Abstract
Emotionally arousing material is typically better remembered than neutral material. Since norepinephrine and cortisol interact to modulate emotional memory, sex-related influences on stress responses may be related to sex differences in emotional memory. Two groups of healthy women – one naturally cycling (NC women, N = 42) and one using hormonal contraceptives (HC women, N = 36) – viewed emotionally arousing and neutral images. Immediately after, they were assigned to Cold Pressor Stress (CPS) or a control procedure. One week later, participants received a surprise free recall test. Saliva samples were collected and later assayed for salivary alpha-amylase (biomarker for norepinephrine) and cortisol. Compared to NC women, HC women exhibited significantly blunted stress hormone responses to the images and CPS. Recall of emotional images differed between HC and NC women depending on noradrenergic and cortisol responses. These findings may have important implications for understanding the neurobiology of emotional memory disorders, especially those that disproportionately affect women.
Keywords: hormonal contraception, norepinephrine, cortisol, emotional memory, salivary alpha-amylase
Introduction
Hormonal contraception has been used by women worldwide for over 50 years, but its effects on hormone responses to different stressors and emotional memory have remained largely unexplored. Estrogen/progestin contraceptives utilize negative feedback to the hypothalamus to inhibit the mid-cycle gonadotropin-releasing hormone (GnRH) surge (Lobo and Stanczyk, 1994). In addition, these synthetic hormones suppress the release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the pituitary to inhibit the gonadal production of endogenous estrogen and progesterone. The suppression of endogenous estrogen and progesterone likely disrupts stress hormone activity (Kudielka and Kirschbaum, 2005), sex/stress hormone interactions and subsequently, memory for emotionally arousing stimuli.
Evidence for the association between sex and stress hormone activity comes from studies demonstrating that hormonal contraception use significantly reduces stress hormone responses to a stressor. Kirschbaum and colleagues (1999) examined hypothalamic-pituitary-adrenal (HPA) responses to the Trier Social Stress Test (TSST) in men, naturally cycling women in both the follicular and luteal phases, and women on oral contraceptives. Women on oral contraceptives had similar salivary cortisol responses to TSST compared to women in the follicular phase of the menstrual cycle, but significantly blunted responses compared to men and women in the luteal phase of the menstrual cycle. These findings suggest that oral contraceptive use can alter HPA activity in response to a psychosocial stressor, and these effects are likely due to the corticosteroid-binding globulin (CBG) enhancing effect of ethinyl estradiol (Fujimoto et al., 1986; Kirschbaum et al., 1999; Kumsta et al., 2007).
In addition,Otterstetter et al. (1999) reported that the use of hormonal contraception can also alter the reactivity of the sympathetic stress system; in response to a maximal exercise task, women on hormonal contraception had significantly lower post-exercise concentrations of plasma norepinephrine relative to naturally cycling women.
What remains unknown, however, is how these oral contraceptive-induced differences in cortisol and salivary alpha-amylase reactivity may affect memory for emotionally arousing stimuli. Several studies, however, have examined how sex and stress hormones interact to influence emotional memory among naturally cycling women. For example, Andreano, Arjomandi, and Cahill (2008) found that the relationship between a post-training release of cortisol and memory for an arousing story changed depending on levels of circulating sex hormones; cortisol positively correlated with story recall only for women in the mid-luteal (high progesterone) phase.
The notion that sex hormones influence stress effects on memory is further supported by human imaging studies. For example, Goldstein and colleagues (2005) demonstrated that women in the late follicular (high estrogen) phase showed significantly decreased responses in several limbic, frontal, and hypothalamic regions compared to those in the early follicular (low estrogen and progesterone) phase. Moreover, van Wingen and colleagues (2008) demonstrated that high levels of synthetic progesterone significantly increased amygdala responses to emotional images relative to neutral images. In addition to these studies showing sex hormone influences on memory, clear sex differences have been shown in stress response circuitry activation between men and naturally cycling women such that mid-cycle hormonal changes in women reduced subcortical arousal, which was coupled with a reduction in cortical arousal (Goldstein et al., 2010).
The findings by Goldstein et al. (2005; 2010) and van Wingen et al. (2008) were further supported by a study that examined the influence of sex hormones on amygdala and hippocampal activity. Andreano and Cahill (2010) scanned naturally cycling women in the early follicular (low estrogen and progesterone) and mid-luteal (high progesterone) phases of the menstrual cycle while they viewed emotional and neutral images. Results showed that women in the midluteal phase had significantly enhanced activity in response to emotional images in the hippocampus and amygdala as compared to those in the early follicular phase. When compared to the results from Goldstein et al. (2005), these findings suggest estrogen and progesterone may have different roles in modulating the brain’s arousal circuitry and potentially, emotional memory processing.
Based on the aforementioned evidence suggesting that sex steroid hormones and stress hormones interact to influence memory, it seems very likely that hormonal contraception should influence memory for emotional material. A recent study from our lab provides preliminary evidence for such an association by demonstrating an association between the use of hormonal contraception and altered memory for information from an emotional story (Nielsen et al., 2011). These findings suggest that additional research is necessary to better understand the effects of hormonal contraception on both hormonal responses to different stressors and subsequently, memory for different types of emotional stimuli.
The purpose of the present investigation was twofold. First, we sought to better characterize the nature of the blunted stress hormone responses that the literature indicates occur with hormonal contraception. Second, we wished to further explore whether and how hormonal contraception influences memory for emotional material.
Because of previous sex-related findings with both stress hormone responses and emotional memory, we examined whether hormone responses to emotionally arousing images and Cold Pressor Stress (CPS) differed between women on hormonal contraception and naturally cycling women. We selected these stressors to elicit a noradrenergic response at encoding (emotionally arousing images) and a post-training glucocorticoid response (CPS). To assess these hormone responses, we measured salivary alpha-amylase (sAA) as a biomarker for norepinephrine (Chatterton et al., 1996) as well as salivary cortisol levels. On the basis of prior research (Kirschbaum et al., 1999; Otterstetter et al., 1999), we predicted that women on hormonal contraceptives would have reduced cortisol responses to CPS and reduced sAA responses to the emotional images compared to naturally cycling women. We also investigated whether their memory for emotional slides differed with a surprise free recall test one week later. Since we wanted to explore differences between naturally cycling (NC) women and women on hormonal contraception (HC) who did or did not exhibit a stress hormone response to the emotional images or to the CPS, we also performed analyses with the women divided into “responder” and “non-responder” groups (Buchanan, Tranel, and Adolphs, 2006). Responder/Non-Responder criteria for sAA and cortisol responses will be discussed in the Methods.
To date, there has been limited work in humans on the relationships between norepinephrine, glucocorticoids, and memory consolidation in general (van Stegeren et al., 2007; 2010; Felmingham et al., 2012) and none with respect to potential sex hormone influences. However, based on previous research that identified sex influences on emotional memory (Andreano and Cahill, 2009), we hypothesized norepinephrine at encoding and post-training glucocorticoids would influence memory differently in naturally cycling women versus women on hormonal contraception.
Methods
Participants
Ninety-eight female students from the University of California, Irvine between the ages of 18–35 (M = 20.37, SD = 2.37) participated in this study, which was approved by the University’s Institutional Review Board. The subjects received course credit. All participants were non-smokers. Participants were asked to refrain from alcohol, caffeine, and cardiovascular exercise for twenty-four hours prior to each experimental session to control for outside influences that could affect baseline salivary alpha-amylase and cortisol levels. To avoid contamination of salivary samples, participants were asked to fast one hour prior to each experimental session as well as refrain from brushing teeth within the hour before their appointment.
Of the participants, 50 were NC women who reported having regular menstrual cycles and 45 were women currently on a combined HC regimen for at least one month. Of these, six women were excluded for not returning for the second experimental session (1 NC, 3 HC), for having baseline sAA levels more than three standard deviations above the mean (1 HC), and for having a cortisol response to CPS that was more than three standard deviations above the mean (1 HC). An additional 11 women (7 NC, 4 HC) were excluded from the final analyses because they exhibited a cortisol response to the control condition for Cold Pressor Stress (CPS).
The final analyses thus included data from 42 NC women (15 Follicular, 27 Luteal) and 36 HC women. All women reported that they were in good health, and the average body-massindex (BMI) values for NC (M = 21.78, SD = 2.71) and HC (M = 22.15, SD = 3.98) women did not differ significantly. Of the women included in the final analyses, seven used prescription medications other than hormonal contraception (5 NC, 2 HC). Of the HC women, 25 took monophasic drugs and 11 used triphasic pills. All the HC women were on drugs that contained ethinyl estradiol, and the content of this synthetic estrogen varied between .015 mg and .03 mg/dose.
Procedures
All experimental sessions were conducted between the hours of 12:00 and 18:00 to minimize the effects of circadian rhythm on sAA and cortisol levels. During the first experimental session, participants filled out a screening questionnaire and three cognitive assessments including the BEM Sex Roles Inventory (BEM; Bem, 1981), the Positive and Negative Affect Schedule (PANAS; Watson, Clark, and Tellegen, 1988), and the Mehrabian test (Mehrabian, 1994). The BEM was implemented to assess masculine and feminine influences/traits within each individual participant, whereas the PANAS was given to measure the participants’ affect at the time of testing. The Mehrabian was implemented to assess levels of trait anxiety (Mehrabian, 1994).
Fifteen minutes after their arrival, participants provided a 1-mL saliva baseline sample (“pre-slideshow”/ “pre-CPS” sample) using the “passive drool” collection method (Shirtcliff et al., 2001). Following the baseline saliva sample, participants moved into a solitary experimental room where they watched a slide show comprised of twelve arousing positive images, twelve arousing negative images, and twelve neutral images from the International Affective Picture System (IAPS) (Lang, Bradley, and Cuthbert, 1997a). Each image was displayed for 10 s and slides were presented in random order. Following each image, participants were asked to rate the image on the degree of emotional arousal and valence; subjects had 5 s to make each rating using a 1 – 9 scale (Adolphs, Denburg, and Tranel, 2001). The scale is described in the “Scoring Recall Performance” section of the Methods. A second 1-mL saliva sample (“post-slideshow” sample) was taken immediately after the 12 minute slideshow.
Participants were then randomly assigned to either a CPS (24 NC, 23 HC) or control condition. Those assigned to the CPS condition immersed their right hand in ice water (1 – 4°C) for up to three minutes, whereas participants in the control condition immersed their right hand in warm water (37°C) for the same length of time. Subjects in both conditions were informed prior to the test that they could remove their hand from the water at any time without penalty. Of the participants included in the final analyses, seven women removed their hand before the end of the three minute CPS session (4 HC, 3 NC). These women were included in the final analyses because they completed at least 30 seconds of the CPS task. Upon completion of CPS or control condition, participants provided a third 1-mL saliva sample. All participants were instructed to refrain from any stressful activities for the remainder of the session. Additional samples were collected 15 and 25 after the CPS or control condition.
One week later, participants returned and provided one 1-mL saliva sample after a fifteen minute acclimation period. This sample was collected to maintain a consistent procedure across the two experimental sessions and was not analyzed for levels of sAA or cortisol. A surprise free recall test for slide recall and associated slide elements was administered shortly afterwards. During the test, participants were asked to write a brief phrase identifying each slide they remembered as well as any information they could recall that was associated with each remembered slide. This additional information was recorded on a separate form. No time or space limit was given for the test. After completing the test, subjects were debriefed and compensated with course credit.
Scoring recall performance
Recall performance was determined by two independent scorers, blind to subject condition. A correct recall was scored if the description of the slide unequivocally matched an image that was presented. There was a high degree of agreement between the two scorers (>99%); when the scorers disagreed, a third independent scorer made the final judgment. Recalled images were classified by arousal and valence based on the participant’s arousal and valence ratings associated with each image. Following each stimulus offset, ratings screens for arousal and valence were presented for 5 s each and participants were required to make their rating within that time frame. If a participant failed to provide a rating for an image, the scorers used standard IAPS ratings to classify the recalled image; ratings were made 96.2% of the time across participants. For arousal, images that were rated as 1 – 5 were considered “non-arousing” and those rated as 6 – 9 were “arousing.” In the case of valence, images rated as 1 – 3 were classified as “negative,” images rated as 4 – 6 were “neutral,” and images rated as 7 – 9 were “positive” (Adolphs, Denburg, and Tranel, 2001).
Saliva collection
Saliva samples were immediately frozen for a minimum of twentyfour hours to allow mucins to precipitate. On the day of the assay, samples were thawed and centrifuged at 2,080 × g for 15 min to extract particulates from saliva. Clear supernatant was decanted into microtubes.
Salivary alpha-amylase measurement
Alpha-amylase levels were measured using Salimetrics (State College, PA) enzyme kinetic assay kits and measured optically using BioTek Instruments, Inc. ELx808 Absorbance Microplate Reader (Winooski, VT).
The sAA response to the IAPS images was calculated by subtracting the amount of sAA in the “pre-slideshow” sample from the amount in the “post-slideshow” sample. Participants with an increase of 3 U/mL or more in their level of sAA were classified as “sAA Responders” (Salimetrics, LLC). All other participants were considered “sAA Non-Responders.”
Cortisol Measurement
Salivary cortisol levels were measured using Salimetrics (State College, PA) cortisol ELISA kits and measured optically using BioTek Instruments, Inc. ELx808 Absorbance Microplate Reader (Winooski, VT). The cortisol response was computed by subtracting the amount of salivary cortisol present in the “pre-CPS” sample from the amount in the “15 min post-CPS” sample, which was taken approximately 15 minutes after termination of CPS. This sample was indicative of the time point when cortisol levels were expected to peak.
Participants who exhibited an increase of at least 0.055 µg/dL from the “pre-CPS” to the “15 min post-CPS sample” were classified as “CPS Cortisol Responders;” all other participants in the CPS condition were classified as “CPS Cortisol Non-Responders.” This criterion was established by analyzing cortisol responses to CPS or the control procedure in over 300 participants from studies in our lab (unpublished data). Based on this criterion, any control participant exhibiting an increase ≥ 0.055 µg/dL was excluded from the experiment prior to the final analyses; thus, control participants are referred to as “Cortisol Control Non-Responders.”
Statistical Analysis
We used chi-square tests of independence to determine whether the number of sAA Responders and CPS Cortisol Responders differed between NC and HC women. Changes in sAA and cortisol were assessed between subjects using one-way analysis of variances (ANOVAs); differences in pre- and post-stressor levels of sAA were assessed within subjects using the same statistical test. We used regression analyses to determine the relationship between baseline levels of stress hormones and responses to the stressors in NC and HC women. Recall scores were analyzed using two-way ANOVAs with contraceptive status and cortisol response (CPS Cortisol Responder v. Cortisol Control Non- Responder) as the between subjects factor. We further used three-way ANOVAs, with contraceptive status, sAA response (Responder v. Non-Responder), and cortisol response (CPS Cortisol Responder v. Cortisol Control Non-Responder) as independent factors, to test for differences in recall for positive and negative images. We used one-way ANOVAs to assess whether there were differences between NC and HC women in their PANAS, BEM, and Mehrabian test scores.
Results
IAPS Ratings and Cognitive Questionnaires
There were no significant differences between the standard ratings for the IAPS images and the subjective ratings made by our participants; also, there were no significant differences between NC and HC women in their subjective ratings of the images. These two groups of women also did not differ significantly on the PANAS, the BEM, or the Mehrabian questionnaires given prior to the experiment.
sAA and Cortisol Reactivity
sAA Reactivity
With respect to the number of sAA Responders and Non-Responders to the IAPS images, NC women (n = 22 and n = 20, respectively) and HC women (n = 17 and n = 19, respectively) did not significantly differ from one another, X2(1, N = 78) = .21, n.s..
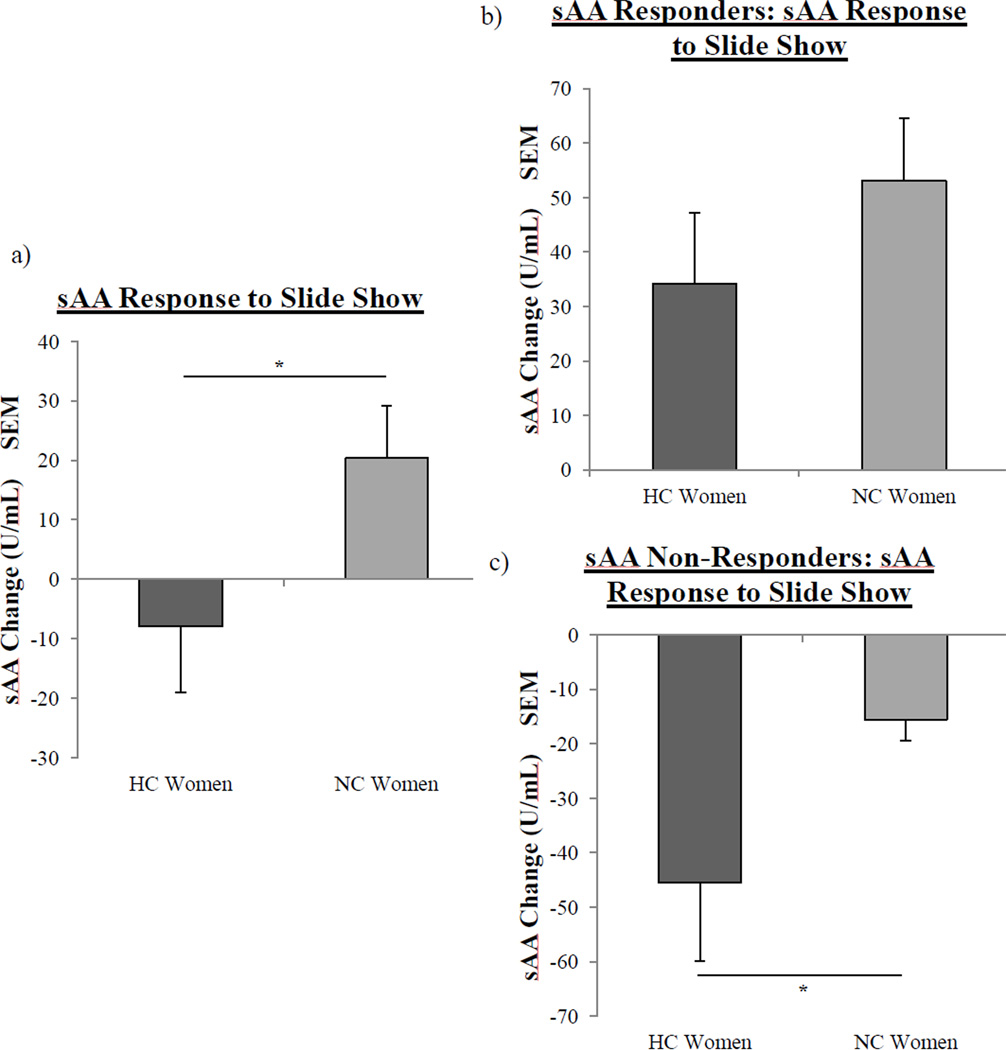
However, NC women (M = 20.4 ± 8.8 U/mL) exhibited a significantly larger sAA response (F(1, 76) = 4.1, p < .05; see Fig 1a) compared to HC women (M = −7.92 ± 11.1 U/mL) in response to the IAPS images. To better characterize the responses in NC and HC women, we examined whether sAA levels differed pre- and/or post-slideshow between the two groups; analyses showed that levels of sAA in NC and HC women did not differ at either time point. In addition, a median split analysis showed that pre-slideshow levels of sAA in NC and HC women did not differ between the “high” and “low” baselines.
Fig. 1.
Salivary alpha-amylase (sAA) responses to the IAPS images in NC and HC women. a, NC women (n = 42) had a significantly larger sAA response to the IAPS images (one asterisk, p < .05, one-way ANOVA) than HC women (n = 36) b, There were no significant differences in the sAA response of NC women (n = 22) and HC women (n = 17) that were sAA Responders. c, In the case of sAA Non-Responders, HC women (n = 19) exhibited a significantly larger decrease in sAA compared to NC women (n = 20) (one asterisk, p < .05). Values ± s.e.m.
However, regression analyses revealed a significant negative correlation between pre-slideshow sAA level and sAA response to images in HC women (F(1,34) = 34.9, p < .0001; R2 = −0.51); no relationship was observed in NC women. The correlations of NC and HC women also differed significantly from one another (p < .05). Therefore, to further investigate these effects, we assessed differences in NC women and HC women that were sAA Responders and sAA Non-Responders.
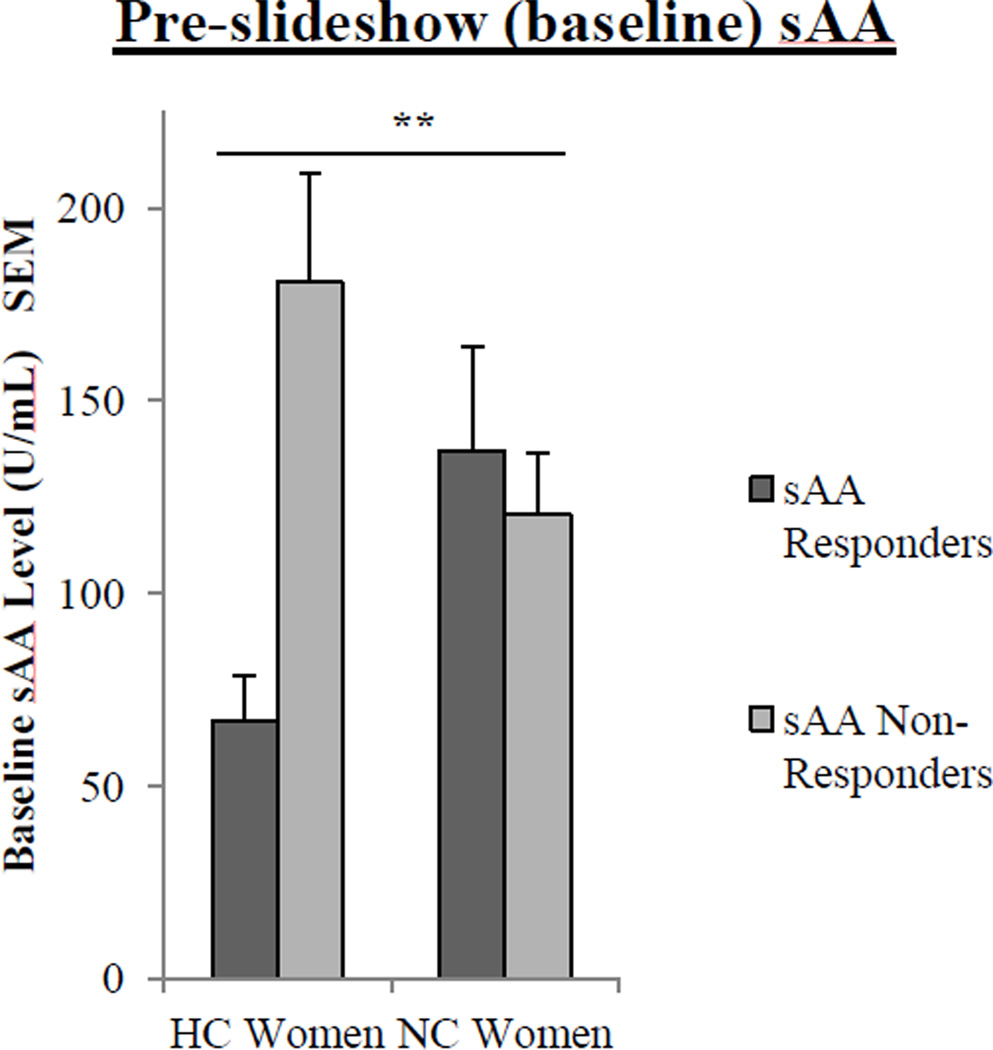
Among sAA Non-Responders, HC women exhibited a significantly larger decrease in their sAA response compared to NC women (F(1, 37) = 4.28, p < .05), but a similar effect was not detected among sAA Responders (see Fig 1b–c). In addition, with respect to pre-slideshow (baseline) levels of sAA, there was a significant interaction between contraceptive status (NC v. HC) and sAA response (sAA responder v. non-responder) (F(2,76) = 8.09, p < .01; Fig 3). Since the sAA Responder/Non-Responder classification was determined by a change in sAA level (pre- to post-slideshow), we next assessed whether sAA levels at these time points differed between sAA Responders and Non-Responders depending on contraceptive status.
Fig. 3.
Pre-slideshow (baseline) salivary alpha-amylase (sAA) levels in HC and NC women who are sAA Responders and sAA Non-Responders. For pre-slideshow sAA levels, a two-way ANOVA revealed a significant interaction between contraceptive status and sAA response to the images (two asterisks, p < .01). Values are means ± s.e.m.
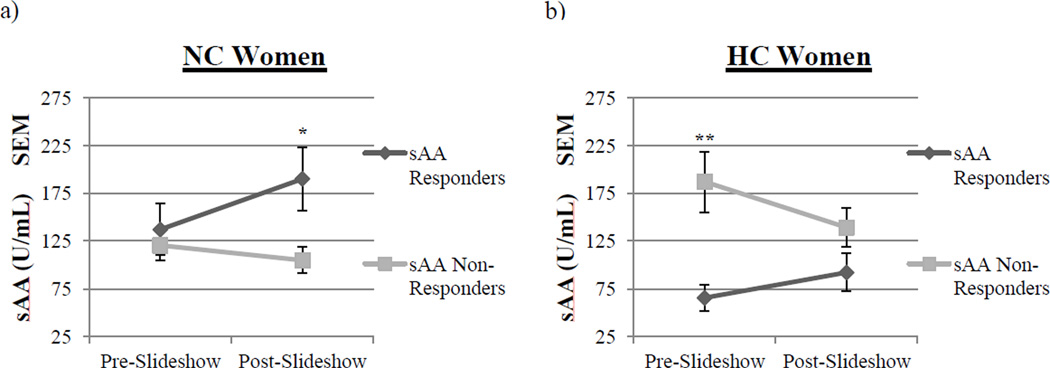
For NC women, there were no differences in pre-slideshow sAA levels between the sAA Responders and Non-Responders (Fig 2a). However, post-slideshow, NC women that were sAA Responders (M = 190.02 ± 32.9 U/mL) had significantly higher sAA levels compared to sAA Non-Responders (M = 104.74 ± 13.7 U/mL; F(1, 40) = 5.35, p < .05; see Fig 2a).
Fig. 2.
Salivary alpha-amylase (sAA) levels in sAA Responders and sAA Non-Responders preand post-slideshow. a, Pre-slideshow, NC women exhibited no differences in the levels of sAA. However, post-slideshow, NC women that were sAA Responders (n = 22) had significantly higher levels of sAA than sAA Non-Responders (n = 20, one asterisk, p <.05, one-way ANOVA). Values are means ± s.e.m. b, Pre-slideshow, HC women that were sAA Non- Responders (n = 19) had significantly higher levels of sAA compared to sAA Responders (n = 17; two asterisks, p <.01, one-way ANOVA). However, post-slideshow, HC women exhibited no differences in levels of sAA. Values are means ± s.e.m.
In HC women, however, we saw a very different relationship; there were no differences between sAA Responders and Non-Responders in post-slideshow sAA levels (Fig 2b). However, pre-slideshow, sAA Non-Responders (M = 180.7 ± 28.6 U/mL) had significantly higher sAA levels compared to Responders (M = 66.9 ± 11.8 U/mL; F(1, 34) = 12.47, p < .01; see Fig 2b). These data suggest that baseline sAA levels strongly influenced the sAA response to the images in HC women, but not NC women.
Cortisol reactivity
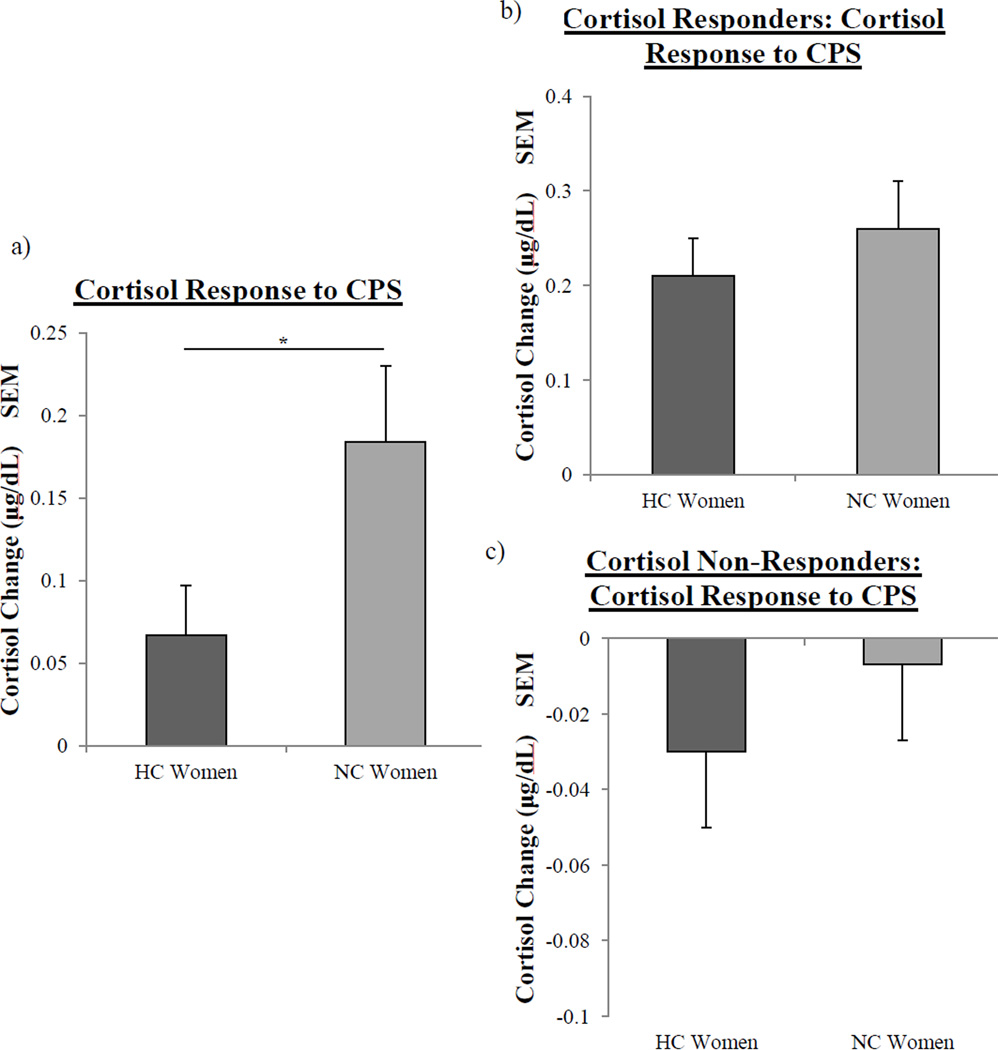
Of the CPS participants, 17 (71%) NC women and 9 (39%) HC women displayed a cortisol increase of 0.055 µg/dL in response to the physical stressor (i.e. from cortisol at baseline (“pre-CPS”) to the 15 minutes post-CPS sample); the number of CPS Cortisol Responders in each group differed significantly, X2(1, N = 47) = 4.86, p < .05, suggesting that NC women were more likely to respond to CPS than HC women.
In addition, NC women had a significantly larger cortisol response to CPS compared to HC women (F(1, 45) = 4.56, p < .05; see Fig 4a). Since the cortisol response is a change between pre-CPS cortisol and 15 min post-CPS cortisol, we examined whether cortisol levels in NC and HC women differed pre-CPS and/or 15 min post-CPS; analyses showed that levels of cortisol in NC and HC women did not differ at either time point. However, regression analyses revealed a significant negative correlation between pre-CPS cortisol level and cortisol response to CPS in HC women (F(1,21) = 8.14, p < .01; R2 = −0.28); a significant positive correlation was observed in NC women (F(1,22) = 8.07, p < .01; R2= 0.27). The correlations of NC and HC women were near significantly different from one another (p = .07). Therefore, we next assessed whether these effects were driven by CPS Cortisol Responders or CPS Cortisol Non-Responders.
Fig. 4.
Salivary cortisol responses to Cold Pressor Stress in NC and HC women. a, NC women (n = 24) had a significantly larger cortisol response to Cold Pressor Stress (one asterisk, p < .05, one-way ANOVA) than HC women (n = 23) b, There were no significant differences in the cortisol response of NC women (n = 17) and HC women (n = 9) that were CPS Cortisol Responders. c, There were no significant differences in the cortisol response of NC women (n = 7) and HC women (n = 14) that were CPS Cortisol Non-Responders. Values ± s.e.m.
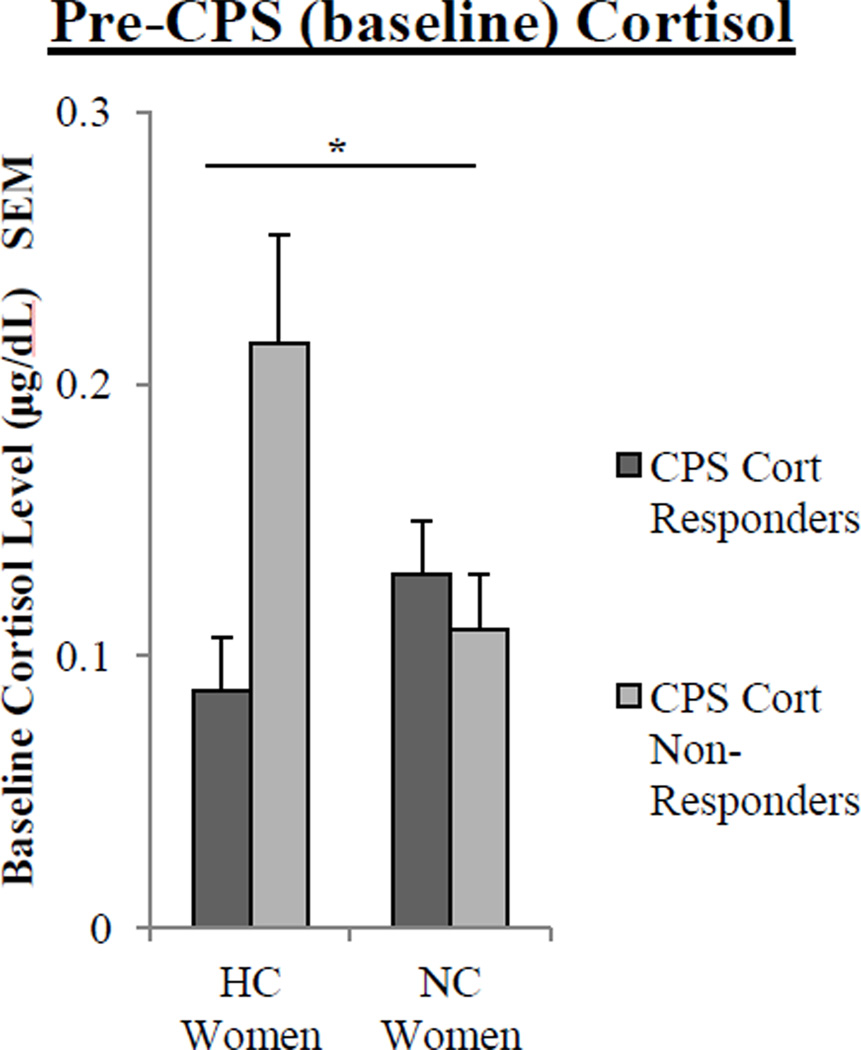
Although we did not observe differences in cortisol responses between CPS Cortisol Responders and CPS Cortisol Non-Responders (Fig 4b–c), there was a significant interaction between contraceptive status and cortisol response (CPS Cortisol Responder v. CPS Cortisol Non-Responder) for pre-CPS (baseline) levels of cortisol (F(2,45) = 5.2, p < .05; Fig 6). Therefore, we assessed whether there were differences in cortisol levels at different time points in CPS Cortisol Responders and CPS Non-Responders depending on contraceptive status.
Fig. 6.
Pre-CPS (baseline) salivary cortisol levels in HC and NC women who are CPS Cortisol Responders and CPS Cortisol Non-Responders. For pre-CPS cortisol levels, a two-way ANOVA revealed a significant interaction between contraceptive status and cortisol response to the CPS (one asterisk, p < .05). Values are means ± s.e.m.
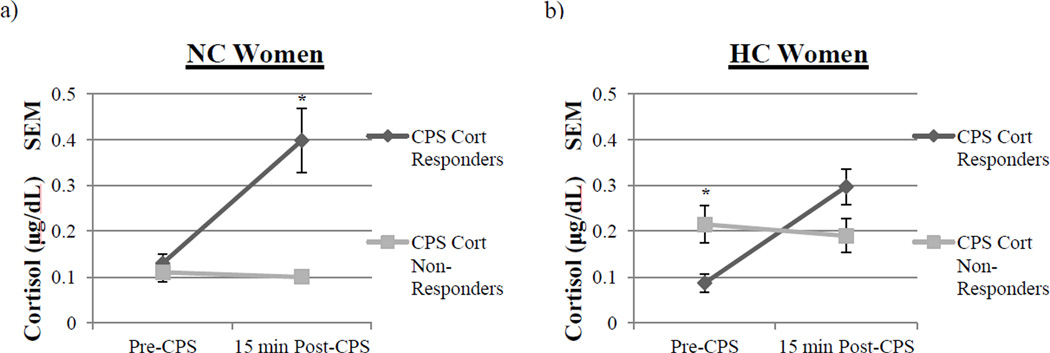
For NC women, there were no differences in pre-CPS cortisol levels between the CPS Cortisol Responders and Non-Responders (Fig 5a). However, for cortisol levels 15 min post- CPS, NC women that were CPS Cortisol Responders (M = 0.398 ± 0.07 µg/dL) had significantly higher cortisol levels compared to CPS Cortisol Non-Responders (M = 0.10 ± .01 µg/dL; F(1, 22) = 7.05, p < .05; see Fig 5a).
Fig. 5.
Cortisol levels in CPS Cortisol Responders and CPS Cortisol Non-Responders pre- and post-CPS. a, Pre-CPS, NC women exhibited no differences in the levels of cortisol. However, post-CPS, NC women that were CPS Cortisol Responders (n = 17) had significantly higher levels of cortisol than CPS Cortisol Non-Responders (n = 7, t, p < .05, one-way ANOVA). Values are means ± s.e.m. b, Pre-CPS, HC women that were CPS Cortisol Non-Responders (n = 14) had significantly higher levels of cortisol compared to CPS Cortisol Responders (n = 9; two asterisks, p < .05, one-way ANOVA). However, post-slideshow, HC women exhibited no differences in levels of cortisol. Values are means ± s.e.m.
In HC women, however, we observed a very different relationship. Pre-CPS, HC women that were CPS Cortisol Non-Responders (M = 0.22 ± .04 µg/dL) had significantly higher cortisol levels compared to CPS Cortisol Responders (M = .087 ± .02 µg/dL; F(1, 21) = 5.56, p < .05; see Fig 5b). However, there were no differences between CPS Cortisol Responders and Non- Responders in 15 min post-CPS cortisol levels (Fig 5b). These data suggest that baseline cortisol levels strongly influenced the cortisol response to CPS in HC women, but not NC women.
Memory findings
To assess recall differences between NC and HC women, we examined memory performance depending on contraceptive status, sAA response to the slideshow, and/or cortisol response to the CPS or control condition. Although our cortisol reactivity analyses explored differences between CPS Responders and CPS Non-Responders, our memory analyses focused on recall performance in those exposed to the stress condition (CPS) or the no-stress control condition. Thus, the factors in the following memory analyses include contraceptive status (NC v. HC women), sAA response (sAA Responder v. sAA Non-Responder), condition (CPS v. Control), and/or cortisol response (CPS Cortisol Responders v. Cortisol Control Non- Responders).
Influence of contraceptive status on memory
We first examined whether contraceptive status influenced memory performance, irrespective of sAA and cortisol responses. A series of one-way ANOVAs showed that NC and HC women did not differ in their total slide recall (n.s.) or their recall of positive (n.s.), negative (n.s.), and neutral images (n.s.). Based on these results, our subsequent analyses examined the influence of contraceptive status, sAA response, stress condition, and cortisol response on memory performance.
Influence of contraceptive status and stress condition on memory
A repeated measures ANOVA (contraceptive status×condition×Valence) revealed a significant main effect of valence (F(2,73) = 8.49, p < .01) and a trend toward a significant interaction between valence, contraceptive status and condition (F(2,73) = 2.84, p =.065) on recall. Since the CPS condition in this analysis was comprised of cortisol responders and cortisol non-responders, the trend toward a significant interaction may have reflected the variability in the cortisol response. We performed a second repeated measures ANOVA (contraceptive status×cortisol response×Valence); the analysis revealed a significant main effect of valence (F(2,52) = 5.12, p < .01) and a near significant interaction between valence, contraceptive status and cortisol response (F(2,52) = 3.03, p = .057). Therefore, our subsequent memory analyses used cortisol response as an independent variable rather than stress condition.
Influence of contraceptive status, sAA response and cortisol response on memory
An analysis of recall performance examined the effects of sex/stress hormone interactions on recall for all images. Separate three-way ANOVAs, with contraceptive status, sAA response, and cortisol response as independent factors, revealed no significant three-way interactions on total recall, negative recall, or neutral recall performance. However, for positive recall, a three way ANOVA with these independent factors revealed a significant main effect of cortisol response (F(1,55) = 5.22, p < .05) and a significant interaction between contraceptive status and cortisol response (F(2,54) = 4.41, p < .05). These effects suggest that recall for positive images may not only be altered by glucocorticoid responses but also by contraceptive use. Therefore, our subsequent set of analyses explored the influence of contraceptive use and stress responses on memory for positively, negatively, and neutrally valenced images.
There were no significant main effects or interactions between contraceptive status and sAA response on recall for positive or negative images. However, a two-way ANOVA with contraceptive status and cortisol response as independent factors revealed a significant main effect of cortisol response (F(1, 55) = 5.34, p < .05) and a significant interaction (F(2, 54) = 4.46, p < .05) on recall for positive images. There were no significant main effects or interactions for recall of negatively valenced images. Based on these results, we further explored the influence of sAA and cortisol responses on memory separately in HC and NC women.
For NC women, there were no significant main effects or interactions between sAA response and cortisol response on recall for neutral, negatively or positively valenced images. However, for HC women, there was a trend toward a significant interaction between sAA response and cortisol response (F(2, 20) = 3.32, p =.085) for recall of negatively valenced images. No effects or interactions were observed for positively or neutrally valenced images.
Since previous research has demonstrated that norepinephrine release at encoding is associated with recall of emotional stimuli, we examined memory for negatively, positively, and neutrally valenced images in all sAA Responders/sAA Non-Responders depending on contraceptive status and cortisol response.
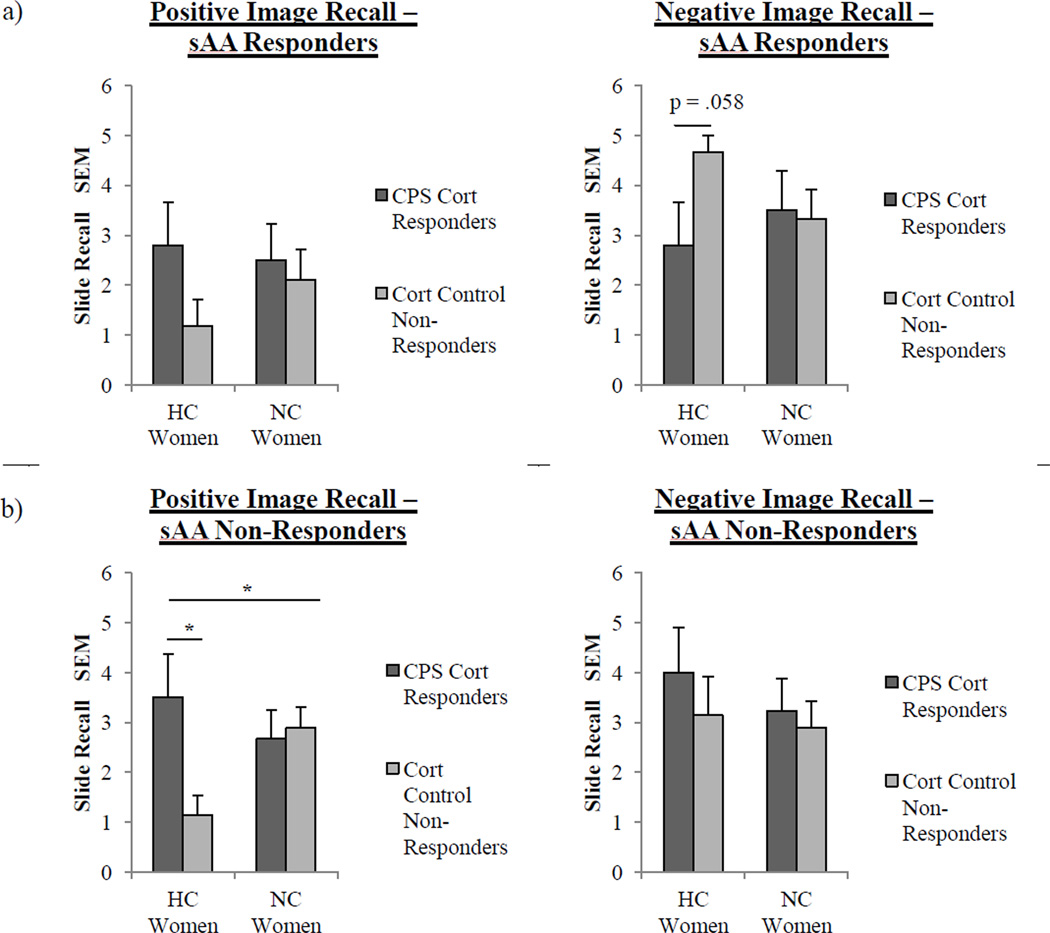
In sAA Responders, we found no main effects or significant interactions with respect to total recall, memory for positively valenced images (Fig 7a), or memory for neutral images (Fig 7a). However, with respect to memory for negatively valenced images, we found a significant interaction between contraceptive status and cortisol response (F(2, 26) = 3.28, p = .08; see Fig 7a). HC women who were Cortisol Control Non-Responders recalled near significantly more negative images (M = 4.67 ± 0.33) than HC women that were CPS Cortisol Responders (M = 2.8 ± 0.86) (F(1, 9) = 4.72, p = .057). We also found that NC women that were CPS Cortisol Responders (M = 2.88 ± 0.51) recalled significantly more neutral images than NC women that were Cortisol Control Non-Responders (1.56 ± 0.34; F(1,15) = 4.79, p < .05).
Fig. 7.
Recall performance for neutral, positively, and negatively valenced images in sAA Responders and sAA Non-Responders. a, sAA Responders: There were no significant effects or interactions between cortisol response and contraceptive use on memory for positively valenced images. However, for negatively valenced image recall, two way-ANOVA revealed a trend toward an overall significant interaction between cortisol response (CPS Cortisol Responder v. Cortisol Control Non-Responder) and contraceptive status (p = .08). Also, HC women that were Cortisol Control Non-Responders (n = 6) recalled near significantly the most negatively valenced images compared to HC women that were CPS Cortisol Responders (n = 5; p = .058, one-way ANOVA). Values ± s.e.m. b, sAA Non-Responders: There were no significant effects or interactions between cortisol response and contraceptive use for negatively valenced images. However, for positively valenced image recall, a two way-ANOVA revealed an overall significant interaction between cortisol response (CPS Cortisol Responder v. Cortisol Control Non-Responder) and contraceptive status (one asterisk, p < .05). Also, HC women that were CPS Cortisol Responders (n = 4) recalled significantly more positively valenced images compared to HC women that were Cortisol Control Non-Responders (n = 7; one asterisk, p < .05, one-way ANOVA). Values are means ± s.e.m.
In sAA Non-Responders, we found no main effects or significant interactions with respect to total recall, memory for negatively valenced images, or memory for neutral images (Fig 7b). However, in the case of memory for positively valenced images, we found a significant interaction between contraceptive status and cortisol response (F(2, 27) = 5.13, p < .05; see Fig 7b). In addition, HC women who experienced a post-training cortisol release (M = 3.5 ± 0.87) recalled significantly more positive images than HC women who did not (M = 1.14 ± 0.4) (F(1, 9) = 8.03, p < .05).
These results suggest that sAA and cortisol influence memory for emotionally arousing stimuli differently depending on the valence of the stimuli. Additionally, the influence of these stress hormones on emotional memory is significantly different in HC and NC women.
Discussion
The present findings indicate that use of hormonal contraception is associated with altered noradrenergic and glucocorticoid responses to stressors. HC women exhibited a significantly blunted cortisol response to Cold Pressor Stress as compared to NC women, consistent with previous research demonstrating that women on hormonal contraception have significantly blunted cortisol responses to a psychosocial stressor, the Trier Social Stress Test (TSST; Kirschbaum et al., 1999). In addition, the present study showed that in HC, but not NC, women, the cortisol response to CPS depended on pre-CPS (baseline) cortisol levels. Higher baseline cortisol levels blunted the cortisol response to CPS in HC women, but not in NC women.
Thus, these results suggest that a relationship exists between baseline cortisol levels and cortisol reactivity to a stressor in HC women that does not exist in naturally cycling women. This is true despite the fact that hormonal contraception use did not increase overall baseline levels of cortisol compared with NC women. Rather, it appears to have altered the relationship between stress and the cortisol response in a subset of HC women, namely, those with baseline cortisol levels in the higher end of the normal range. The findings further indicate that the blunted cortisol response seen in HC women in previous studies stemmed not from reduced cortisol activation to a stressor, as one might imagine, but from a more rapid decrease in cortisol levels across the measurement period in those subjects who failed to show any cortisol activation to the stressor.
A highly similar pattern of results was obtained when we examined the noradrenergic response to the emotional images. As was the case with cortisol’s response to CPS, we found that HC women had a significantly blunted overall noradrenergic response (as indexed by sAA) to the images as compared to NC women. Also, as was the case for cortisol, the overall blunted sAA response stemmed not from reduced sAA activation in those HC women who responded, but in a more rapid decline of sAA levels in those who did not. Furthermore, in HC women we observed a relationship between sAA baseline levels and sAA reactivity that was similar to that observed with cortisol. HC women with high baseline sAA levels failed to respond to the images, whereas those with lower baseline levels did respond. In contrast, no relationship was observed between sAA baseline levels and sAA reactivity in NC women. Note that hormonal contraception use appeared to alter the relationship between the baseline levels of noradrenaline and the noradrenergic response to a stressor without altering overall baseline noradrenergic activity. To our knowledge, this is the first study to demonstrate that the use of hormonal contraception can alter noradrenergic responding during the encoding of emotionally arousing material.
We observed in HC women a negative relationship between baseline levels of both norepinephrine (indexed by sAA) and cortisol and responses to the images and CPS, respectively. Although overall baseline levels of both norepinephrine and cortisol were the same in NC and HC women, our data indicate that a subset of HC women with high baseline stress hormone levels responded to the stressors in a manner not seen in NC women with similar baseline stress hormone levels. How/why HC alters the stress response in the high-baseline subset of women is unknown, but appears to be a very important question for future work.
A secondary goal of this study was to explore stress hormone effects on emotional memory in HC and NC women. To date, no studies have explored the interactions between norepinephrine at encoding, post-training release of glucocorticoids, and contraceptive status on emotional memory consolidation. We previously hypothesized that since hormonal contraception alters levels of endogenous estrogen and progesterone as well as hormone responses to different stressors, sex/stress hormone interactions shown to affect emotional memory and amygdala function are likely to be affected (Andreano and Cahill, 2010; van Wingen et al, 2008). Results from the present study support this hypothesis in that the influence of stress hormones on emotional memory depended on both contraceptive status and valence of the stimuli.
HC and NC women exhibited differential recall of positively and negatively valenced emotional images. This difference in emotional memory depended on their noradrenergic response to the images and their glucocorticoid response to CPS or the control condition. Previous research has indicated that arousal at encoding and post-training glucocorticoids interact to enhance emotional memory (Roozendaal et al., 2006a, 2006b; Buchanan and Lovallo, 2001; Cahill and Alkire, 2003); however, we observed a different pattern in both NC and HC women.
In NC women, we observed no emotional memory enhancement for negatively or positively valenced stimuli, regardless of their noradrenergic response at encoding or cortisol release post-training. A possible explanation may be that there were menstrual cycle influences on the effects of these stress hormone interactions on emotionally arousing memory. We collected self-report menstrual cycle data, but we were unable to verify menstrual cycle position by salivary assays for endogenous estrogen and progesterone. Therefore, we did not account for menstrual cycle phase in our analyses. Also, the present study was intended to broadly assess differences between naturally cycling women and women on hormonal contraception. However, future studies should explore whether norepinephrine at encoding and post-training cortisol influence emotional memory differently for women in the follicular (low estrogen/progesterone) phases and luteal (high estrogen/progesterone) phase.
While we did not find an emotional memory enhancement in NC women, we did observe a memory enhancement for neutral images. NC women who were sAA Responders/CPS Cortisol Responders recalled more neutral images compared to sAA Responders/Cortisol Control Non-Responders. While this result was unexpected, previous work with both rodents (Roozendaal et al., 2006a)) and humans (Abercrombie et al., 2003; Rimelle et al., 2003; Schwarze et al., 2012) suggests that memory for neutral material can be enhanced when arousal at encoding interacts with a post-training release of glucocorticoids. In these studies, however, the arousal was not inherent to the stimuli, but rather, the experimental context (Roozendaal et al., 2006a).
Our data supports this notion; in NC women, the arousal associated with the emotional images seemed to be sufficient to interact with a CPS-induced cortisol release to enhance memory for neutral images. Future work will need to examine whether this phenomenon in NC women is influenced by menstrual cycle phase and if it exists under different experimental parameters (i.e. arousal at encoding induced by nociceptive stimuli as opposed to emotionally arousal images).
Our analyses showed that in HC women, memory for negative images was near significantly enhanced when norepinephrine was released at encoding in the absence of a post-training cortisol release. However, recall of positive images was significantly enhanced when cortisol was released post-training in the absence of a norepinephrine release at encoding. These results suggest that in HC women, norepinephrine at encoding and cortisol released post-training do not interact to enhance emotional memory. Instead, in HC women these stress hormones seem to act independently to enhance memory for emotional material depending on the valence of the stimulus. Clearly further studies are needed to further document and clarify these implications of HC use for stress hormone interactions and emotional memory.
This study is only the second to investigate the influence of hormonal contraception on emotional memory, and the results are consistent in several ways with those of our previous findings (Nielsen et al., 2011). Comparing recall of an emotional versus neutral story, we previously found that women on hormonal contraception had enhanced memory for central information, but not peripheral details, from a negative emotional story; whereas naturally cycling women exhibited enhanced memory for peripheral details but not the gist from the same story. The present data are consistent with this finding in that we again found enhanced recall of emotionally negative information in HC women who experienced noradrenergic activation, and no cortisol activation, while viewing the stimuli. The present study was not designed to address the issue of retention of gist versus detail addressed by Nielsen et al. (2011).
Nielsen et al. (2011) also showed that the memory differences between women on hormonal contraceptives and naturally cycling women could not be attributed to differences in arousal as both groups had equivalent pupil dilation changes (pre- to post-slideshow changes in pupil diameter) in response to the emotional story. In the current study, HC women overall did exhibit a blunted noradrenergic (sAA) response to the slide show compared to naturally cycling women; however, there was no difference in the noradrenergic response between the HC and NC women who were “sAA Responders.” Thus, as was the case in our previous study, the differential recall of HC and NC women in the “sAA Responders” cannot easily be attributed simply to a differential emotional, or stress, reaction to the emotional material.
There are several limitations to this study. First, HC women were self-selected users, and as a result, the findings could potentially undermine any mood or cognitive differences between NC and HC women (Mordecai, Rubin, and Maki, 2008). However, it should be noted that there were no significant differences on the cognitive measures given in this study.
Another caveat to this study is that we did not account for whether HC women were tested during the “on” or “off” active contraception weeks. This may have important implications as research has shown differential memory effects in contraceptive users that were in “on” or “off” weeks (Mordecai, Rubin, and Maki, 2008). Future studies on how hormonal contraception affects sex/stress hormone interactions and subsequently, emotional memory, should account for this and explore possible differences between the two groups.
In spite of these limitations, this study would appear to have important implications for research on neurobiological mechanisms of emotional memory and disorders associated with learning and memory for emotional events (eg. PTSD). The present findings demonstrate that women on hormonal contraception not only have altered noradrenergic and glucocorticoid responses to different stressors compared to naturally cycling women; but in these women, the data also suggest that norepinephrine at encoding and post-training cortisol act independently to enhance emotional memory depending on the valence of the stimulus. These results suggest that hormonal contraception alters emotional memory processes, and more specifically, those pertaining to consolidation. Thus, to better understand the underlying mechanisms of these observed phenomena, future work should further investigate how combined contraceptive use specifically alters the neurobiology of emotional memory. These future studies would not only contribute to our knowledge of the cognitive effects of hormonal contraceptive use; they would also facilitate a better understanding of emotional memory consolidation.
Highlights.
-
>
We examine effects of hormonal contraception on stress responses and emotional memory
-
>
Contraceptive users had blunted responses to stressors versus naturally cycling Women
-
>
Contraceptive use reduces norepinephrine and cortisol responses to acute Stressors
-
>
Emotional recall depended on contraceptive use and stress hormone responses
-
>
Contraceptive use alters memory for emotional material depending on stress Responses
Acknowledgements
This work was supported by NIMH 575082 to LC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionall laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Denburg NL, Tranel D. The amygdala’s role in long-term declarative memory for gist and detail. Behavioral Neuroscience. 2001;115(5):983–992. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Andreano J, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33(6):874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning and Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53:1286–193. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem SL. Bem sex-role inventory. Redwood City, CA: Consulting Psychologists Press, Inc; 1981. [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning and Memory. 2006;13(3):382–387. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Chatterton RTJ, Wogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clinical Psychology. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Tran TP, Fong WC, Bryant RA. Sex differences in emotional memory consolidation: the effect of stress-induced salivary alpha-amylase and cortisol. Biological Psychology. 2012;89(3):539–544. doi: 10.1016/j.biopsycho.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Fujimoto NY, Willanueva AL, Hopper B, Moscinski M, Rebar RW. Increased adrenocortical responsiveness to exogenous ACTH in oral contraceptive users. Advances in Contraception. 1986;2:343–353. doi: 10.1007/BF02340051. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabireli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamuspituitary- adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: influence of menstrual cycle and oral contraceptives. Psychopharmacology. 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wüst S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32(8–10):1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville: The Center for Research in Psychophysiology, University of Florida; 1997a. [Google Scholar]
- Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. American Journal of Obstetrics and Gynecology. 1994;170:1499–507. doi: 10.1016/s0002-9378(94)05011-8. [DOI] [PubMed] [Google Scholar]
- Mehrabian A. Manual for the revised trait arousability (converse of stimulus screening) scale. University of California, Los Angeles. 1994:1–10. [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Hormones and Behavior. 2008;54:286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YL, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of Learning and Memory. 2011;96:378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterstetter O, Szymanski LM, Kamimori GH, Kessler CM, Gold MR, Fernhall B. Hemeostatic responses to maximal exercise in oral contraceptive users. American Journal of Obstetrics and Gynecology. 1999;181(4):958–963. doi: 10.1016/s0002-9378(99)70332-7. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Biggio G, Concas A. Oral contraceptives and neuroactive steroids. Pharmacology, Biochemistry, and Behavior. 2006;84:628–634. doi: 10.1016/j.pbb.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Rimelle U, Domes G, Mathiak K, Houtzinger M. Cortisol has different effects on human memory for emotional and neutral stimuli. Cognitive Neuroscience and Neuropsychology. 2003;14(18):2485–2488. doi: 10.1097/00001756-200312190-00038. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006a;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences USA. 2006b;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze U, Bingel U, Sommer T. Event-related nociceptive arousal enhances memory consolidation for neutral scenes. The Journal of Neuroscience. 2012;32(4):1481–1487. doi: 10.1523/JNEUROSCI.4497-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic in the human amygdala. Neurobiology of Learning and Memory. 2007;87(1):57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joёls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiology of Learning and Memory. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry. 2008;13(3):325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]