Abstract
The genetic manipulation of skeletal muscle cells in vitro is notoriously difficult, especially when using undifferentiated muscle cell lines (myoblasts) or primary muscle stem cells (myosatellites). We therefore optimized methods of gene transfer by overexpressing green fluorescent protein (GFP) in mouse C2C12 cells and in a novel system, primary rainbow trout myosatellite cells. A common lipid-based transfection reagent was used (Lipofectamine 2000) along with three different viral vectors: adeno-associated virus serotype 2 (AAV2), baculovirus (BAC) and lentivirus. Maximal transfection efficiencies of 49% were obtained in C2C12 cells after optimizing cell density and reagent:DNA ratio, although GFP signal rapidly dissipated with proliferation and was not maintained with differentiation. The transduction efficiency of AAV2 was optimized to 65% by extending incubation time and decreasing cell density, although only 30% of cells retained expression after passing. A viral comparison revealed that lentivirus was most efficient at transducing C2C12 myoblasts as 97% of cells were transduced with only 106 viral genomes (vg) compared to 54% with 108 vg AAV2 and 23% with 109 vg BAC. Lentivirus also transduced 90% of primary trout myosatellites compared to 1–10% with AAV2 and BAC. The phosphoglycerate kinase 1 promoter was 10-fold more active than the cytomegalovirus immediate-early promoter in C2C12 cells and both were effective in trout myosatellites. Maximal transduction of C2C12 myotubes was achieved by differentiating myoblasts previously transduced with lentivirus and the pgk promoter. Thus, our optimized protocol proved highly effective in diverse muscle cell systems and could therefore help overcome a common technological barrier.
Keywords: muscle, myoblast, myosatellite, transfection, transduction
Introduction
Gene therapy is a promising approach for treating many disorders of skeletal muscle. In fact, many preclinical approaches for treating muscular dystrophies, sarcopenia and cancer cachexia, to name a few, have already been developed [1], some of which are currently being used in clinical trials [2]. Despite its potential, the use of viral vectors in gene therapeutics presents several challenges related to immunogenicity, inflammation, DNA size restriction, and variability or efficiency of transgene expression [3–5]. A better understanding of the underlying molecular mechanisms responsible for these phenomena is arguably critical to the field and may reveal novel approaches with direct clinical implications. It would also be aided by studies using myogenic cell lines or even primary muscle stem cells (a.k.a. myosatellite cells), which itself presents challenges unique to the cell type.
Genetically manipulating muscle cells, especially proliferating myoblasts, is notoriously difficult. Although myogenic cultures are generally amenable to transfection, previous studies report poor expression efficiencies when using non-viral approaches including cationic lipid reagents or calcium phosphate precipitation [6, 7]. Indeed, these methods regularly produce very low transfection efficiencies (<10%) when not optimized and rarely exceed 50% [8–10]. Moreover, differentiation status of the cells as well as cell culture conditions, such as the presence or absence of serum and matrix coating of culture plates, impact transfection efficiency [9, 11]. Culture conditions are well known to influence phenotypic cellular behaviors including proliferation and differentiation, adding a level of complexity to the system that could potentially compromise the quantification of cellular responses. The use of viral vectors could presumably address many of these issues as different vectors, serotypes and gene promoters have been demonstrated to efficiently transduce skeletal muscle in vivo [12]. Their efficiency in vitro, however, is less well known and to date has not been optimized. Furthermore, viral transduction is mediated by interactions between viral components and host proteins including receptors and other proteins that influence infection efficiency [13, 14] whose presence may vary between cell types (e.g. different cell lines or primary cells) and possibly with differentiation status. Studies are therefore needed to identify and optimize viral-based approaches for transducing muscle cells in vitro.
To investigate the feasibility of achieving high transgene expression in cultured muscle cells, we compared a common lipid-based transfection system with similarly common viral gene transfer methods, optimizing techniques for best expression efficiency with each delivery system. Our study characterizes different approaches for genetically manipulating C2C12 mouse myoblasts as well as an emerging primary system, rainbow trout myosatellite cells [15, 16]. Our novel results suggest that the combined use of lentivirus with a phosphoglycerate kinase 1 (pgk) promoter is far superior to using Lipofectamine 2000, adeno-associated virus serotype 2 (AAV2), baculovirus or the cytomegalovirus immediate-early (cmv) promoter. This combination transduces proliferating as well as differentiated cells with very high efficiency. We additionally demonstrate, also for the first time, lentiviral transduction of primary rainbow trout myosatellite cells and that maximal transduction of C2C12 myotubes can best be achieved by infecting myoblasts before inducing differentiation. The methods and systems described are highly useful for investigating molecular signaling in muscle cells, for overcoming current impediments to gene therapy and for genetically manipulating an emerging biomedical and comparative model system.
Results
Non-viral transfection
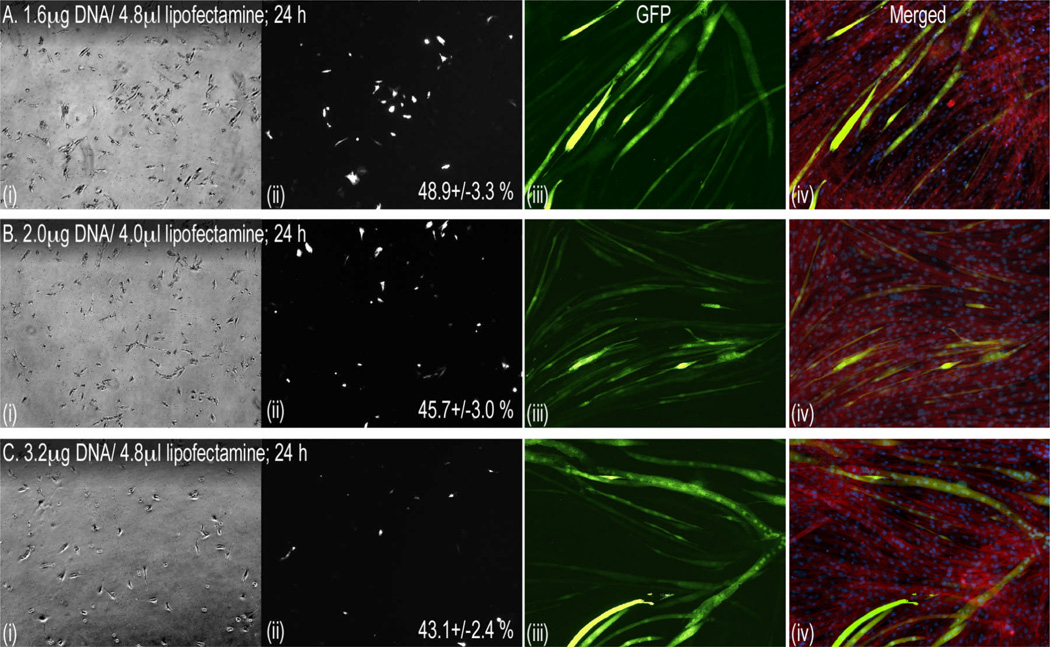
Myoblast transfection efficiency typically varies between 10 and 30% [7, 9, 11]. We therefore used a popular and commercially available transfection reagent, Lipofectamine 2000, and optimized conditions for maximal transfection efficiency in C2C12 myoblasts by altering cell confluency and the Lipofectamine:DNA ratio. A cell confluency of 40% proved optimal as densities above or below this produced cell death or lower transfection efficiencies (data not shown). We then varied the plasmid Lipofectamine:DNA ratio, using 40% confluent cells, and achieved the highest transfection efficiency of 48.9 +/− 3.3% EGFP-expressing cells at a ratio of 1:3 (Fig. 1). In fact, higher ratios of 1:2 and 1:1.5 had slightly lower (non-significant) transfection efficiencies of 45.7 +/− 3.0% and 43.1 +/− 2.4%, respectively (Fig. 1). Despite the high initial transfection efficiency, however, GFP expression decreased with proliferation resulting in <10% of cells retaining expression after differentiation.
Figure 1. Optimizing transfection of C2C12 myoblasts.
Subconfluent cells were passed 24 h before transfecting with the indicated DNA/Lipofectamine ratios using the manufacturer’s protocol. Ratios above and below those indicated were either toxic or were significantly less efficient (see Results). Myoblasts were imaged 24 h (40×) after transfection, grown to confluency for 4 additional days and differentiated with serum-withdrawal (iii & iv). Shown in each panel set (A–C) are phase-contrast (i) and fluorescent images of corresponding myoblasts (ii), differentiated myotubes (iii, GFP, 100×) and merged images (iv) of GFP, DAPI-stained nuclei and sarcomeric actin labeled with phalloidin red. Transfection efficiency (mean % +/− SEM; no significant differences) is indicated for each treatment and was determined by counting fluorescent and total cell numbers in 9 random views from each well/treatment.
Optimization of AAV2 in myoblasts
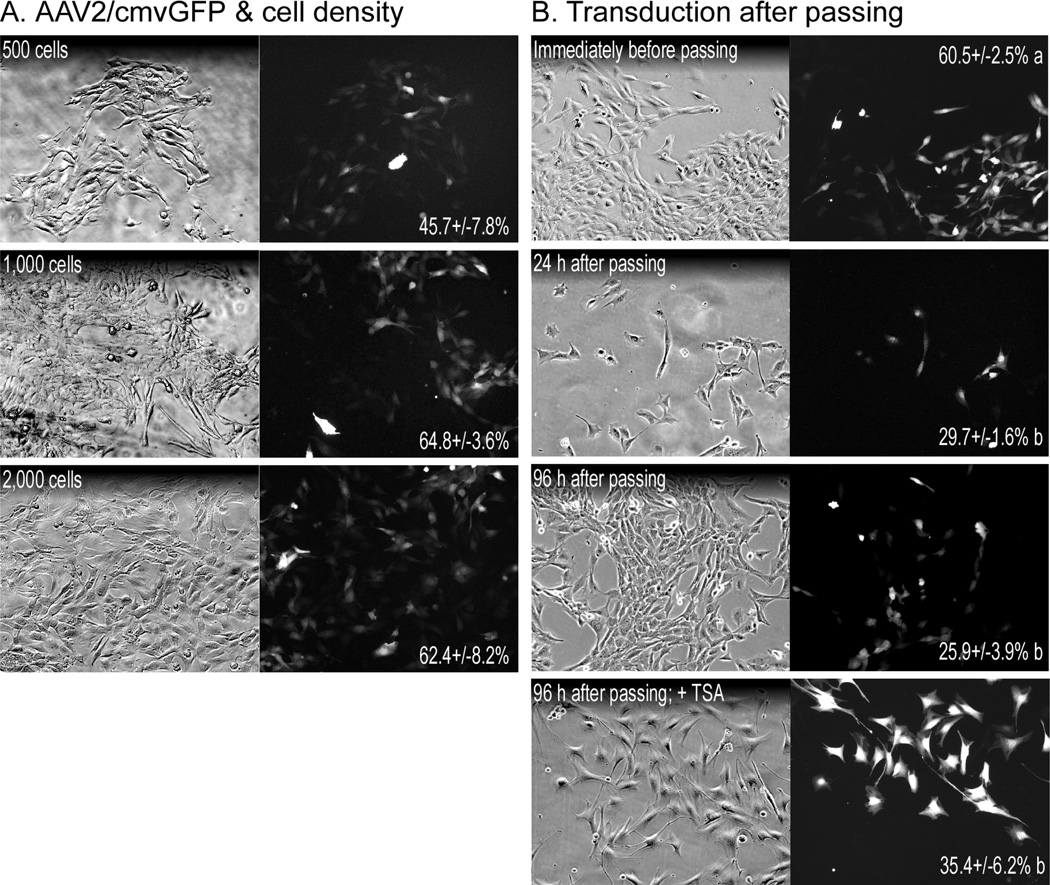
Preliminary tests with high cell densities produced low transduction efficiencies (~10% or lower with 30–50% confluency, data not shown). We therefore tested very low cell densities, 130, 260 and 520 cells/cm2, as this prolonged the time before 100% cell confluency is reached and in turn viral incubation times (Fig. 2A). The highest transduction efficiency of 64.8 +/− 3.6% was achieved at an initial cell density of 260 cells/cm2 (1000 cells) compared to 45.7 +/− 7.8% at 130 cells/cm2 and 62.4 +/− 8.2% at 520 cells/cm2, indicating that an initial plating density of 260–520 cells/cm2 is optimal. Because cells plated at optimal densities were nearly confluent when maximal transduction efficiency was reached, we then determined whether transduced myoblasts could be passed while retaining the GFP signal. Cells were plated at a density of 260 cells/cm2, incubated for five days with virus and passed into virus-free media before GFP transduction efficiency was analyzed at various time points (Fig. 2B). The lower of the optimal densities was used as it was less likely to reach confluency during the incubation time. Transduction efficiency was 29.7 +/− 1.6% and 25.9 +/− 3.9% in cells cultured 24 h and 96 h after passing, respectively, compared to the initial 60.5 +/− 2.5%. This loss in GFP expression is similar to the low expression in transfected myoblasts and prevents the use of AAV2 in most experiments where physiological endpoints (e.g. proliferation, differentiation, protein synthesis, etc.) are needed.
Figure 2. Adeno-associated virus (AAV) transduction of C2C12 myoblasts.
(A) Cells were plated at low densities, 500, 1000 and 2000 cells/well (~130, 260 & 520 cells/cm2, respectively), and incubated for 5 days with AAV2/cmvGFP (108 vg/well). Transduction efficiency (mean % +/− SEM; no significant differences) is indicated for each treatment and was determined by counting fluorescent and total cell numbers in 9 random views from each well/treatment. (B) The 1000 cell group was then diluted 5-fold, passed into new wells and subsequently cultured for 96 h in virus-free medium. After 72 h, some cells were treated with 1 µM TSA for an additional 12 h before imaging at 100×. Transduction efficiency (mean % +/− SEM; different letters = significant difference, same letters = no difference; A & B analyzed separately; p≤0.05) is indicated for each treatment and was determined by counting fluorescent and total cell numbers in 9 random views from each well/treatment.
Trichostatin A (TSA) effects on AAV2 & baculovirus (BAC) transduction in undifferentiated and differentiated C2C12 cells
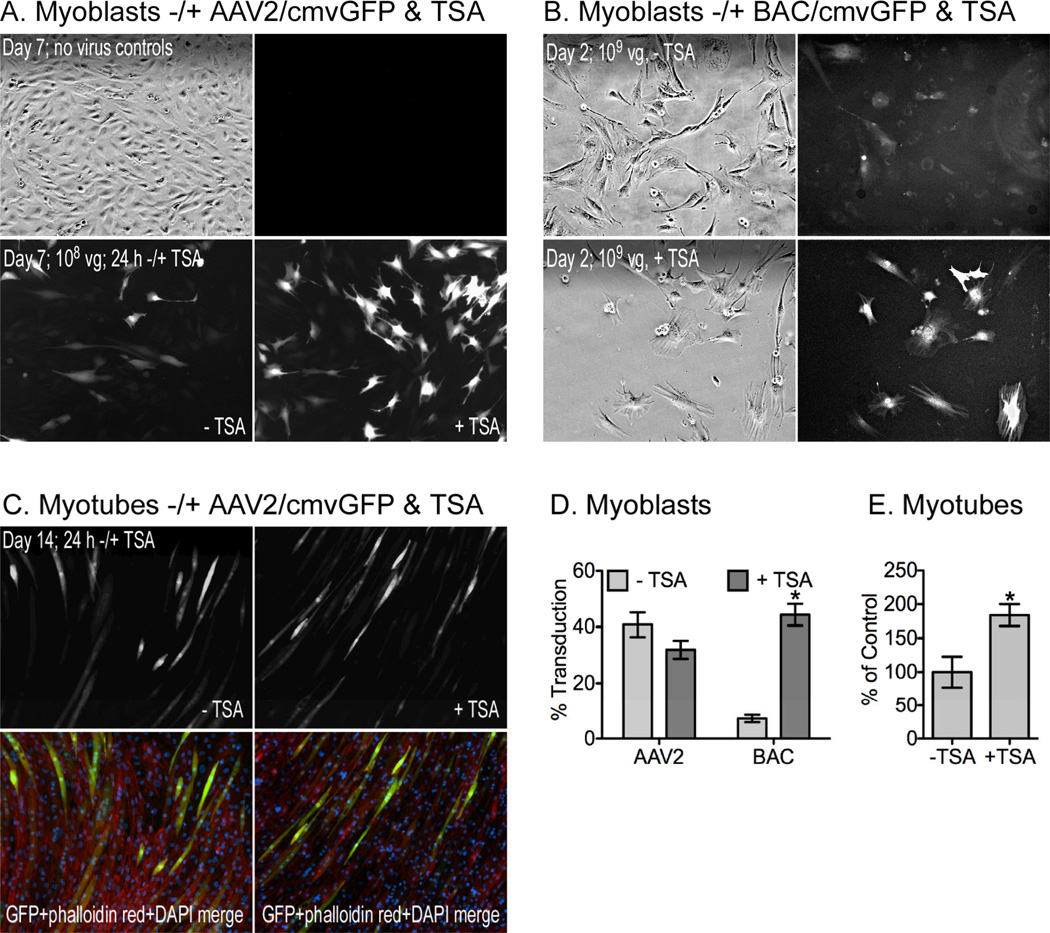
The histone deacetylase inhibitor TSA enhances gene expression in transduced cells [17–19] and although it also inhibits cell proliferation and stimulates differentiation [20–22], it could nevertheless prove useful in some experiments with muscle cells. In fact, TSA increased the percentage of EGFP-expressing cells from 25.9 +/− 3.9% to 35.4 +/− 6.2% in myoblasts previously infected with AAV2 and then passaged (Fig. 2B). We then tested TSA effects on cells transduced with either BAC or AAV2 to determine if its effects were limited to differentiation status as both C2C12 myoblasts and myotubes were tested.
Myoblasts were seeded at 260 cells/cm2 and incubated with either AAV2 or BAC for 5 days or 24 hours, respectively, before adding 1 µM TSA (Fig. 3A/B). In AAV2-infected cells, the level of expression appears brighter (Fig. 3A), although the total number of transduced cells did not differ (Fig. 3D). Similar results also occurred when AAV2-infected myoblasts were differentiated (Figs. 3CE). This suggests that TSA had no effect on AAV2 transduction efficiency, but enhanced the intensity of GFP expression. There was a six-fold increase (7.2 +/− 1.3% to 44.4 +/− 3.9%) in the apparent transduction efficiency when TSA was added to cells treated with BAC (Fig 3D). This increase is also likely due to enhanced expression as cells were incubated with BAC for only 24 hours (Fig. 3B).
Figure 3. TSA effects on AAV2 and baculovirus (BAC) transduction.
Proliferating C2C12 myoblasts were plated at low densities (260 cells/cm2) and incubated with (A) AAV2/cmvGFP or (B) BAC/cmvGFP for 7 or 2 days, respectively. Cells were treated −/+ 1 µM TSA during the final 24 h before imaging at 100×. (C) The C2C12 cells not treated with TSA were subsequently differentiated in low serum for an additional 7 days and as before, were treated −/+ TSA during the final 24 h. (D,E) The number of total and fluorescent myoblasts were counted and the average field fluorescent intensity of the myotubes was quantified with Image J as described in the Materials and Methods (mean +/− SEM; asterisks = significant difference, −/+ TSA vector groups analyzed independently; p≤0.05).
Viral and promoter challenge
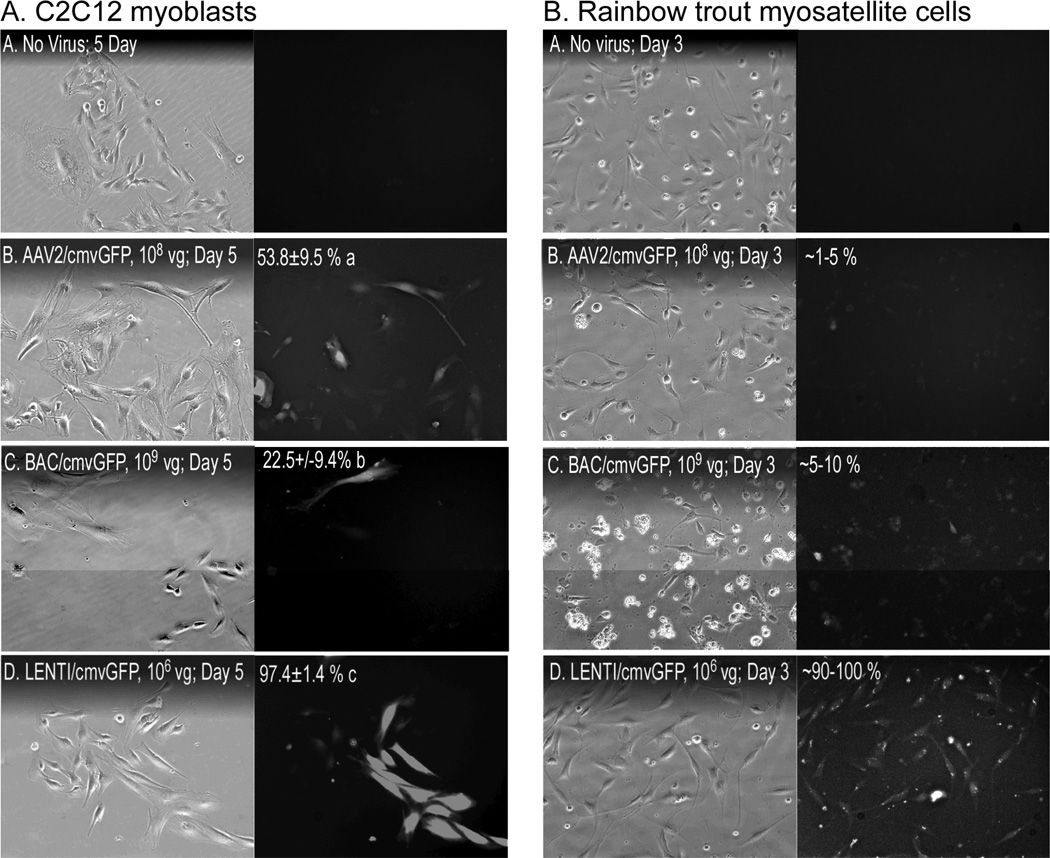
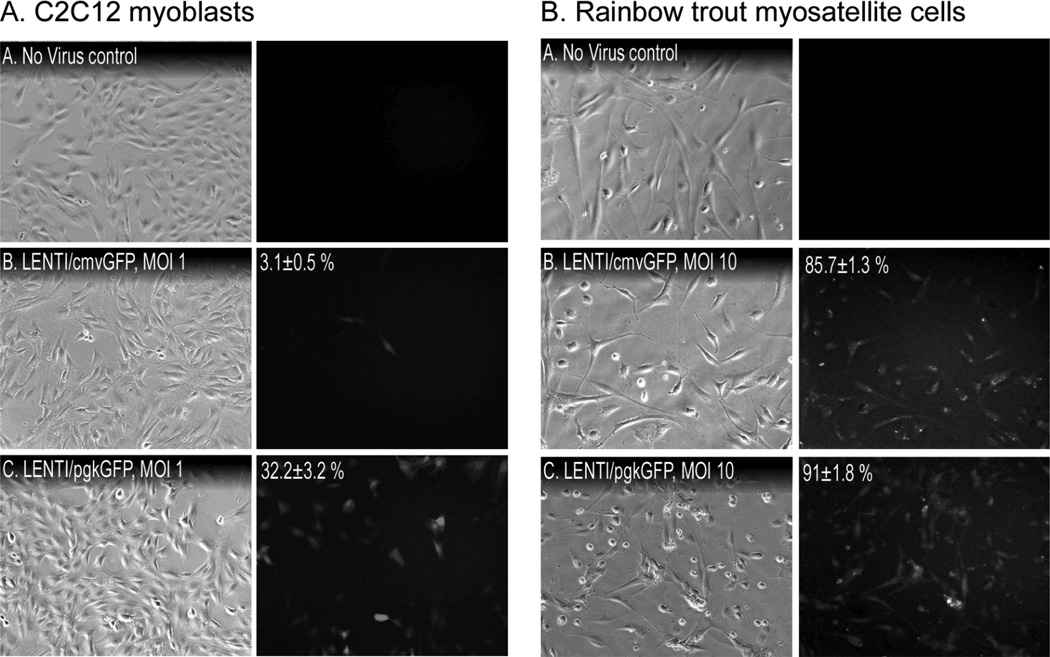
To determine which viral vector was most effective in transducing C2C12 myoblasts, cells were incubated with 108 vg AAV2, 109 vg BAC or 106 vg lentivrus for five days, each expressing GFP under the control of a cmv promoter. These vector concentrations were determined empirically from preliminary experiments (data not shown) and the protocols used varied between viral vectors (e.g. incubation time, cell pretreatments, etc.) The results therefore represent comparisons using conditions optimized for each vector system. As expected, transduction efficiency varied significantly between vectors as BAC and AAV2 transduced 22.5 +/− 9.4% and 53.8 +/− 9.5% of the cells, respectively, while lentivirus was far more effective with 97.1 +/− 1.4% (Fig. 4A). These differences were even more apparent when using primary myosatellite cells from rainbow trout as neither AAV2 nor BAC were particularly effective and produced transduction efficiencies of only 1–10% (Fig. 4B). By contrast, nearly all myosatellites were transduced by lentivirus, which appears to be equally efficient when using muscle cells from even diverse vertebrate classes.
Figure 4. Viral challenge.
(A) C2C12 myoblasts and (B) primary myosatellite cells isolated from rainbow trout were incubated for 5 or 3 days, respectively, with the indicated amounts of viral vectors (LENTI, lentivirus; BAC, baculovirus). Transduction efficiency (mean % +/− SEM; different letters = significant difference, same letters = no difference; columns A & B analyzed separately; p≤0.05) was determined by counting fluorescent and total cell numbers in 9 random views of each well/treatment.
Both cmv and pgk promoters are commonly used in gene therapy studies [23], although to date, comparative assessments of their efficiencies have not been performed in muscle cells in vitro. Mouse C2C12 myoblasts and primary rainbow trout myosatellites were therefore transduced with lentivirus carrying either a cmvGFP or pgkGFP construct to determine the relative efficacies of each promoter. Cells were infected using a multiplicity of infection (MOI) of 1 or 10 to facilitate comparisons of promoter efficacies between systems. Transduction efficiency was generally lower in trout myosatellite cells as no fluorescence was detected in cells treated with a MOI of 1, regardless of promoter (data not shown). Infecting C2C12 myoblasts with 1 MOI of LENTIpgkGFP produced 10-fold more fluorescent cells than did LENTIcmvGFP after 72 h (Fig. 5A; 32.2 +/− 3.2% v. 3.1 +/− 0.5%, respectively). Although 100% transduction efficiency was achieved when cells were treated with 10 MOI of either vector (data not shown), these data indicate that the pgk promoter is superior in this culture system. Furthermore, the two promoters proved equally efficacious in trout myosatellites when using 10 MOI (Fig. 5B; 91.0 +/− 1.8% v. 85.7 +/− 1.3%, respectively). These results together suggest that although lentiviral vectors transduce mammalian cells much better than trout cells, nearly 100% transduction can be achieved in the latter system by using a relatively high MOI. They also suggest that the pgk promoter is superior to the cmv promoter in proliferating muscle cells.
Figure 5. Comparison of pgk and cmv promoters.
(A) C2C12 myoblasts and (B) primary trout myosatellite cells were incubated for 72 h with lentivirus (LENTI) containing either cmv/GFP or pgk/GFP at the indicated MOI. Transduction efficiency (mean % +/− SEM; values in C2C12 experiments were significantly different, p≤0.05) was determined by counting fluorescent and total cell numbers in 9 random views of each well/treatment.
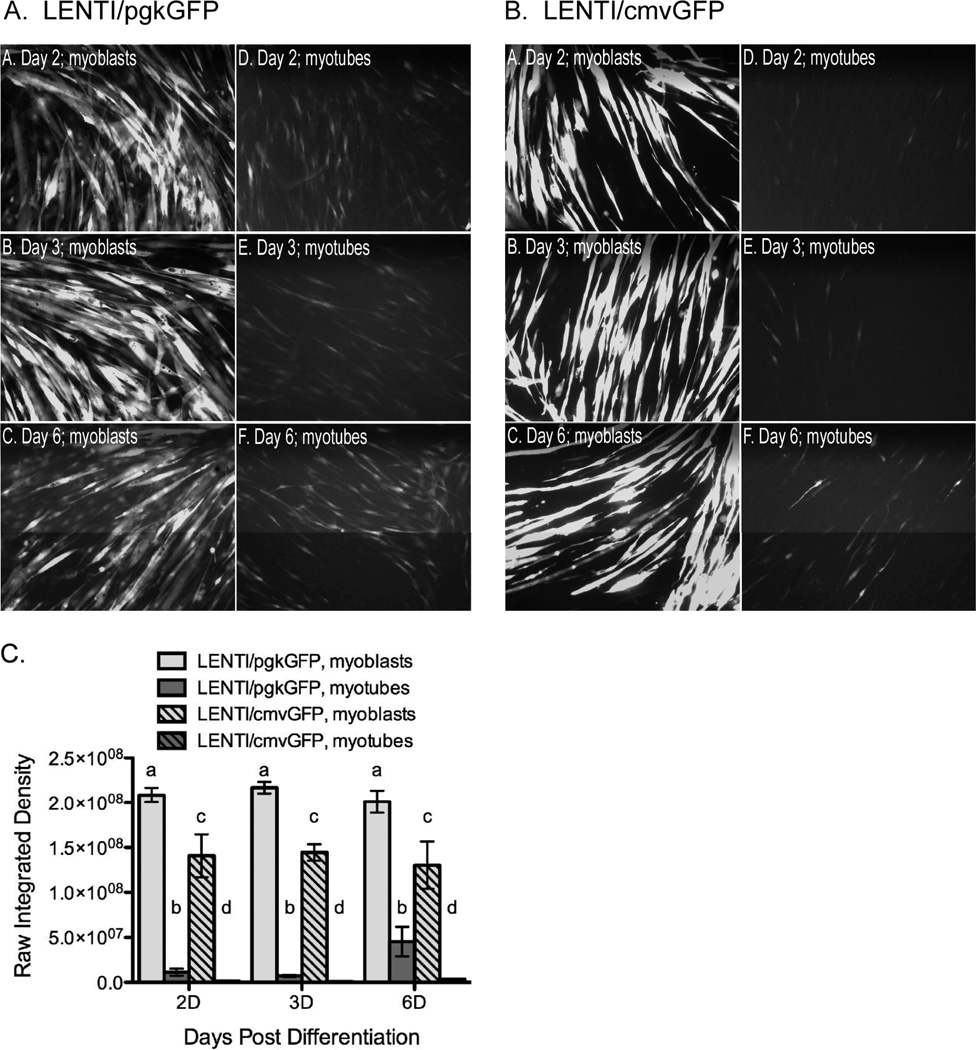
We then determined which promoter was optimal in differentiated C2C12 myotubes by either treating myoblasts and subsequently inducing differentiation or by treating fully differentiated myotubes directly. GFP expression was consistently higher in cells treated with pgkGFP than cmvGFP regardless of time point or differentiation status of the cell when treated (Fig. 6). In fact, this increase in expression was evident even though we used a 10-fold lower viral titer for pgkGFP (105 vg) than cmvGFP (106 vg). The intensity of GFP expression was ten fold higher in myotubes directly treated with pgkGFP compared to cmvGFP (107 and 106 optical density units (ODUs), respectively). Although GFP expression also increased with time in the myotubes, even after the virus was removed, this increase was still 10 to 100 fold lower when compared to myoblasts treated with pgkGFP or cmvGFP and then differentiated (2×108 and 1.4×108 ODUs, respectively). These results together suggest that maximal transduction efficiency and transgene expression in differentiated myotubes is best achieved by first infecting myoblasts with lentivirus that carry the pgk promoter and then differentiating the transduced cells.
Figure 6. Optimization of myotube transduction; treating before or after differentiation.
C2C12 myoblasts and myotubes were transduced with LENTI/pgkGFP (A) or LENT/cmvGFP (B) at 105 and 106 vg/well, respectively. Myoblasts were transduced 3 days prior to a 7-day differentiation period without virus. They were then imaged 2, 3 or 6 days later as indicated. C2C12 myoblasts were also differentiated 7 days before incubating with vector for 3 additional days. Cells were then imaged 2, 3 or 6 days later as indicated. (C) Field fluorescent intensity of myotubes (mean +/− SEM; different letters = significant difference, same letters = no difference; each time point was assessed independently; p≤0.05) were quantified with Image J as described in the Materials and Methods.
Discussion
Gene therapy is a promising therapeutic approach to combat various skeletal muscle diseases including muscular dystrophies, sarcopenia and cancer cachexia, although success has been limited due to obstacles associated with gene delivery and immunogenicity [2, 5, 24]. Better tools for investigating these obstacles in vitro would help to overcome these obstacles and could additionally help in studying intracellular signaling in skeletal muscle. However, the genetic manipulation of cultured muscle cells, whether myoblast cell lines, primary myosatellites or fully differentiated myotubes, is notoriously difficult. The two primary methods used include chemical/lipid-based transfection and viral vectors. Chemical transfections are fast and cost-effective in most cell culture systems, but produce low transfection efficiencies in muscle cells [7, 9, 11, 25]. Viral vectors are capable of introducing transgenes in a tissue-specific manner and may or may not integrate transgenes into the host genome, depending on the specific vector, and are powerful tools for gene therapy [26]. They are more difficult to construct, however, and vary in effectiveness with different cell systems. Thus, our goal was to determine an optimal system for introducing transgenes into proliferating and differentiated muscle cells using common gene delivery systems, a popular muscle cell line. We also investigated gene transfer using an emerging in vitro model, primary rainbow trout myosatellite cells.
The poor transfection efficiency of lipid-based protocols with muscle cells prevents their use in many cell-based assays, such as proliferation or differentiation assays, as only a minority of cells are transfected. Although we optimized transfections to ~50% using Lipofectamine 2000, GFP expression was lost as cells proliferated and was poorly retained after differentiation. In addition, the reagent was toxic at very low cell densities. We were therefore unable to generate a protocol capable of being used in proliferation and differentiation assays. These problems are hardly unique as in general, transfection efficiencies with cationic lipids are inversely related to transgene size [27, 28]. Indeed, cellular mortality and low transfection efficiencies were previously reported when using lipid based reagents on mesenchymal stem cells [29] and primary human and mouse myoblasts [30, 31]. Alternative approaches are therefore needed if cell-based assays are to be used with genetically manipulated muscle cells. The use of selection markers to generate stable cell lines is an obvious alternative. This cannot be done quickly, however, and introduces other problems including phenotypic variability between monoclonal cell lines.
Adeno-associated viral vectors have great promise for gene therapy as they infect both dividing and non-dividing cells, they rarely integrate into host genomes and they are relatively immune tolerant [24]. These systems are not without limitations, however, as the viral packaging size is small and synthesis of the second DNA strand is a rate-limiting step [32, 33]. Serotypes 2, 6 and 8 are of particular interest as AAV2 infects a wide variety of cell types while AAV6 and AAV8 are specific for striated muscle unless used at very high titers [34–36]. In this study, we used AAV2 due to the comparatively lower transduction efficiency of AAV6 in vitro [29]. In fact, a preliminary trial in our lab using both vector systems clearly demonstrated AAV2 superiority, even when vector was maintained on cells for six days (data not shown).
Plating cells at low densities, which prevents rapid confluency and extends incubation times, enabled us to consistently obtain transduction efficiencies of ~60% using AAV2. The drawback to the system, however, is that transgene expression did not continue after cells were passed, which would be required for cell-based assays using this procedure. Baculovirus can transduce a broad range of mammalian cells as well [17, 37, 38] and has emerged as a promising gene delivery vector due to low cytotoxicity and the ability to carry large inserts [38, 39]. It also transduced myoblasts and in a shorter time than AAV2, although a 10-fold higher titer was needed to produce the same relative transduction efficiency. The histone deacetylase inhibitor TSA significantly increased GFP expression in cells transduced with either vector, although this was due to improved expression resulting in increased detection rather than transduction efficiency. In fact, TSA is commonly used to boost transfection efficiency when in fact it has no influence on transfection per se, but on gene expression. TSA is also toxic to cells if maintained for long periods and can lower proliferation rates. Its use in cell-based assays is therefore impractical. Nevertheless, neither AAV2 nor baculovirus were particularly effective and required either long incubation times or high titers to produce efficiencies less than those typically generated by transfecting cells. Baculovirus transduction efficiency can be increased, however, by removing sodium bicarbonate from the culture media [40, 41], although it still does not reach the transduction efficiencies obtained with lentivirus.
Using optimal conditions for each vector, the viral challenge clearly indicated that after 5 days, lentivirus was by far the most efficient vector at transducing C2C12 myoblasts despite using titers 100- and 1000-fold lower than those of AAV2 and baculovirus, respectively (Fig. 4). Tissue specificity is dependent upon host receptors, host proteins that influence infection efficiency, cell cycle and the presence of specific transcription factors to drive transgene expression so it was surprising that lentivirus was equally effective at transducing primary trout myosatellites. These cells are phenotypically very similar to mammalian myosatellites, can be isolated in greater quantities and are considered an “emerging biomedical model” [15, 16]. Kawasaki et al. [42] previously demonstrated lentiviral, AAV and adenoviral transduction of zebrafish sertoli cells, although AAV serotype was not indicated, and determined that adenoviral vectors were superior. This further suggests that tissue specificity as well as species divergence should be considered when using viral vectors with non-mammalian tissues. Nevertheless, our demonstration that lentiviral vectors transduce trout myosatellites is extremely novel and will help both biomedical and comparative biologists to fully exploit this unique model system.
Various studies have shown that promoters can influence viral transduction efficiency [23, 43, 44]. We therefore tested the efficacy of two commonly used promoters, cmv and pgk, to determine which is more active (Fig. 5). These promoters are useful due to their ubiquitous activity in most cell types, although a recent study suggests that cmv activity is more variable in different cell types in vitro [45]. In fact, both were active in C2C12 cells and in primary trout myosatellites, although a much higher MOI was required to transduce the latter. The pgk promoter was most effective in C2C12 cells and although cmv and pgk appeared equally efficient in trout myosatellites, promoter activity of both was comparatively weak and could explain the need to use a 10-fold higher MOI to detect significant fluorescence.
Cellular uptake of viral vectors is enhanced during proliferation [46]. This partially explains why myotubes are difficult to transduce. Our studies suggest that the most effective way to transduce myotubes is to infect proliferating myoblasts before inducing differentiation (Fig. 6). Lentiviral superiority over the other vectors is likely due to the fact that their genomes, the vector provirus, are integrated into host genomes, which explains how GFP expression is maintained several days after differentiation. Our results also demonstrate that lentivirus itself does not alter the differentiation potential of C2C12 myoblasts. Brooks et al. [47] reported cmv methylation and silencing in mature skeletal muscle. Similar inactivation could potentially prove problematic with muscle cells in vitro, although this likely does not explain the differences reported herein as both cmv and pgk were equally active in fully differentiated myotubes despite pgk superiority in myoblasts.
Lentiviral entry into myoblasts was apparently rapid as treating them for just two hours before inducing to differentiate also resulted in high transduction efficiencies (90%, data not shown). Although the intensity of GFP expression in these cells was lower than in cells incubated with virus for three days, this nevertheless indicates that lentivirus effectively and rapidly infects myoblasts, at lower virus titers, and that transgene expression is maintained well after differentiation.
In summary, Lenti/pgk is optimal when transducing C2C12 myoblasts at a conflucency of 260 cells/cm2 with a low viral titer of 106 and a three-day incubation period. These parameters also work well when using trout myosatellite cells and can be used to introduce transgenes in myoblasts that will be subsequently differentiated into myotubes, which again is preferred to transducing myotubes directly. Finally, TSA can be used to boost expression of transgenes, but it does not increase transfection or transduction efficiency and its use limits how the treated cells themselves are subsequently used.
Materials and Methods
Cell culture
C2C12 myoblasts and 293 cells were maintained at 37°C, under the presence of 5% CO2 and in growth media (Dulbecco’s modified Eagle’s medium, DMEM; Hyclone) containing 10% fetal bovine serum (FBS, Atlanta Biologicals) and an antibiotic/antimycotic (Sigma). Confluent C2C12 myoblasts cells were induced to differentiate with serum withdrawal to 2% FBS. Primary myosatellite cells were also isolated from rainbow trout. These cells are activated skeletal muscle satellite cells (a.k.a. muscle stem cells) and like primary myosatellites from other vertebrates, spontaneously differentiate in culture and in response to serum. Fish were obtained from the Washington State University hatchery, a Center of Reproductive Biology core facility, and were reared and used in strict accordance to protocols preapproved by the institutional animal care and use committee. Cells were isolated using a procedure previously described [15, 16]. Briefly, fish were collected, weighed and killed by a sharp blow to the head and immersed in 70% ethanol for 30 s. The skin was removed and the dorsal white muscle was carefully dissected and collected in cold DMEM containing 9 mM NaHCO3, 20 mM HEPES (pH 7.4), 15% horse serum (HS) and an antibiotic/antimyotic (Sigma). Muscle was weighed and cut into small pieces with scissors and centrifuged at 300×g for 15 min to remove floating pieces. It was then resuspended in a 0.25% collagenase solution for 70 min at 18°C. Cells were pelleted as before, washed and incubated with 0.1 % trypsin (Sigma) for 20 min at 18°C. After again pelleting and washing, the resulting cell suspension was centrifuged for 1 min at 300 × g the supernatant was diluted 4-fold with DMEM containing 15% HS and digested again with trypsin for 20 min before pouring into extraction medium made up of DMEM containing 15% HS, antibiotic/antimyotic and gentamicine. Cells were then centrifuged for 20 min at 300 × g, resuspended in basal medium containing DMEM and Hepes and sequentially filtered through 70 and 40 µm nylon cell strainers. The resulting filtrate was centrifuged for 20 min at 300 × g and cells were resuspended and counted before plating. Myosatellite cells were then cultured on plates pretreated with 100 µg/ml poly-l-lysine (Sigma) for 3 h at 18°C. Wells were then washed with sterile water and treated with 5 µg/ml laminin (Sigma) diluted in PBS overnight at 18°C. The laminin solution was then aspirated and cells were plated the following day and incubated at 18°C in sealed plates to prevent evaporation.
Lipofectamine Transfection
C2C12 cells were plated at a confluency of 40–50% (~35,000 cells/well) in a 12-well plate (3.8 cm2) one day prior to transfection. Different ratios (µl:ug) of Lipofectamine 2000 reagent (Invitrogen) to CMV-EGFP plasmid DNA were then used for transfection according to the manufacturer’s protocol. The specific reagent:DNA ratios used varied from 1:1 to 3:1 and were paired with 2–12µl Lipofectamine 2000. Preliminary experiments established the preferred ratios shown in Figure 1 (4.8µl:1.6µg, 4µl:2µg and 4.8µl:3.2µg) and each experiment was performed in triplicate using serum-free DMEM. Culture media was changed 4 hours after transfection and replaced with DMEM/10% FBS without antibiotics. Images were taken 24 h after transfection using a Nikon Eclipse Ti-S inverted microscope and DS-Qi1Mc Nikon camera at 40× (9 images/well). Transfection efficiency was determined by counting fluorescent and total cell numbers in 3 wells/treatment. The cells were then allowed to proliferate and differentiate in DMEM/2% FBS to determine the number of cells that retain GFP expression after differentiation.
AAV2 production
293 cells were plated at a confluency of 80% (approximately 3 million cells) in a 100 × 20 mm tissue culture plate (56 cm2) the day prior to transfection. A three-plasmid calcium phosphate co-transfection protocol was used in accordance with the manufacturer’s protocol (Stratagene). All plasmids (pAAV-CMV/EGFP, pAAV-RC & pHelper) were used at a working concentration of 1 µg/ul. The transfection mixture was added to the cells for 18 h and then the media was replaced with serum free DMEM for an additional 48 hours before collecting cells and conditioned medium. Three consecutive freeze-thaw cycles were utilized to lyse cells and release virus, followed by centrifugation to remove cell debris. The supernatant was saved and passed through Hi-Trap Heparin column (GE Healthcare) at a rate of 1 ml/min to bind AAV. The column was washed with Hank’s Balanced Salt solution (HBSS) and eluted with 500 mM NaCl/HBSS. The resulting virus was concentrated using a 50 kDa Centricon spin filter (Millipore) and titered using PCR. A standard curve was generated using the GFP plasmid (10 pg to 1 ng) and gene specific primers (forward, 5’ATGGTGAGCAAGGGCGAGGAG3’; reverse, 5’CGCTTTACTTGTACAGCTCGTCC3’). Serial dilutions of each virus and the standards were amplified for 30 cycles of 94°C for 20s, 60°C for 30s and 72°C for 40s. The intensity of each band was measured using a Kodak IM2000mm and titers (vg/µl) were determined from the standard curve.
Lentivirus production
Lentivirus was manufactured as described previously [48]. The HIV vectors were self-inactivating pRRL [49] vector backbones containing a central polypurine tract and a woodchuck post-transcriptional regulatory element. Two promoters were used to drive EGFP expression including the HIV-based PGK promoter and a CMV promoter. Both promoters contained identical Kozak sequences (5’-TCGCCACCATGGTG-3’) and were 515 bp and 532 bp in size, respectively. HIV-based vectors were pseudotyped with VSV-G envelope and produced by transient transfection of 293T cells and concentrated 100-fold as previously described [48]. Lentivirus was titered by transducing HT1080 cells and counting the positive cells with a flourescent activated cell sorter and by using the PCR assay described above.
Baculovirus production
Recombinant baculovirus expressing EGFP under CMV promoter was constructed by inserting the CMV promoter with EGFP in the Not I site of the pfastbac HTb plasmid (Invitrogen). The plasmid was transformed into DH10Bac cells and after 48 h, the bacmid was isolated from white colonies. It was then used to transfect SF9 insect cells (Invitrogen) with Cellfectin reagent (Invitrogen). Conditioned medium was subsequently used to transduce SF9 in order to create second-generation baculovirus. The resulting virus was concentrated by centrifugation on a 40% sucrose cushion at 56,000×g for 2 h. The virus pellet was resuspended in PBS and the titer was determined using PCR as described above.
Viral Transduction
C2C12 myoblasts were plated in a 12-well plate a day prior to transduction. For AAV2-GFP transduction, 4.8 µM camptothecin (Sigma) was added to the cells before adding virus, which was maintained on the cells for five days. Myoblasts were then either treated with 1 µM trichostatin A (TSA, Sigma) or differentiated in low serum. For lentiviral transduction, cells were added with the desired titer of the virus along with 4 µg/ml protamine sulfate (Sigma). For baculovirus transduction, virus was added to the medium at the titer desired. Virus was left on the undifferentiated cells for 1–5 days before imaging or differentiating, depending on the experiment and vector, or for 3 days when treating C2C12 myotubes. For most experiments, an equal number of viral genomes were added to cells except when comparing cmv and pgk promoters in different cell types. Promoter activity naturally varies between cell types especially when using cells from different vertebrate classes. We therefore used multiplicity of infection (MOI) values of 1 and 10 when comparing the transduction efficiency of lentivirus carrying cmv/GFP or pgk/GFP in the different cell types.
Quantifying Transduced Cells
Images for all transduction experiments were taken at 100× except fish cells that were taken at 200×. In all figures, the chosen images approximated the mean values quantified or displayed cells where fluorescence was very evident, especially in cases of low transfection/transduction efficiency. Analysis of C2C12 myoblasts involved counting fluorescent and total cell numbers in 9 random views from 3 wells/treatment. Images of myotubes were analyzed using ImageJ (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA). In brief, color images were converted to color-free 8 bit images, background was subtracted using a radius of 100 pixels and the threshold was set to include only the brightest pixels. Threshold limits were set to 15 and 255 for all images and the integrated density of each image was measured for a total of 9 images/treatment.
Actin and Hoescht staining
Myotubes and myoblasts were stained as described [15, 16]. Briefly, cells were washed with PBS, fixed in 4% paraformaldehyde, washed again and permeabilised with 0.2% triton X-100. Acti-stain 555 (Cytoskeleton) was added to the fixed cells at a concentration of 100 nM for 30 minutes at room temperature. After washing with PBS, nuclei of fixed cells were stained with Hoescht dye (5 µg/ml) for 15 minutes at room temperature.
Statistical Design
Statistical differences were determined either by a Student’s t-test or by an analysis of variance coupled to Bonferroni or Tukey post hoc test for mean comparisons when appropriate (P≤ 0.05).
Acknowledgements
These studies were supported by grants from the National Science Foundation (0840644 & 1147275) to Buel D. Rodgers, from the National Institutes of Health (DK077806) to Grant D. Trobridge and from a National Institute of General Medical Sciences training grant (T32GM083864) for Melissa F. Jackson. We want to especially thank Dilip K. Garikipati who assisted in different aspects of these studies.
References
- 1.Muir LA, Chamberlain JS. Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med. 2009;11:e18. doi: 10.1017/S1462399409001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A, Herzog RW. Innate Immune Responses to AAV Vectors. Front Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaffer DV, Koerber JT, Lim KI. Molecular engineering of viral gene delivery vehicles. Annu Rev Biomed Eng. 2008;10:169–194. doi: 10.1146/annurev.bioeng.10.061807.160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 6.Jordan M, Wurm F. Transfection of adherent and suspended cells by calcium phosphate. Methods. 2004;33:136–143. doi: 10.1016/j.ymeth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Neville C, Rosenthal N, Hauschka S. DNA transfection of cultured muscle cells. Methods Cell Biol. 1997;52:405–422. doi: 10.1016/s0091-679x(08)60389-1. [DOI] [PubMed] [Google Scholar]
- 8.Yamano S, Dai J, Moursi AM. Comparison of transfection efficiency of nonviral gene transfer reagents. Mol Biotechnol. 2010;46:287–300. doi: 10.1007/s12033-010-9302-5. [DOI] [PubMed] [Google Scholar]
- 9.Dodds E, Dunckley MG, Naujoks K, Michaelis U, Dickson G. Lipofection of cultured mouse muscle cells: a direct comparison of Lipofectamine and DOSPER. Gene Ther. 1998;5:542–551. doi: 10.1038/sj.gt.3300604. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi RA, Dickson G. Liposome-mediated gene transfer into normal and dystrophin-deficient mouse myoblasts. J Neurochem. 1995;64:2230–2238. doi: 10.1046/j.1471-4159.1995.64052230.x. [DOI] [PubMed] [Google Scholar]
- 11.Balci B, Dinçer P. Efficient transfection of mouse-derived C2C12 myoblasts using a matrigel basement membrane matrix. Biotechnol J. 2009;4:1042–1045. doi: 10.1002/biot.200800269. [DOI] [PubMed] [Google Scholar]
- 12.Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2010:143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RA, Palacios JA, Cassell GD, Adam S, Giacca M, Weitzman MD. The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J Virol. 2007;81:12936–12945. doi: 10.1128/JVI.01523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 15.Garikipati DK, Rodgers BD. Myostatin stimulates myosatellite cell differentiation in a novel model system: evidence for gene subfunctionalization. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1059–R1066. doi: 10.1152/ajpregu.00523.2011. [DOI] [PubMed] [Google Scholar]
- 16.Garikipati DK, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: evidence for endocrine-regulated transcript processing. J Endocrinol. 2012;215:177–187. doi: 10.1530/JOE-12-0260. [DOI] [PubMed] [Google Scholar]
- 17.Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci U S A. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada T, Uchibori R, Iwata-Okada M, Takahashi M, Nomoto T, Nonaka-Sarukawa M, Ito T, Liu Y, Mizukami H, Kume A, Kobayashi E, Ozawa K. A histone deacetylase inhibitor enhances recombinant adeno-associated virus-mediated gene expression in tumor cells. Mol Ther. 2006;13:738–746. doi: 10.1016/j.ymthe.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Dion LD, Goldsmith KT, Tang DC, Engler JA, Yoshida M, Garver RI. Amplification of recombinant adenoviral transgene products occurs by inhibition of histone deacetylase. Virology. 1997;231:201–209. doi: 10.1006/viro.1997.8538. [DOI] [PubMed] [Google Scholar]
- 20.Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottière HM. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut. 2000;46:507–514. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida M, Beppu T. Reversible arrest of proliferation of rat 3Y1 fibroblasts in both the G1 and G2 phases by trichostatin A. Exp Cell Res. 1988;177:122–131. doi: 10.1016/0014-4827(88)90030-4. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 23.Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 24.Kwon I, Schaffer DV. Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res. 2008;25:489–499. doi: 10.1007/s11095-007-9431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhuber B, Huang DI, Daniels MP, Torgan CE. High efficiency transfection of primary skeletal muscle cells with lipid-based reagents. Muscle Nerve. 2002;26:136–140. doi: 10.1002/mus.10171. [DOI] [PubMed] [Google Scholar]
- 26.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics. 2003;4 doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 27.Kreiss P, Cameron B, Rangara R, Mailhe P, Aguerre-Charriol O, Airiau M, Scherman D, Crouzet J, Pitard B. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999;27:3792–3798. doi: 10.1093/nar/27.19.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7:31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 29.McMahon JM, Conroy S, Lyons M, Greiser U, O'shea C, Strappe P, Howard L, Murphy M, Barry F, O'Brien T. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev. 2006;15:87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- 30.Pampinella F, Lechardeur D, Zanetti E, MacLachlan I, Benharouga M, Lukacs GL, Vitiello L. Analysis of differential lipofection efficiency in primary and established myoblasts. Mol Ther. 2002;5:161–169. doi: 10.1006/mthe.2002.0528. [DOI] [PubMed] [Google Scholar]
- 31.Vitiello L, Bockhold K, Joshi PB, Worton RG. Transfection of cultured myoblasts in high serum concentration with DODAC:DOPE liposomes. Gene Ther. 1998;5:1306–1313. doi: 10.1038/sj.gt.3300729. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, Miller AD, Miller DA, Chamberlain JS. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci U S A. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu YC. Baculoviral vectors for gene delivery: a review. Curr Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- 39.Kukkonen SP, Airenne KJ, Marjomäki V, Laitinen OH, Lehtolainen P, Kankaanpää P, Mähönen AJ, Räty JK, Nordlund HR, Oker-Blom C, Kulomaa MS, Ylä-Herttuala S. Baculovirus capsid display: a novel tool for transduction imaging. Mol Ther. 2003;8:853–862. doi: 10.1016/j.ymthe.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Shen HC, Lee HP, Lo WH, Yang DG, Hu YC. Baculovirus-mediated gene transfer is attenuated by sodium bicarbonate. J Gene Med. 2007;9:470–478. doi: 10.1002/jgm.1037. [DOI] [PubMed] [Google Scholar]
- 41.Shen HC, Yeh CN, Chen GY, Huang SF, Chen CY, Chiu YC, Hu YC. Sustained baculovirus-mediated expression in myogenic cells. J Gene Med. 2008;10:1190–1197. doi: 10.1002/jgm.1245. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki T, Saito K, Mitsui K, Ikawa M, Yamashita M, Taniguchi Y, Takeda S, Mitani K, Sakai N. Introduction of a Foreign Gene into Zebrafish and Medaka Cells Using Adenoviral Vectors. Zebrafish. 2009;6 doi: 10.1089/zeb.2009.0596. [DOI] [PubMed] [Google Scholar]
- 43.Hong S, Hwang DY, Yoon S, Isacson O, Ramezani A, Hawley RG, Kim KS. Functional analysis of various promoters in lentiviral vectors at different stages of in vitro differentiation of mouse embryonic stem cells. Mol Ther. 2007;15:1630–1639. doi: 10.1038/sj.mt.6300251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Husic N, Lin Y, Christensen H, Malik I, McIver S, Daniels CM, Harris DA, Kotzbauer PT, Goldberg MP, Snider BJ. Optimal promoter usage for lentiviral vector-mediated transduction of cultured central nervous system cells. J Neurosci Methods. 2010;189:56–64. doi: 10.1016/j.jneumeth.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One. 2010;5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita M, Emerman M. Retroviral infection of non-dividing cells: Old and new perspectives. Virology. 2006;344 doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. The journal of gene medicine. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 48.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trobridge GD, Wu RA, Hansen M, Ironside C, Watts KL, Olsen P, Beard BC, Kiem HP. Cocal-pseudotyped lentiviral vectors resist inactivation by human serum and efficiently transduce primate hematopoietic repopulating cells. Mol Ther. 2010;18:725–733. doi: 10.1038/mt.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]