawed on ice, and centrifuged at 1000 × g for 15 min at 4 °C. The serum diamine oxidase (DAO) activity was detected with dianisidine developer, and the serum DAO activity assay was performed according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
HE staining of tissues
Both the small intestinal tissue and lung tissues were fixed with 4% paraformaldehyde for more than 24 h. After dehydrating and embedding, the tissues were cut into 5 μm slices. The slices were stained with HE. The pathogenesis of lung injury and small intestinal mucosal injury was observed under microscope. The intestinal mucosal injury was evaluated by the Chiu scoring system. The Chiu’s score was graded as: Grade 0: normal mucosa; Grade 1: formation of subepithelial detachments at the tip of the villi with capillary congestion; Grade 2: subepithelial detachments exert a moderate amount of upward push on the mucosal epithelium; Grade 3: large subepithelial detachments exert a massive amount of upward push on the mucosal epithelium along the villi and a few denuded villus tips are observed; Grade 4: the villi are denuded to the level of lamina propria and dilated capillaries; Grade 5: presence of ulceration, disintegration of lamina propria, and hemorrhage[11]. Ten fields for each sample were observed, and the average score was recorded as the pathology score of the small intestinal tissue.
Changes in the organelles of small intestine
The small intestine segments were fixed with 2.5% glutaraldehyde. After dehydration, the samples were embedded in epoxy resins, and then were cut into ultrathin sections. The changes in the organelles were observed under transmission electron microscope.
Measurement of serum inflammatory mediators
The frozen serum was thawed on ice, and centrifuged at 1000 × g for 15 min at 4 °C. Levels of IL-6, IL-8 and TNF-α were detected by ELISA according to the manufacturer’s instructions (Wuhan Boster Bio-engineering Limited Company, Wuhan, China).
Immunohistochemical study
Tissue sections (slice thickness 3 μm) were dried for 4 h at 60 °C-65 °C, and then were deparaffinized and rehydrated. Sections were immersed in phosphate buffered saline (PBS) three times (5 min/time). Then antigen retrieval was performed followed by cooling at room temperature. The slides were immersed in PBS three times (5 min/time). After that, the sections were incubated with methanol containing 3% hydrogen peroxide for 20 min, and then were left at room temperature for 10 min followed by washing with PBS three times (5 min/time). The slides were blocked with normal goat serum solution at room temperature for 10 min, and the superfluous serum surrounding slides was wiped off. After that, the slides were incubated with primary antibody (50 μL) at 4 °C overnight. After washing with PBS three times (5 min/time), sections were incubated in horseradish peroxidase-polymer goat anti-Ms/Rb immunoglobulin G at room temperature for 20 min followed by rinsing in PBS three times (5 min/time). The slides were developed with 3,3’-diaminobenzidine solution for about 3-5 min, rinsed with tap water, and then counterstained with hematoxylin. Acid alcohol solution (1%) was used for differentiation for 1 s, followed by rinsing with tap water for 10-15 min. Finally, specimens were dehydrated and mounted, and examined with a microscope equipped with an image analysis system. Yellow or brownish-yellow staining represents positive staining.
Western blotting analysis
Levels of Fas and Bcl2 proteins in lung tissue were quantified by Western blotting as described previously with some minor modifications[12]. Briefly, about 10 mg frozen lung tissue were immersed in the tissue lysates at a ratio of 1:10 w/v, then submitted to ultrasound homogenation for 1 min followed by centrifugation at 13 000 rpm for 5 min at 4 °C. The supernatant was collected, and total protein concentration was measured by the Bradford method. About 15 μg total proteins was loaded in each well and separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis for 1 h. After running, the proteins were transferred onto the polyvinylidene difluoride membranes (Millipore, United States). The membranes were blocked with 5% fat-free milk, and then were incubated with Tris-buffered-daline Tween20-containing the primary antibodies (1:500 dilution) overnight at 4 °C. After extensive washing, the second antibody was added, and the immunocomplexes were then detected using an enhanced chemiluminescence Western blotting detection kit (GE, Healthcare). The relative densities of the protein bands were analyzed using the Quantity One software from Bio-Rad. Data were represented in relative arbitrary units.
Statistical analysis
All the data analyses were carried out using the statistical analysis software SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, United States). Data were presented as the mean ± SD. Significant differences between the groups were analyzed by analysis of variance, and differences were considered significant at P < 0.05.
RESULTS
Fluid volume and blood gas parameters
Compared with Group A, the pH, MAP, heart rate and oxygen partial pressure of Group B were significantly decreased; however, a significant increase in lactate value was observed (P < 0.05). The pH, MAP, heart rate and oxygen partial pressure were increased obviously, and the lactate value was clearly decreased in Group C as compared with Group B. Furthermore, a significant increase in pH value and oxygen partial pressure and decrease in lactate value were also observed in Group D compared with Group C (P < 0.05). There was no significant difference in MAP and heart rate between Group D and Group C. Total fluid volume and the amount of norepinephrine used in Group D were significantly less than in Group C (P < 0.05). There was no significant difference in partial pressure of carbon dioxide among the four groups (P > 0.05; Table 1).
Table 1.
Comparison of blood gas and fluid volume among four groups (mean ± SD)
| Group | pH value | MAP (mmHg) | Heart rate | PaO2 (mmHg) | PaCO2 (mmHg) | Lactate (mmol/L) | Fluid volumes (mL) | Norepinephrine (μg/kg per minute) |
| A | 7.38 ± 0.12 | 101 ± 8 | 240 ± 13 | 93.9 ± 2.23 | 38.65 ± 1.78 | 1.50 ± 0.34 | 0 | 0 |
| B | 6.89 ± 0.13a | 37 ± 11a | 65 ± 20a | 50.19 ± 3.78a | 34.54 ± 1.89 | 6.98 ± 2.45a | 0 | 0 |
| C | 7.21 ± 0.15c | 95 ± 10c | 260 ± 15c | 62.34 ± 2.46c | 35.09 ± 2.01 | 4.37 ± 1.36c | 12 ± 3c | 3.5 ± 1.8c |
| D | 7.32 ± 0.17e | 97 ± 11 | 255 ± 12 | 88.98 ± 3.17e | 36.49 ± 1.84 | 3.59 ± 1.53c | 5 ± 2e | 0.8 ± 0.3e |
P < 0.05 vs Group A;
P < 0.05 vs Group B;
P < 0.05 vs Group C. MAP: Mean arterial pressure.
OH, MDA, MPO and SOD levels and lung W/D values
In Group B, the levels of OH, MDA, and MPO were significantly increased, and the level of SOD was significantly decreased compared with Group A (P < 0.05). Compared with Group B, lower levels of OH, MDA and MPO, and a higher level of SOD in Group C were also observed, but there was no significant difference between the two groups (P > 0.05). However, compared with Group C, there were also significant decreases in the levels of OH, MDA and MPO, and a significant increase in the SOD level in Group D (P < 0.05). The lung W/D value in Group B was significantly increased compared with group A (P > 0.05), and a higher lung W/D value was observed in Group C compared with Group B, but there was no significant difference between the two groups (P > 0.05). However, there was a significant decrease in the lung W/D value in Group D compared with Group C (P < 0.05; Table 2).
Table 2.
Comparison of wet/dry wight and redox indicator among four groups (mean ± SD)
| Group | Wet/dry wight | Inhibition of •OH (U/mg protein) | MDA (nmol/mg protein) | MPO (U/g wet tissue) | SOD (U/mg protein) |
| A | 3.85 ± 0.34 | 87.52 ± 3.45 | 2.98 ± 0.64 | 1.69 ± 0.14 | 365.78 ± 10.26 |
| B | 6.89 ± 0.23a | 12.89 ± 1.52a | 10.17 ± 1.12a | 6.79 ± 1.02a | 250.32 ± 8.56a |
| C | 6.96 ± 0.25 | 13.14 ± 2.24 | 9.56 ± 1.37 | 6.02 ± 1.16 | 262.98 ± 5.47 |
| D | 4.62 ± 0.27c | 66.31 ± 2.98c | 4.45 ± 1.13c | 2.58 ± 0.21c | 331.15 ± 9.64c |
P < 0.05 vs Group A;
P < 0.05 vs Group C. •OH: Hydroxyl radical; MDA: Malonaldehyde; MPO: Mmyeloperoxidase; SOD: Superoxide dismutase.
HE staining of lung tissues
HE-stained lung tissue sections were observed under a light microscope. The normal alveolar structure was found in Group A, and no hyperemia, neutrophil infiltration, and interstitial edema in the interstitium were observed (Figure 1A). In Group B, disorders of the alveolar structures, the collapse of alveoli, loss of integrity of the alveolar wall, severe neutrophil infiltration in the alveoli, and alveolar capillary congestion were observed, and the alveolar walls were thickened by edema (Figure 1B). In Group C, the extent of neutrophil accumulation and the alveolar-capillary exudate were reduced compared with Group B, but there was no significant improvement in alveolar edema (Figure 1C). Compared with Group B, significant decreases in alveolar damage were found in Group D (Figure 1D), and there was significant improvement in alveolar edema compared with Group C.
Figure 1.
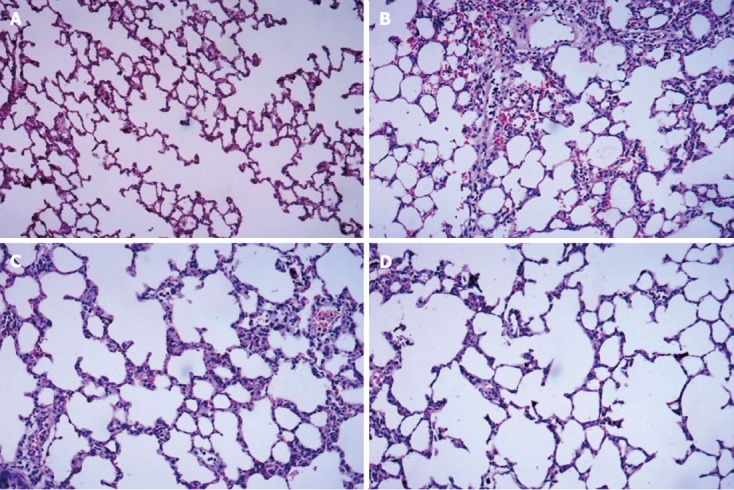
Hematoxylin and eosin staining of dorsal lobe of right lung tissue. A: Control group; B: Septic shock group; C: The early fluid resuscitation-treated group; D: Combined early fluid resuscitation and 2% hydrogen inhalation-treated group. Lung tissues were fixed with 4% paraformaldehyde for more than 24 h. After dehydrating and embedding, they were cut into 5 μm slices, and were stained with hematoxylin and eosin. The pathogenesis of lung injury was observed under a microscope (original magnification × 200). Normal alveolar structure was found in Group A. In Group B, disorders of the alveolar structures, severe neutrophil infiltration in the alveoli and alveolar capillary congestion were observed. In Group C, the extent of neutrophil accumulation and the alveolar-capillary exudate were reduced compared with Group B. However, significant decreases in alveolar damage were found in Group D, and there was significant improvement in alveolar edema compared with Group C.
Cytokine profiles in lung tissues
Compared with Group A, significant increases in levels of IL-6, IL-8 and TNF-α were found in Group B (P < 0.05). The expression of IL-6, IL-8 and TNF-α was significantly decreased in Group C as compared with Group B. In addition, the expression of IL-6, IL-8 and TNF-α was even lower in Group D than in Group C, and the difference was significant (Table 3).
Table 3.
Level of inflammatory mediators in the bronchial alveolar lavage fluid of four groups (mean ± SD)
| Group | IL-6 (pg/mL) | IL-8 (pg/mL) | TNF-α (pg/mL) |
| A | 35.88 ± 3.12 | 24.29 ± 4.23 | 19.65 ± 1.78 |
| B | 446.89 ± 37.24a | 411.19 ± 32.78a | 158.54 ± 16.89a |
| C | 387.79 ± 31.58c | 347.34 ± 29.37c | 104.09 ± 9.18c |
| D | 118.32 ± 11.38e | 96.45 ± 10.25e | 47.58 ± 3.65e |
P < 0.05 vs Group A;
P < 0.05 vs Group B;
P < 0.05 vs Group C. TNF-α: Tumor necrosis factor-α; IL: Interleukin.
Expression of Fas and Bcl2-immunoreactivity in lung tissues
The positive expression of Fas and Bcl2 protein in lung tissue was demonstrated by the presence of brown-yellow granules, mostly located in the nucleolus cytoplasm (Figure 2). Most bronchial epithelial cells, alveolar epithelial cells and inflammatory cells were Fas-positive and Bcl-positive cells, and Fas and Bcl2 were expressed at low levels in normal lung tissue (Figure 2A and E). The expression of Fas proteins was up-regulated, while the expression of Bcl2 proteins was down-regulated in Group B (Figure 2B and F) compared with Group A. In Group C, however, the expression of Fas proteins was down-regulated, and the expression of Bcl2 proteins was up-regulated (Figure 2C and G) compared with Group B (P < 0.05). In addition, the lower expression of Fas proteins and the higher expression of Bcl2 proteins were observed in Group D (Figure 2D and H) compared with Group C (P < 0.05; Table 4).
Figure 2.
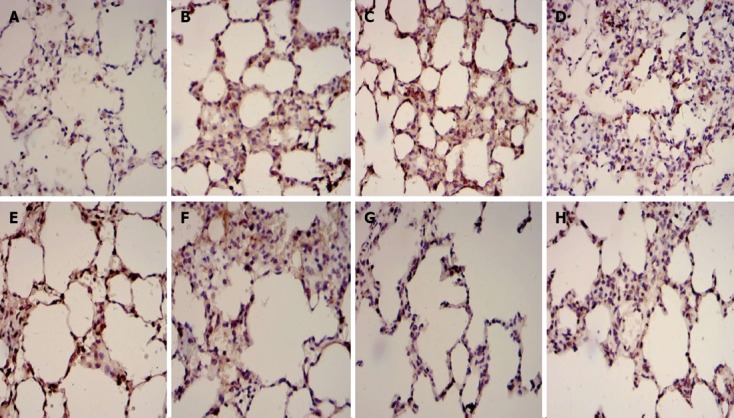
Immunohistochemical examples for Fas and Bcl2 in dorsal lobe of right lung tissue. A: Fas in control group; B: Fas in septic shock group; C: Fas in early fluid resuscitation-treated group; D: Fas in early fluid resuscitation + 2% hydrogen inhalation-treated group; E: Bcl2 in control group; F: Bcl2 in septic shock group; G: Bcl2 in early fluid resuscitation-treated group; H: Bcl2 in early fluid resuscitation + 2% hydrogen inhalation-treated group. Examples were examined under a light microscope equipped with an image analysis system. Yellow or brownish-yellow staining represents positive staining. The expression of Fas proteins was up-regulated, while the expression of Bcl2 proteins was down-regulated in Group B compared with Group A. In Group C, however, the expression of Fas proteins was down-regulated, and the expression of Bcl2 proteins was up-regulated compared with Group B. In addition, the lower expression of Fas proteins and the higher expression of Bcl2 proteins were observed in Group D compared with Group C.
Table 4.
Comparison of average optical density and the positive area ratio of Fas and Bcl2 among the four groups (n = 15, mean ± SD)
| Group |
Average optical density (10-3) |
Positive area ratio (%) |
||
| Fas | Bcl2 | Fas | Bcl2 | |
| A | 0.802 ± 0.221 | 1.260 ± 0.128 | 2.737 ± 0.881 | 11.012 ± 0.792 |
| B | 1.549 ± 0.343a | 0.638 ± 0.072a | 6.441 ± 0.331a | 4.121 ± 0.313a |
| C | 1.329 ± 0.311c | 1.103 ± 0.099c | 4.876 ± 0.887c | 4.376 ± 0.250c |
| D | 1.255 ± 0.400e | 1.141 ± 0.043e | 3.247 ± 0.621e | 10.432 ± 0.791e |
P < 0.05 vs Group A;
P < 0.05 vs Group B;
P < 0.05 vs Group C.
Levels of Fas and Bcl2 protein in lung tissues
The level of Fas protein was up-regulated, while the level of Bcl2 protein was down-regulated in Group B compared with Group A (P < 0.01). However, in Group C, level of Fas protein was down-regulated, and the level of Bcl2 protein was up-regulated compared with Group B (P < 0.01; Figure 3). In addition, a lower level of Fas protein and a higher level of Bcl2 protein were found in Group D compared with Group C (P < 0.05; Figure 3).
Figure 3.
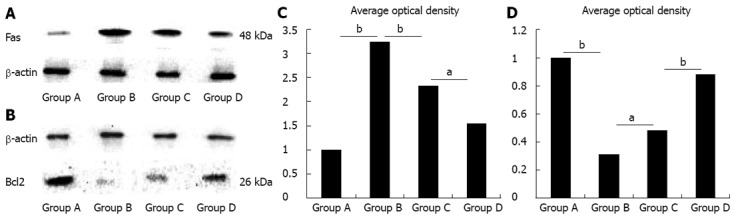
Levels of Fas and Bcl2 proteins in dorsal lobe of right lung tissues quantified by Western blotting. β-actin was used as a loading control. The relative Fas (A) and Bcl2 (B) protein levels of lung tissues from the four groups were expressed as arbitrary units after normalization with β-actin (C: Fas; D: Bcl2). Data were considered significant at aP < 0.05 and highly significant at bP < 0.01 vs the control group. The level of Fas protein was up-regulated, while the level of Bcl2 protein was down-regulated in Group B vs Group A. However, in Group C the level of Fas proteins was down-regulated, and the level of Bcl2 proteins was up-regulated vs Group B (P < 0.01). In addition, the lower level of Fas protein and the higher level of Bcl2 protein was found in Group D compared with Group C (P < 0.05).
Levels of DAO in intestinal tissues
The level of DAO in Group B was significantly increased compared with Group A (P < 0.05), and the level of DAO was even higher in Group C (P < 0.05). But the level of DAO in Group D was significantly decreased compared with both Group B and Group C (P < 0.05; Table 5).
Table 5.
Levels of serum diamine oxidase and inflammatory mediators in the bronchial alveolar lavage fluid of four groups (mean ± SD)
| Group | DAO (kU/L) | IL-6 (pg/mL) | IL-8 (pg/mL) | TNF-α (pg/mL) |
| A | 4.32 ± 0.33 | 50.38 ± 3.24 | 35.93 ± 4.37 | 25.32 ± 2.18 |
| B | 6.54 ± 0.68a | 478.33 ± 30.78a | 458.19 ± 31.32a | 167.41 ± 14.47a |
| C | 6.89 ± 0.77c | 394.21 ± 28.15c | 395.34 ± 28.46c | 121.05 ± 8.01c |
| D | 5.14 ± 0.44ce | 145.26 ± 12.33e | 123.12 ± 10.23e | 36.27 ± 3.54e |
P < 0.05 vs Group A;
P < 0.05 vs Group B;
P < 0.05 vs Group C. TNF-α: Tumor necrosis factor-α; IL: Interleukin; DAO: Diamine oxidase.
HE staining of small intestinal tissues
In Group A, the structure of the small intestinal mucosa was intact, and normal intestinal mucosa was observed (Figure 4A). In Group B, glands of the small intestine were significantly damaged, and severe edema of mucosal villi, neutrophil infiltration, epithelial cell sloughing off, and structural changes in the epithelium of the small intestine and even small bowel ulceration were observed (Figure 4B). The damage mentioned above was far more severe in Group C (Figure 4C). But in Group D, the damage was significantly reduced (Figure 4D). The Chiu’s score of Group B was significantly higher than Group A (P < 0.05), and the Chiu’s score of Group C was even higher compared with Group B. But the Chiu’s score of Group D was significantly decreased compared with Group B and Group C (Table 6).
Figure 4.
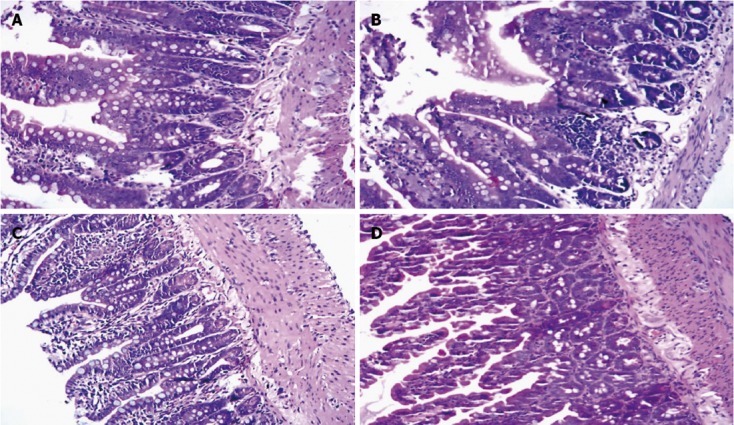
Hematoxylin and eosin staining of small intestine tissue. A: Control group; B: Septic shock group; C: Early fluid resuscitation-treated group; D: Early fluid resuscitation + 2% hydrogen inhalation-treated group. Small intestinal tissues were fixed with 4% paraformaldehyde for more than 24 h. After dehydrating and embedding, they were cut into 5 μm slices, and were stained with hematoxylin and eosin. The pathogenesis of small intestine injury was observed under a light microscope (original magnification × 200). In Group A, normal intestinal mucosa was observed. In Group B, glands of small intestine were significantly damaged, and severe edema of mucosal villi, neutrophil infiltration, epithelial cell sloughing off, and even small bowel ulceration were observed. The damage mentioned above was far more severe in Group C. But in Group D, the damage was significantly reduced.
Table 6.
Chiu’s score and levels of malonaldehyd and myeloperoxidase of small intestine (mean ± SD)
| Group | Chiu’s score | MDA (nmol/mg protein) | MPO (U/g wet tissue) |
| A | 0.37 ± 0.33 | 2.09 ± 0.33 | 1.55 ± 0.12 |
| B | 3.30 ± 0.89a | 6.19 ± 1.38a | 6.54 ± 0.99a |
| C | 3.79 ± 1.12c | 6.89 ± 1.26c | 7.05 ± 1.01c |
| D | 2.12 ± 0.67ce | 2.98 ± 0.45ce | 2.32 ± 0.34ce |
P < 0.05 vs Group A;
P < 0.05 vs Group B;
P < 0.05 vs Group C. MDA: Malonaldehyd; MPO: Myeloperoxidase.
Transmission electron microscopic analysis of small intestinal samples
In Group A, normal microvilli on the surface of epithelial cells of the intestine were observed. The mitochondria, lysosome and rough endoplasmic reticulum maintained normal morphology (Figure 5A). In Group B, disarrangement of the epithelial surface and intestinal microvillus reduction were observed. Mitochondria present in the cytoplasm also showed vacuolization, and the cristae of mitochondria were reduced. Heterochromatin nuclei showed margination phenomena, membrane rupture, and widened nuclear gap (Figure 5B). The microvilli on the surface of epithelial cells of the intestine were sparse in Group C; there was obvious reduction of the cristae of mitochondria, severe vacuolization of mitochondria and an abundance of marginated heterochromatin nuclei. The amount of rough endoplasmic reticulum was reduced, and more severe rough endoplasmic reticulum swelling and expansion were observed (Figure 5C). In Group D, the microvilli were missing to a small extent, and the heterochromatin nuclei showed only mild margination (Figure 5D).
Figure 5.
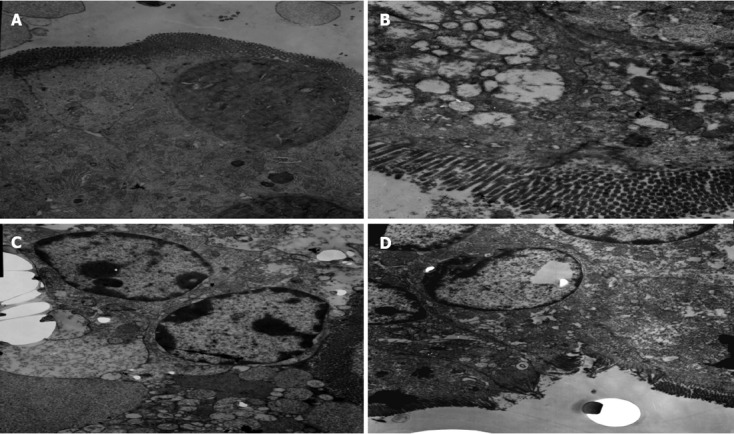
Changes in the organelles of small intestinal tissues under transmission electron microscope. A: Control group; B: Septic shock group; C: Early fluid resuscitation-treated group; D: Early fluid resuscitation + 2% hydrogen inhalation-treated group. The changes in the organelles were observed under transmission electron microscope. In Group A, normal microvilli on the surface of epithelial cells of the intestine were observed. In Group B, intestinal microvillus reduction was observed. Mitochondria showed vacuolization and heterochromatin nuclei showed margination phenomena. The microvilli on the surface of epithelial cells of the intestine were sparse in Group C, and reduction of the cristae of mitochondria, an abundance of marginated heterochromatin nuclei, and severe rough endoplasmic reticulum swelling and expansion were observed. In Group D, the microvilli were missing to a small extent, and the heterochromatin nuclei showed only mild margination.
Levels of MDA and SOD in small intestinal tissues
The levels of MDA and SOD in Group B were significantly increased compared with Group A (P < 0.05). Levels of MDA and SOD were higher in Group C than in Group B. In Group D, MDA and SOD were significantly decreased compared with both Group B and Group C (Table 6).
Cytokine profiles in small intestinal tissues
Levels of IL-6, IL-8 and TNF-α were significantly increased in Group B compared with Group A (P < 0.05). The expression of IL-6, IL-8 and TNF-α was significantly lower in Group C than in group B. In addition, the expression of IL-6, IL-8 and TNF-α in Group D was even lower compared with Group C, and the difference was significant (P < 0.05; Table 5).
DISCUSSION
In the present study, we investigated the effects of combined early fluid resuscitation and 2% hydrogen on the lung and small intestine in a LPS-induced septic shock rat model. Our study showed that early fluid resuscitation and inhalation of 2% hydrogen could decrease the oxidative damage and inhibit the over-expression of inflammatory factors (IL-6, IL-8 and TNF-α) in the lung tissue of the septic shock rats. This treatment could also down-regulate the expression of proapoptotic protein Fas, and up-regulate the expression of anti-apoptotic protein Bcl2 in the lung tissue. These data suggest that the application of combined early fluid resuscitation and inhalation of 2% hydrogen may protect the lung from septic shock damage by inhibiting the inflammatory reaction and apoptosis in comparison with traditional fluid resuscitation alone. Early fluid resuscitation and inhalation of 2% hydrogen not only decreased the oxidative damage and the levels of IL-6, IL-8 and TNF-α in plasma, but also protected the intestinal mucosa from mechanical injury.
Effects of combined early fluid resuscitation and hydrogen inhalation on lung injury in a LPS-induced septic shock rat model
Previous studies have indicated that MPO is a peroxidase enzyme released by activated polymorphonuclear neutrophils, which can be used as an indicator of the level of tissue neutrophils. MDA is one of the toxic metabolites of ROS induced lipid peroxidation, and SOD is one of the most important free radical scavenging enzymes. Levels of MDA and SOD can reflect the extent of oxidative stress. In the present study, the levels of MDA, SOD and MPO were used as an indicator for oxidative stress[13-15]. The results showed that the levels of MDA and MPO were significantly increased, however, the level of SOD was significantly decreased in the septic shock rats, and severe neutrophil infiltrates were observed in the alveoli. These data suggest that changes in the lung oxidant-antioxidant status may be associated with lung injury in septic shock rats. There was no obvious improvement in oxidative stress in the traditional fluid resuscitation-treated group compared with the septic shock group, but there was significant improvement in the indicators above in the early fluid resuscitation and inhalation of 2% hydrogen treatment group. Early fluid resuscitation and inhalation of 2% hydrogen attenuated septic shock-induced organ injury, and decreased neutrophil infiltrate in the alveoli. Significant improvements in alveolar edema and reduced alveolar damage were also observed. Previous studies have indicated that hydrogen, as a selective antioxidant with a therapeutic antioxidant activity, can easily pass through the cell membrane and reach the organelles[16,17]. Hydrogen can effectively protect against organ damage induced by selectively clearing •OH. These findings indicate that 2% hydrogen may selectively reduce •OH and provide a beneficial effect on septic shock.
Amplification of inflammatory responses and cell apoptosis also play an important role in the pathogenesis of sepsis-induced acute lung injury (ALI)[18,19]. In the present study, the levels of IL-6, IL-8 and TNF-α in BALF were significantly increased in the LPS-induced ALI rats compared with the control group. Our data showed that the early fluid resuscitation and inhalation of 2% hydrogen treatment reduced the levels of IL-6, IL-8 and TNF-α in BALF in comparison with the traditional fluid resuscitation-treated group, and these results are consistent with the previous study[1]. In the process of sepsis-induced ALI, the expression of Fas protein in the lung epithelial cell was up-regulated, and blocking FAS-mediated apoptosis could reduce the LSP-induced lung injury[20]. As one of the most important anti-apoptotic factors, Bcl-2 plays an important role in regulating the progress of apoptosis, and it is considered as a cytoprotective factor[21]. In the present study, decreases in lung cell apoptosis were observed in the early fluid resuscitation-treated group, and further decreases in lung cell apoptosis were observed in the early fluid resuscitation and inhalation of 2% hydrogen treated group. These results demonstrated that co-treatment could down-regulate the levels of Fas protein and up-regulate the levels of Bcl2 protein, which may inhibit ALI by inducing apoptosis, and may protect lung function.
Effects of combined early fluid resuscitation and inhalation of 2% hydrogen on small intestine in a LPS-induced septic shock rat model
Clinical observations and animal experiments have shown that the gastrointestinal tract is one of the most important target organs during septic shock. In early shock, blood flow in the intestinal wall is significantly decreased because of the redistribution of blood, and intestinal mucosal barrier dysfunction is induced by hypoxia and ischemia. Previous studies show that intestinal mucosal edema and bacterial translocation are closely related to the occurrence of MODS[22]. In the present study, bowel mucosal structural changes were observed in the septic shock rats, including significant damage of the small intestine, severe edema of mucosal villi, sloughing off of epithelial cells, structural changes in the epithelium of the small intestine and even small bowel ulceration. Serum DAO activity, which was used as a marker of intestinal integrity, was significantly increased[23]. All these indicators suggest that the intestinal mucosal mechanical barrier is severely damaged during septic shock. Early fluid resuscitation treatment alone was not effective in reducing pathological changes of the small intestine; on the contrary, pathological changes were more severe, including more severe bowel wall edema, higher Chiu’s score, and higher level of serum DAO activity in the early fluid resuscitation-treated group compared with the septic shock group. But 2% hydrogen gas treatment significantly reduced the pathological changes of the small intestine, indicating that early fluid resuscitation and inhalation of 2% hydrogen could protect the intestinal mucosal mechanical barrier, inhibit bacterial translocation, and then effectively prevent enterogenous sepsis, and protect the function of other organs in the body[24,25].
Increased endothelial cell ischemia and hypoxia were observed during septic shock. The endothelial cells could release large amounts of cytokine and chemokine, and induce large amounts of neutrophil aggregation. During the removal of harmful pathogens, large amounts of oxygen free radical, which increase the intestinal damage, were released[26]. After fluid resuscitation treatment, large amounts of oxygen free radical were generated in the course of ischemia and reperfusion injury. Thus, oxygen free radicals were considered as one of the major causes of intestinal damage during septic shock. Our data indicate that oxygen free radicals were involved in the severe intestinal mucosal damage, and pathological changes were more severe after fluid resuscitation treatment. Early fluid resuscitation and inhalation of 2% hydrogen could significantly decrease the level of MDA and MPO, and mitigate the oxidative damage, indicating that 2% hydrogen may selectively reduce •OH, reducing ischemia/reperfusion-induced organ damage.
Intestinal mucosal injury can lead to the release of inflammatory mediators which play an important role in the process of shock-induced intestinal tissue injury[27]. The inflammatory mediators were one of the initiation factors of MODS, and could worsen organ dysfunction[28]. During the shock period, the intestinal tract is the main organ to produce TNF-α[29]. Release of TNF-α increases chemotaxis, aggregation and adhesion to endothelial cells of neutrophils. Then more inflammatory mediators such as IL-6 and IL-8 are also released, leading to aggravated intestinal mucosal injury[30]. In the present study, the levels of TNF-α, IL-6 and IL-8 in serum were significantly increased in septic shock rats, but the levels of TNF-α, IL-6 and IL-8 in serum were decreased after early fluid resuscitation treatment, while early fluid resuscitation and inhalation of 2% hydrogen gas further decreased the levels of TNF-α, IL-6 and IL-8 in serum compared with early fluid resuscitation only. These data demonstrated that the association of early fluid resuscitation and inhalation of 2% hydrogen gas may protect small intestine function by reducing inflammatory mediators.
In summary, combined early fluid resuscitation and inhalation of 2% hydrogen is beneficial for septic shock and septic shock-associated lung or small intestine injury, which is associated with a decrease of oxidative stress and inhibition of the inflammatory response. We conclude that combined early fluid resuscitation and inhalation of 2% hydrogen may be an effective therapeutic strategy for ALI and MODS in patients with septic shock.
COMMENTS
Background
Sepsis shock is a common complication of infection, severe trauma, and major operation in patients. However, the effective therapy method needs to be developed.
Research frontiers
Early fluid resuscitation has been demonstrated to have some protective effects on the sepsis shock -induced acute lung injury, but the effective is not significant. Recent studies indicate that hydrogen inhalation can significantly decrease the oxidative stress in septic animal model. The objective of this study is to explore the effects of combined early fluid resuscitation and hydrogen inhalation on septic shock induced lung and intestine injuries.
Innovations and breakthroughs
This is the first study to report that combined early fluid resuscitation and hydrogen inhalation exerts a potency to effectively attenuate lung and intestine injuries.
Applications
The paper provides the experiment basis for the clinical application of early fluid resuscitation and hydrogen inhalation to prevent sepsis induced lung and intestine injury.
Terminology
Sepsis shock is a common complication of infection, severe trauma, and major operation in patients. Sepsis shock leads to severe, hypoxia, and reperfusion injury of lung and intestine ischemia. The inflammatory factors in these tissues will be activated and may consequently develop the multiple organ dysfunction syndrome, which is associated with high morbidity and mortality rates. Early fluid resuscitation and hydrogen have the potential to clear these inflammatory factors.
Peer review
This study shows that combined early fluid resuscitation and hydrogen inhalation can significantly decrease septic shock induced lung and intestine injuries compared with early fluid resuscitation alone. The study is innovative and with potential therapeutic interest.
Footnotes
Supported by National Natural Science Foundation of China, No. 81101415
P- Reviewer Schmidt R S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, Wang G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34:90–97. doi: 10.1097/SHK.0b013e3181cdc4ae. [DOI] [PubMed] [Google Scholar]
- 2.Biswal S, Remick DG. Sepsis: redox mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2007;9:1959–1961. doi: 10.1089/ars.2007.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velasco N. [Gut barrier in the critically ill patient: facts and trends] Rev Med Chil. 2006;134:1033–1039. doi: 10.4067/s0034-98872006000800014. [DOI] [PubMed] [Google Scholar]
- 4.Brandt S, Regueira T, Bracht H, Porta F, Djafarzadeh S, Takala J, Gorrasi J, Borotto E, Krejci V, Hiltebrand LB, et al. Effect of fluid resuscitation on mortality and organ function in experimental sepsis models. Crit Care. 2009;13:R186. doi: 10.1186/cc8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 7.Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta. 2012;1820:586–594. doi: 10.1016/j.bbagen.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong L, Hou L, Wang G. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock. 2010;34:495–501. doi: 10.1097/SHK.0b013e3181def9aa. [DOI] [PubMed] [Google Scholar]
- 9.Xie K, Yu Y, Huang Y, Zheng L, Li J, Chen H, Han H, Hou L, Gong G, Wang G. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37:548–555. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- 10.Sennoun N, Montemont C, Gibot S, Lacolley P, Levy B. Comparative effects of early versus delayed use of norepinephrine in resuscitated endotoxic shock. Crit Care Med. 2007;35:1736–1740. doi: 10.1097/01.CCM.0000269028.28521.08. [DOI] [PubMed] [Google Scholar]
- 11.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 12.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An KW, Shin HS, Choi CY. Physiological responses and expression of metallothionein (MT) and superoxide dismutase (SOD) mRNAs in olive flounder, Paralichthys olivaceus exposed to benzo[a]pyrene. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:534–539. doi: 10.1016/j.cbpb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 14.McFarland VA, Inouye LS, Lutz CH, Jarvis AS, Clarke JU, McCant DD. Biomarkers of oxidative stress and genotoxicity in livers of field-collected brown bullhead, Ameiurus nebulosus. Arch Environ Contam Toxicol. 1999;37:236–241. doi: 10.1007/s002449900510. [DOI] [PubMed] [Google Scholar]
- 15.Oldham KM, Bowen PE. Oxidative stress in critical care: is antioxidant supplementation beneficial? J Am Diet Assoc. 1998;98:1001–1008. doi: 10.1016/S0002-8223(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 17.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Zhang L, Jin Z, Jin E, Fujiwara M, Ghazizadeh M, Asoh S, Ohta S, Kawanami O. Anti-apoptotic PTD-FNK protein suppresses lipopolysaccharide-induced acute lung injury in rats. Exp Mol Pathol. 2007;83:377–384. doi: 10.1016/j.yexmp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Lee JS, Liles WC, Frevert CW, Mongovin S, Wong V, Ballman K, Sutlief S, Martin TR. Fas-mediated acute lung injury requires Fas expression on nonmyeloid cells of the lung. J Immunol. 2005;175:4069–4075. doi: 10.4049/jimmunol.175.6.4069. [DOI] [PubMed] [Google Scholar]
- 21.O’Reilly MA, Staversky RJ, Huyck HL, Watkins RH, LoMonaco MB, D’Angio CT, Baggs RB, Maniscalco WM, Pryhuber GS. Bcl-2 family gene expression during severe hyperoxia induced lung injury. Lab Invest. 2000;80:1845–1854. doi: 10.1038/labinvest.3780195. [DOI] [PubMed] [Google Scholar]
- 22.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–1106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 23.Luk GD, Bayless TM, Baylin SB. Plasma postheparin diamine oxidase. Sensitive provocative test for quantitating length of acute intestinal mucosal injury in the rat. J Clin Invest. 1983;71:1308–1315. doi: 10.1172/JCI110881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Rustum RS, Abu-Rustum SE, Abdo BK, Jamal MH. Inner myometrial laceration causing a massive postpartum hemorrhage: a case report. J Reprod Med. 2006;51:135–137. [PubMed] [Google Scholar]
- 25.Magann EF, Evans S, Chauhan SP, Lanneau G, Fisk AD, Morrison JC. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290–293. doi: 10.1097/01.AOG.0000151993.83276.70. [DOI] [PubMed] [Google Scholar]
- 26.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1993;218:444–453; discussion 453-454. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamion F, Richard V, Lacoume Y, Thuillez C. Intestinal preconditioning prevents systemic inflammatory response in hemorrhagic shock. Role of HO-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G408–G414. doi: 10.1152/ajpgi.00348.2001. [DOI] [PubMed] [Google Scholar]
- 28.Sobhian B, Jafarmadar M, Redl H, Bahrami S. Hemorrhage- and resuscitation-related alterations in gastrointestinal circulation: effect of a low dose of L-NMMA. Shock. 2005;23:243–247. [PubMed] [Google Scholar]
- 29.Tani T, Fujino M, Hanasawa K, Shimizu T, Endo Y, Kodama M. Bacterial translocation and tumor necrosis factor-alpha gene expression in experimental hemorrhagic shock. Crit Care Med. 2000;28:3705–3709. doi: 10.1097/00003246-200011000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Weinbroum AA, Hochhauser E, Rudick V, Kluger Y, Karchevsky E, Graf E, Vidne BA. Multiple organ dysfunction after remote circulatory arrest: common pathway of radical oxygen species? J Trauma. 1999;47:691–698. doi: 10.1097/00005373-199910000-00013. [DOI] [PubMed] [Google Scholar]


