Abstract
AIM: To examine how the expression of caudal type homebox transcription factor 2 (Cdx2) is regulated in the development of malignancy in Barrett’s esophagus.
METHODS: Cdx2, mucin (MUC) series (MUC2, MUC5AC and MUC6), p53 and E-cadherin expression in Barrett’s esophagus and adenocarcinoma specimens were examined by immunostaining. Isolated clusters of cells from (1) MUC2 and Cdx2-positive intestinal metaplastic mucosa; (2) MUC5AC and MUC6-positive, and MUC2 and Cdx2-negative high-grade dysplasia (HD), or intramucosal adenocarcinoma (IMC); and (3) MUC5AC, MUC6 and Cdx2-positive poorly-differentiated invasive adenocarcinoma (PDA) were analyzed by methylation-specific polymerase chain reaction using sets of primers for detecting methylation status of the Cdx2 gene.
RESULTS: Most of the non-neoplastic Barrett’s esophageal mucosa showing intestinal-type metaplasia with or without low-grade dysplasia was positive for E-cadherin, MUC series and Cdx2, but negative for p53. A portion of the low-grade to HD was positive for E-cadherin, MUC5AC, MUC6 and p53, but negative for MUC2 and Cdx2. The definite IMC area was strongly positive for MUC5AC, MUC6 and p53, but negative for MUC2 and Cdx2. Methylation of the Cdx2 promoter was not observed in intestinal metaplasia, while hypermethylation of part of its promoter was observed in hot dipped and IMC. Hypermethylation of a large fraction of the Cdx2 promoter was observed in PDA.
CONCLUSION: Cdx2 expression is restored irrespective of the methylation status of its promoter. Apparent positive immunohistochemical results can be a molecular mark for gene silencing memory.
Keywords: Barrett¡’s esophagus, Caudal type homebox transcription factor 2, Intestinal metaplasia, Promoter hypermethylation
INTRODUCTION
Barrett’s esophagus, first described in 1950 and refined in 1957, is a condition whereby the distal esophageal squamous epithelium is replaced by metaplastic columnar epithelium[1]. Three types of morphologically distinct metaplastic columnar epithelia are recognized in Barrett’s esophagus: gastric-fundic, gastric-cardiac (junctional type), and intestinal (specialized type) metaplasia[2]. Reflecting a finding that patients with intestinal-type epithelium are at increased risk of developing adenocarcinoma, the American College of Gastroenterology has recently proposed a restricted definition of Barrett’s esophagus: “a change in the esophageal epithelium of any length that can be recognized at endoscopy and is confirmed to have intestinal metaplasia at biopsy”[3]. Although a recent cohort study has demonstrated that the frequency of cancer development in Barrett’s esophagus is not related to the presence of intestinal metaplasia, metaplastic columnar epithelium, per se, is generally accepted as a precancerous process predisposed to develop discrete neoplastic lesions such as the gastric or foveolar type, the adenomatous or intestinal type, hybrid type dysplasia, and intramucosal [high-grade dysplasia (HD) or intramucosal adenocarcinoma (IMC)] and invasive cancers[4]. Cancers derived from Barrett’s esophagus are histopathologically classified into two major categories: gastric and intestinal[5]. Since most Barrett-related IMC cases are either gastric or intestinal with distinct phenotypic stability during progression, two separate (gastric and intestinal) pathways of carcinogenesis have been proposed[5]. Importantly, during the progression of the intestinal pathway, a gradual decrease in transcription factor caudal type homebox transcription factor 2 (Cdx2, a caudal-related homeobox gene essential for skeletal and intestinal development has been noted, suggesting its tumor suppressor role in Barrett’s esophagus[5].
We encountered a case of invasive esophageal adenocarcinoma developing into intestinal-type dysplasia and IMC, and examined Cdx2 expression and its promoter methylation status in close histopatho- logical relation to the progression stages with the use of microdissection and methylation-specific polymerase chain reaction (MSP).
MATERIALS AND METHODS
Patient
An 81-year-old Japanese man was admitted to our hospital complaining of heartburn especially after eating sweet fare. The patient had undergone stomach surgery (distal partial gastrectomy) due to gastric ulcer nearly forty years earlier. Because of gastric regurgitation, he had undergone endoscopic examination of the upper digestive tract, which revealed severe reflux esophagitis with widespread Barrett’s esophagus. A biopsy was taken from irregularly elevated lesions inside the Barrett’s esophagus, and a histological examination confirmed esophageal adenocarcinoma in the lesions. An esophagectomy was carried out, and the right hemicolon was rebuilt. The patient has been free of recurrence for two years since the operation.
Immunohistochemistry
The specimens of Barrett’s esophagus were subjected to immunohistochemistry using diaminobenzidine as the chromogen. Deparaffinized sections of formalin-fixed tissue were stained with mucin (MUC) series (Novocastra Laboratories Ltd., Newcastle upon Tyne, United Kingdom), p53 (Lab Vision, Kalamazoo, United States) and E-cadherin (Dako, Denmark) antibody diluted at 1:100 after heat-induced antigen retrieval and with Cdx2 (Dako, Denmark) antibody diluted at 1:50. Anti-rabbit immunoglobulin G (IgG) was used as the secondary antibody for p53 and anti-mouse IgG was used for MUC series, Cdx2 and E-cadherin.
Agarose-bead mediated template preparation
Paraffin-embedded samples were deparaffinized in xylene and subjected to microdissection under light microscopic observation (Leica Microsystems, LMD7000) with the aid of both E-cadherin immunostaining and Cdx2 immunostaining. The microdissected samples were liquefied in low-melting agarose (3.2%) at 1:1, and agarose beads were made by chilling on ice. Beads were treated with proteinase K, followed by bisulfite conversion, as previously described[6].
Polymerase chain reaction amplification and sequencing
Bead fragments were analyzed by MSP using sets of primers for accessing the methylation status of the Cdx2 gene. The promoter region of the human Cdx2 genomic sequence (GenBank accession no. AL591024) was searched for CpG islands with an online search engine (www.ebi.ac.uk/emboss/cpgplot). One of the CpG islands (AL591024 nt 28391-28683) was further analyzed for methylation status by MSP. In the first-step polymerase chain reaction (PCR) amplification, a 183-bp amplicon containing 71-bp CpG sites, was amplified with two primers, (forward) 5’-GCCAAGGGGCCTAGGGCTGGA-3’, and (reverse) 5’-GTTCACCTCCTAATACAAGCCTTTG-3’ (Table 1), under the following conditions: 98˚C 2 min, 30 cycles (98 °C 10 s, 50 °C 15 s, 68 °C 39 s). The primers used for second-step PCR were, (forward) 5’-GGAGCTGCCCCGACAGGAGCG-3’, and (reverse) 5’-CGCGCCCAGCTCGGn TTTCAGCAA-3’ (Table 1), under the following conditions: 98 °C 2 min, 25 cycles (98 °C 10 s, 60 °C 15 s, 68 °C 30 s). The PCR mixture contained Mighty AMP® DNA polymerase (Takara, Tokyo, Japan) and bead fragments in a final volume of 25 μL. The PCR products were electrophoresed in a 3% agarose gel, stained with ethidium bromide and visualized under ultraviolet light.
Table 1.
Primer sequences used in polymerase chain-based assays, product size and annealing temperature
| Primer sequence | Size (bp) | Temp (°C) | |
| First PCR | (F)5’-GTTAAGGGGTTTAGGGTTGGA | 183 | 60 |
| Nested PCR | (R)5’-CAAAAACTTATATTAAAAAATAAAC | ||
| Methylated | (F) 5’-GGAGTTGTTTCGATAGGAGCGC | 71 | 60 |
| (R) 5’-TTACTAAAACCGAACTAAACGCG | |||
| Unmethylated | (F) 5’-GGAGTTGTTTTGATAGGAGTGT | 71 | 60 |
| (R) 5’-TTACTAAAACCAAACTAAACACA |
Temp: Temperature; PCR: Polymerase chain reaction; R: Reverse; F: Forward.
Ethics
Written informed consent was obtained from this patient, and this study was reviewed and approved by the local ethics committee at Ehime University.
RESULTS
Pathological findings
Grossly, a superficial spreading IMC surrounded by low-grade dysplasia and intestinal-type metaplasia extended between 30 mm from the oral and 105 mm from the anal surgical margins (Figure 1). One elevated nodule was noted inside this superficial spreading region (Figure 1). Microscopically, the background non-neoplastic esophageal mucosa was replaced, very extensively, by gastric foveolar type mucosa with (Figure 1) and without intestinal metaplasia (Figure 1). The superficial spreading IMC region was mostly composed of definite well-differentiated tubular adenocarcinoma or HD, surrounded by dysplastic change (low-grade dysplasia). The oral elevated nodular ridge was a solid, poorly-differentiated, invasive adenocarcinoma with lymphatic invasion, but no venous invasion or metastasis within the esophageal mucosa was observed. Immunohistochemically, the original esophageal squamous epithelium was positive for E-cadherin, but negative for all the MUC series (MUC2, MUC5AC and MUC6), Cdx2 and p53. Most of the non-neoplastic Barrett’s esophageal mucosa showing intestinal-type metaplasia with or without low-grade dysplasia was positive for E-cadherin, MUC2, MUC5AC, MUC6 and Cdx2, but negative for p53. A portion of the low-grade to high-grade dysplasia was positive for E-cadherin, MUC5AC, MUC6 and p53, but negative for MUC2 and Cdx2. The definite IMC area was strongly positive for MUC5AC, MUC6 and p53, but negative for MUC2 and Cdx2. Figure 2 shows the transitional area between the intestinal metaplasia with low-grade dysplasia and the definite IMC area. A portion of the poorly-differentiated adenocarcinoma was positive for MUC5AC, MUC6, Cdx2 and p53, but negative for MUC2 (Figure 3). Table 2 shows a summary of the immunohistochemical findings.
Figure 1.
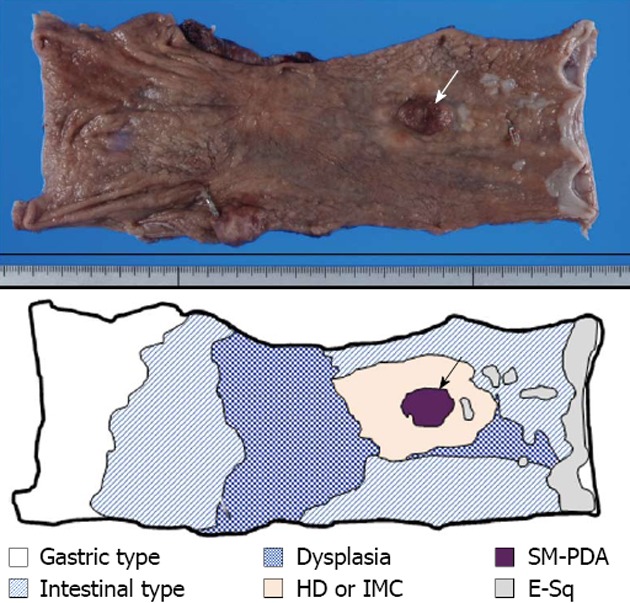
Macroscopic findings of excised esophagus. Surgical specimen shows the presence of superficial spreading carcinoma, extending between 30 mm from the oral and 105 mm from the anal surgical margins. The superficial spreading region is mostly composed of high-grade dysplasia (HD), or intramucosal adenocarcinoma (IMC), surrounded by dysplastic change (E-sq low-grade dysplasia). Inside this superficial spreading region, one observable elevated nodule (arrow) is composed of solid and submucosal invasive poorly-differentiated invasive adenocarcinoma (SM-PDA) with lymphatic invasion. The background non-neoplastic esophageal mucosa is extensively replaced by glandular mucosa with and without intestinal metaplasia.
Figure 2.
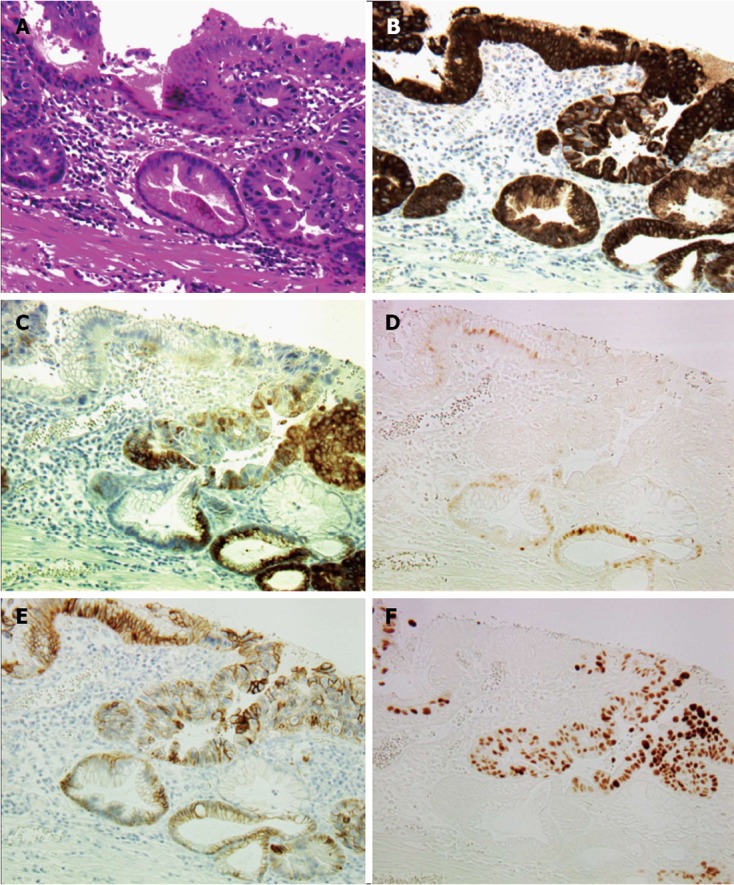
Histological findings of transitional area between intestinal metaplasia and high-grade dysplasia or intramucosal adenocarcinoma (× 200). A: Hematoxylin and eosin staining of transitional area. Intestinal metaplasia (IM) stretches from the upper left to the lower right corner; B: Mucin (MUC) 5AC immunostaining. Strong MUC5AC expression is observed in both IM and high-grade dysplasia (HD), or intramucosal adenocarcinoma (IMC) areas; C: MUC6 immunostaining. MUC6 expression is observed mostly in parts of the HD or IMC areas; D: Caudal type homebox transcription factor 2 (Cdx2) immunostaining. Cdx2 expression is observed only in the nuclei of the cells in the IM area; E: E-cad immunostaining. E-cad expression is observed on the membranes of cells in both IM and HD or IMC areas; F: p53 immunostaining. Strong p53 expression is observed in the nuclei of the cells in the HD or IMC area.
Figure 3.
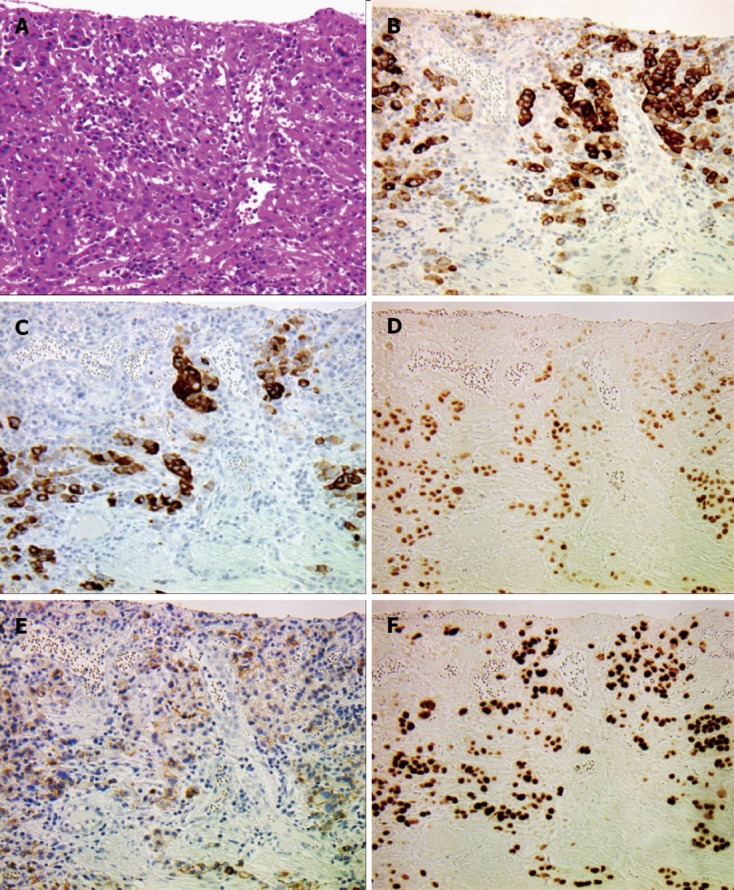
Histological findings of diffuse poorly-differentiated invasive adenocarcinoma (× 200). A: Hematoxylin and eosin staining of poorly-differentiated invasive adenocarcinoma (PDA). Cancer cells are diffusely scattered with prominent stromal reaction; B: Mucin (MUC) 5AC; C: MUC6; D: Caudal type homebox transcription factor 2 expression is positive in the PDA area; E: E-cad expression is markedly reduced in the PDA area; F: Strong p53 expression is observed in the nuclei of the cells in the PDA area.
Table 2.
Summary of immunohistochemical findings
| G-type | I-type (IM) | HD or IMC | PDA | |
| MUC2 | - | + | - | - |
| MUC5AC | + | - | ++ | + |
| MUC6 | + | - | ++ | + |
| p53 | - | - | ++ | ++ |
| E-cadherin | ++ | ++ | ++ | + |
| Cdx2 | - | + | - | + |
G-type: Gastric metaplasia; IM: Intestinal metaplasia; HD or IMC: High-grade dysplasia or intramucosal adenocarcinoma; PDA: Poorly-differentiated invasive adenocarcinoma; MUC: Mucin.
Microdissection and MSP of the Cdx2 promoter
MSP revealed no methylation in Cdx2-positive Barrett’s mucosa with intestinal metaplasia (Figure 4, intestinal type). Microdissected samples from the Cdx2-negative IMC area showed that a fraction of the cells was hypermethylated (Figure 4, IMC). Although Cdx2 expression was found by immunohistochemical analysis, samples from the poorly-differentiated invasive (PDA) area showed a hypermethylation pattern (Figure 4, submucosal invasive PDA).
Figure 4.
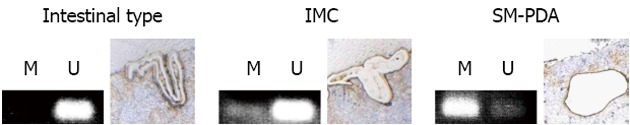
Detection of methylated cytosine by methylation-specific polymerase chain reaction analysis of caudal type homebox transcription factor 2 CpG-island region. Tissue samples were stained with E-cadherin and caudal type homebox transcription factor 2 (Cdx2) to assist cell identification. Cells, either isolated from intestinal metaplasia, intramucosal adenocarcinoma (IMC) or submucosal invasive poorly-differentiated adenocarcinoma (SM-PDA) by laser-assisted microdissection, were subjected to bisulfite conversion and subsequent methylation-specific polymerase chain reaction (MSP). MSP products using primers that specifically amplify only unmethylated DNA are indicated by visible polymerase chain reaction products in line unmethylated pattern (U), while visible polymerase chain reaction products in line methylated pattern (M) indicate those amplified by primers specific for methylated DNA. In intestinal-type metaplasia sections, MSP shows a U pattern, while MSP shows U patterns with partial M patterns in high-grade dysplasia or IMC sections. MSP shows a mostly M pattern in PDA where strong Cdx2 expression is observed (Figure 3D).
DISCUSSION
Cdx2 is an intestine-specific transcription factor expressed in cells constituting the mucosal epithelium from the duodenum to the rectum[7]. While Cdx2 is negative for the normal foveolar mucosa of the stomach and the squamous epithelium in the esophagus stemming from the foregut, its heterotopic expression in Barrett’s esophagus is observed especially in cases with intestinal-type metaplasia[8]. Among most of the terminal differentiation-specific transcription factors, Cdx2 is known to play a tumor suppressor role in cancer progression in the distal colon, a role, which in adults, is functionally and geographically distinct from the homeotic role of Cdx2 during gut development[9]. In our present case, and in confirming its tumor suppressor role, Cdx2 expression diminished during the progression from intestinal-type metaplasia to distinct IMC. Mirroring Cdx2 expression at the protein level by immunohistochemistry, the hypermethylation of the Cdx2 gene promoter was revealed (Figure 4, IMC). Since primers used for MSP are set to amplify the Cdx2 gene promoter with hypermethylation, i.e., when all CpGs are methylated, a large fraction of the cells may acquire partial or scatter-type CpG methylation and, therefore, the Cdx2 gene promoter may have been underestimated in our MSP. In support of our current study, Khor et al[5] also demonstrated the gradual downregulation of Cdx2 expression during progression in adenomatous dysplasia, at least in the intestinal pathway of the Barrett esophageal cancers. These data suggest that Cdx2 also plays a tumor-suppressor role in the metaplasia-dysplasia-carcinoma sequence in Barrett’s esophagus. In our present case, irrespective of the hypermethylation status of the Cdx2 gene promoter, Cdx2 expression was restored in PDA as analyzed by immunohistochemistry (Figure 3F). To achieve final gene-silencing, chromatin condensation followed by modifications of histone proteins are essential[10], we therefore hypothesize that epigenetic alterations other than demethylation may lead to Cdx2 gene reactivation during the progression phase. Indeed, our previous study showed that hypermethylation of the E-cadherin gene promoter and MeCP2, a methyl-CpG binding domain protein, synergistically silenced gene expression in colorectal cancers[6]. Therefore, it is evident that hypermethylation of the gene promoter, per se, is essential for establishing gene silencing, but not sufficient for blocking gene expression. Since in our present case, Cdx2 reactivation did not correlate with differentiated intestinal phenotype, but was observed in invasive or aggressive phenotypes, the tumor suppressive effect of Cdx2 on these invasive cancer cells might be lost. These somewhat complicated epigenetical events may partly explain the dispersion of Cdx2 expression. Therefore, when characterizing cancer cells by immunophenotyping, any apparent positive immunohistochemical results should be interpreted carefully with the help of the hypermethylation status as a molecular mark for gene silencing memory[10,11].
ACKNOWLEDGMENTS
We thank Ms. Yuki Takaoka for technical assistance.
COMMENTS
Background
Barrett’s esophagus, a pathological condition in which the esophageal squamous epithelium is replaced by metaplastic columnar mucosa, is known to predispose to the development of dysplasia and subsequent cancers.
Research frontiers
Caudal type homebox transcription factor 2 (Cdx2) has recently been shown to play a tumor-suppressor role in the ‘metaplasia-dysplasia-carcinoma sequence’ in Barrett’s esophagus.
Innovations and breakthroughs
Recent reports have evaluated the phenotypic stability and role of Cdx2 in the neoplastic progression of different types of dysplasias. This suggests that nonintestinalized columnar metaplasia may be an unstable intermediate state at risk for neoplastic progression.
Applications
When characterizing cancer cells by immunophenotyping, any apparent positive immunohistochemical results should be interpreted carefully with the help of the hypermethylation status as a molecular mark for gene silencing memory.
Peer review
The authors examined the expression of Cdx2 and its methylation in Barrett metaplasia and esophageal adenocarcinoma. It revealed that irrespective of the hypermethylation status of the Cdx2 gene promoter, Cdx2 expression was restored in poorly-differentiated invasive adenocarcinoma. The results are interesting and when characterizing cancer cells by immunophenotyping, any apparent positive immunohistochemical result should be interpreted carefully.
Footnotes
Supported by Grant-in Aid from Ministry of Education, Sports and Culture (GP Program for Basic Science), Japan
P- Reviewers Pei ZH, Smith MDC S- Editor Gou SX L- Editor Webster JR E- Editor Xiong L
References
- 1.Bani-Hani KE, Bani-Hani BK. Columnar-lined esophagus: time to drop the eponym of “Barrett”: Historical review. J Gastroenterol Hepatol. 2008;23:707–715. doi: 10.1111/j.1440-1746.2008.05386.x. [DOI] [PubMed] [Google Scholar]
- 2.Phillips RW, Frierson HF, Moskaluk CA. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol. 2003;27:1442–1447. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery E. Refining diagnostic criteria for high-grade dysplasia in Barrett esophagus. Am J Clin Pathol. 2009;132:7–9. doi: 10.1309/AJCPPCJC71IFRVVG. [DOI] [PubMed] [Google Scholar]
- 4.Kelty CJ, Gough MD, Van Wyk Q, Stephenson TJ, Ackroyd R. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;42:1271–1274. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 5.Khor TS, Alfaro EE, Ooi EM, Li Y, Srivastava A, Fujita H, Park Y, Kumarasinghe MP, Lauwers GY. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett Esophagus? Am J Surg Pathol. 2012;36:331–342. doi: 10.1097/PAS.0b013e31823d08d6. [DOI] [PubMed] [Google Scholar]
- 6.Darwanto A, Kitazawa R, Maeda S, Kitazawa S. MeCP2 and promoter methylation cooperatively regulate E-cadherin gene expression in colorectal carcinoma. Cancer Sci. 2003;94:442–447. doi: 10.1111/j.1349-7006.2003.tb01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Jaffee IM, Rahmani M, Singhal MG, Younes M. Expression of the intestinal transcription factor Heaton in carcinoid tumors is a marker of midgut origin. Arch Pathol Lab Med. 2006;130:1522–1526. doi: 10.5858/2006-130-1522-EOTITF. [DOI] [PubMed] [Google Scholar]
- 9.Bonhomme C, Duluc I, Martin E, Chawengsaksophak K, Chenard MP, Kedinger M, Beck F, Freund JN, Domon-Dell C. The Cdx2 homeobox gene has a tumour suppressor function in the distal colon in addition to a homeotic role during gut development. Gut. 2003;52:1465–1471. doi: 10.1136/gut.52.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raynal NJ, Si J, Taby RF, Gharibyan V, Ahmed S, Jelinek J, Estécio MR, Issa JP. DNA methylation does not stably lock gene expression but instead serves as a molecular mark for gene silencing memory. Cancer Res. 2012;72:1170–1181. doi: 10.1158/0008-5472.CAN-11-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–496. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]


