Abstract
Active cognitive control of working memory is central in most human memory models, but behavioral evidence for such control in nonhuman primates is absent and neurophysiological evidence, while suggestive, is indirect. We present behavioral evidence that monkey memory for familiar images is under active cognitive control. Concurrent cognitive demands during the memory delay impaired matching-to-sample performance for familiar images in a demand-dependent manner, indicating that maintaining these images in memory taxed limited cognitive resources. Performance with unfamiliar images was unaffected, dissociating active from passive memory processes. Active cognitive control of memory in monkeys demonstrates that language is unnecessary for active memory maintenance.
Keywords: primate cognition, rehearsal, cognitive control, short-term memory, familiarity
1. Introduction
Human working memory can be compared to the display on an airport x-ray machine. Only a few bags can be viewed simultaneously and images of new baggage displace older images unless an operator exerts active control to freeze or manipulate the current view. Current models of human working memory differ in many aspects, but agree that the defining characteristic of working memory is active cognitive control (e.g., Baddeley, 2003; Cowan, 2008). Information is rapidly lost unless actively maintained, such as by verbal rehearsal in a “phonological loop” (Baddeley, 2003). Because maintenance by top-down cognitive control consumes limited resources, cognitive operations that compete for these resources cause forgetting in a demand-dependent manner. For example, the comparatively difficult task of deciding whether two abstract shapes are identical impairs memory performance more than does passively viewing the same shapes (Logie, 1986). Adding numbers impairs memory performance more than passively viewing numbers (Phillips & Christie, 1977). Cognitive control over working memory is likely a major factor in general intelligence (Unsworth & Engle, 2007), and may account for many cognitive differences between humans and nonhumans (Wynn & Coolidge, 2004). Thus, cognitive control is a critical and defining feature of human working memory.
Researchers have made substantial progress characterizing the capacity (Elmore et al., 2011; Heyselaar, Johnston, & Pare, 2011) and neural substrates (Constantinidis, Franowicz, & Goldman-Rakic, 2001; Fuster & Alexander, 1971; Heuer & Bachevalier, 2011; Miller, Erickson, & Desimone, 1996) of short-term memory in nonhuman primates. But it is unclear whether these studies characterize a cognitively-controlled system similar to human working memory (Washburn & Astur, 1998). The definitions of working memory in humans and nonhumans often differ. In the human literature, definitions of working memory focus on cognitive control (Baddeley, 2003; Cowan, 2008). In the nonhuman literature, working memory is often operationalized as memory relevant only to the current trial, as opposed to reference memory for the rules of the task (Shettleworth, 1998, chapter 6). Other criteria for identifying working memory can also lead to confusion. For example, working memory is not equivalent to short-term memory (Jeneson & Squire, 2012). Humans can use working memory over relatively long delays if rehearsal is not interrupted (Milner, 1970), and short-delay memory tasks can require long-term memory if the amount of to-be-remembered information exceeds working memory capacity (Hannula, Tranel, & Cohen, 2006; Jeneson, Mauldin, Hopkins, & Squire, 2011). Additionally, localized brain activity should not be uncritically equated with specific cognitive processes (Uttal, 2001). Cells in the prefrontal cortex of monkeys fire when monkeys view to-be-remembered images and continue to fire during the memory interval (Constantinidis et al., 2001; Fuster & Alexander, 1971; Miller et al., 1996). It is tempting to equate this monkey neural activity with human working memory based on fMRI studies that find activation of prefrontal cortex associated with active working memory in humans (D’esposito, Postle, Ballard, & Lease, 1999; Stern, Sherman, Kirchhoff, & Hasselmo, 2001). But this equation ignores the potential for cognitive differences between species. It is possible that monkeys and humans remember information differently even when performance or neural activity is superficially similar. For example, the inference of active working memory based on prefrontal activity is empirically contradicted by the fact that prefrontal activity is also found in experimentally naïve monkeys during passive viewing of images (Meyer, Qi, & Constantinidis, 2007). Resolving these ambiguities will require more definitive behavioral methods for assessing cognitive control in monkey working memory.
Surprisingly, there is no strong behavioral evidence for cognitively-demanding memory maintenance in monkeys. In humans, memory performance is impaired by performing a distractor task and more cognitively-demanding distractor tasks produce more impairment (Logie, 1986; Phillips & Christie, 1977), demonstrating that working memory requires limited cognitive resources. In monkeys, distractors presented during the memory interval, such as flashing lights (Prendergast et al., 1998), irrelevant images (Miller & Desimone, 1993; Miller et al., 1996), or a motor task (Washburn & Astur, 1998), can impair memory performance. However, unlike the case in humans, distractor tasks that required sustained activity and attention produced no more impairment than ones that only required passive viewing (Washburn & Astur, 1998). This indicates that the performance impairment in monkeys caused by these distractors was due to passive displacement of information rather than by competition for limited cognitive resources used to maintain information in working memory. Related investigations have tested for active control of memory in monkeys using directed forgetting paradigms or by providing opportunities for “rehearsal” of studied images. In humans, these approaches demonstrate cognitive control (Hourihan, Ozubko, & Macleod, 2009; Wright et al., 1990), but in monkeys they have not (Cook, Wright, & Sands, 1991; Washburn & Astur, 1998).
Previous tests may not have found evidence for active memory maintenance in monkeys because of the limited range of conditions under which these tests were conducted. Not all types of memory require active maintenance. For example, familiarity alone can support accurate recognition performance in many memory tests. Familiarity codes only whether stimuli have been seen previously (Yonelinas, 2002), and is an automatic, effortless process (Jacoby, 1991). In humans, the ability to distinguish items based on familiarity is unaffected by reduction in cognitive control by secondary tasks (Yonelinas & Jacoby, 1994) or intoxication (Bisby, Leitz, Morgan, & Curran, 2010). By contrast, when familiarity alone cannot support accurate performance, these manipulations do impair memory (Bisby et al., 2010; Yonelinas & Jacoby, 1994). Accordingly, we gave monkeys two memory tests that differed in the extent to which they could be solved by familiarity alone. In tests using a small set of familiar, frequently-repeating images (hereafter, familiar images), target images from previous trials were reused as distractors in later trials. This made all images highly familiar and created a high level of interference among test images, presumably making it almost impossible to distinguish target images from distractors based on relative familiarity. We hypothesized that active maintenance of memory for the target image would be required in these tests. We also administered control tests using a large set of unfamiliar, infrequently-repeating images (hereafter, unfamiliar images) from which recently studied targets could easily be discriminated from distractors at test based on relative familiarity. Thus, the critical difference between the familiar and unfamiliar image sets was whether monkeys could discriminate studied images from unstudied images based on familiarity.
2. Experiment 1: Primary findings
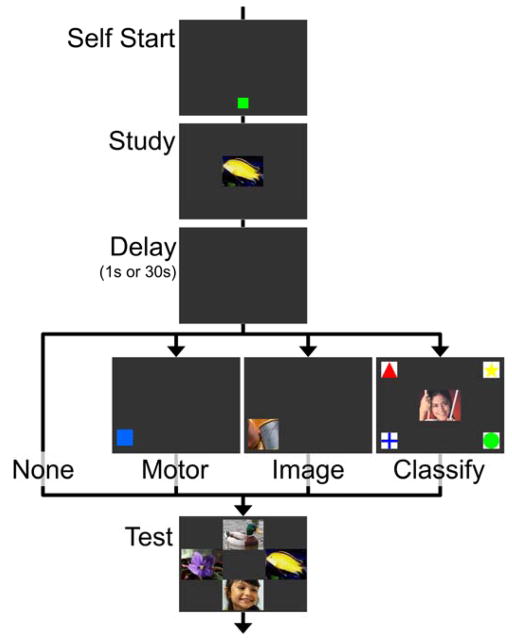
We presented monkeys with visual matching-to-sample recognition tests on touchscreen computers and required them to complete one of three distractor tasks during the memory interval (Fig. 1). The three tasks required the same motor response but varied in cognitive demand: 1) touch a blue square that appeared in a randomly-selected corner of the screen (motor only), 2) touch a photograph that appeared in a randomly-selected corner of the screen (motor + image perception), or 3) classify a photograph as depicting a bird, fish, flower, or person by touching the appropriate symbol in one of the four corners of the screen (motor + image perception + classification). Touching a uniform blue square should require the least cognitive processing. Viewing unfamiliar photographs may elicit more cognitive processing than viewing a blue square because the photograph is more visually complex and presumably more interesting. Finally, classifying photographs should require the most cognitive processing because the monkeys had to accurately assign the images to one of four categories to proceed to the memory test. If remembering required active maintenance of the studied image during the memory interval, accuracy should be impaired least by the motor task and most by the classification task. Passive retention should be unaffected by these manipulations of concurrent cognitive demand.
Figure 1. Memory tests with four levels of concurrent cognitive demand.
Monkeys were required to remember an image over a memory interval that was either empty, or filled by one of three tasks: 1) motor: touch a blue square, 2) image: touch a non-classifiable image, or 3) classify: classify a central image as a bird, fish, flower, or person by touching the corresponding symbol. Motor and image stimuli could appear in any of the four screen corners. All three concurrent tasks required the same motor response.
2.1 Methods
2.1.1 Subjects and apparatus
Six adult, male rhesus monkeys (Macaca mulatta; mean age = 8.2 years) experienced in matching-to-sample and classification tasks (Basile & Hampton, in press) were pair-housed except during testing, fed full food rations, and given ad libitum water access. Monkeys were tested in their home cages on portable touchscreen computers (Basile & Hampton, 2010). Procedures were approved by the Emory Institutional Animal Care and Use Committee and complied with US law.
2.1.2 Stimuli
Stimuli were color photographs of exemplars from categories the monkeys had previously learned to classify as birds, fish, flowers, or people. The to-be-remembered stimuli consisted of two sets: a small set of four images, highly-familiar from previous testing, and a large set of 1400 relatively-unfamiliar images. Each category was equally represented within each set. For the concurrent task, images for the classify condition were drawn from the large set of 1400 images, and non-classifiable images for the image condition consisted of a set of 400 relatively-novel images.
2.1.3 Procedure
Monkeys completed four 300-trial sessions, two with the small set of familiar images and two with the large set of unfamiliar images, alternating and counterbalanced for testing order across monkeys. Half the trials in each session contained no secondary task; the other half were equally divided among motor, image, and classification tasks. The four levels of cognitive demand, the four categories, and the four possible response locations were intermixed pseudorandomly within each session. To-be-classified images were never from the same category as the sample and were not presented as distractors for that trial. Trials proceeded as in Fig. 1, separated by a 10-sec ITI. Because matching-to-sample accuracy in monkeys is typically higher with large sets of unfamiliar images than with small sets of familiar images (Basile & Hampton, 2010; Mishkin & Delacour, 1975), we matched baseline performance by testing the large set of unfamiliar images at a 30-sec delay and the small set of familiar images at a 1-sec delay (values determined during pre-testing). At test, selection of the sample produced a positive audio stimulus and a 75% chance of food, whereas selection of a distractor produced a negative audio stimulus and a 2-sec timeout. To ensure that monkeys were attending to, and processing, the concurrent task, incorrect responses in the concurrent tasks aborted the trial. Proportions were arcsine transformed prior to statistical analysis (Aron & Aron, 1999).
2.2 Results and Discussion
The distraction tasks affected memory performance for the two image sets differently (Fig. 2; two-factor repeated measures ANOVA; interaction: F(3,15) = 57.83, p < .001, partial η2 = .920). Concurrent cognitive demand during the memory interval impaired performance for familiar images, but left performance for unfamiliar images intact (Fig. 2; one-factor repeated-measures ANOVA; familiar images: F(3,15) = 72.034, p < .001, partial η2 = .935; unfamiliar images: F(3,15) = 0.715, p = .558). The classification task took longer to complete than did either the motor or image tasks, which did not differ from each other (paired t-tests, two-tailed, Bonferroni corrected = 0.017; motor vs classify: t5 = 6.11, p = .002, d = 2.50; image vs classify: t5 = 6.20, p = .002, d = 2.53; motor vs image: t5 = 0.59, p = .58). Critically, the concurrent tasks that required more cognitive effort produced more impairment (paired t-tests, two-tailed, Bonferroni corrected = 0.017; none vs motor: t5 = 6.81, p = .001, d = 2.78; motor vs image: t5 = 2.17, p = .083; image vs classify: t5 = 9.05, p < .001, d = 3.69; note that five of six monkeys performed numerically worse after image than motor interference, but the group difference was not significant). The differential impairment was not due to the familiar images being harder to remember than the unfamiliar images, because accuracy with the two sets was matched using different memory intervals and did not differ when concurrent cognitive demand was absent (Fig. 2, t5 = 1.37, p = .230). Together, these results suggest that memory for familiar, but not unfamiliar, images was impaired by a concurrent cognitive demand in a demand-dependent manner.
Figure 2. Memory performance for familiar but not unfamiliar images is impaired by concurrent cognitive demand in a demand-dependent manner in monkeys.
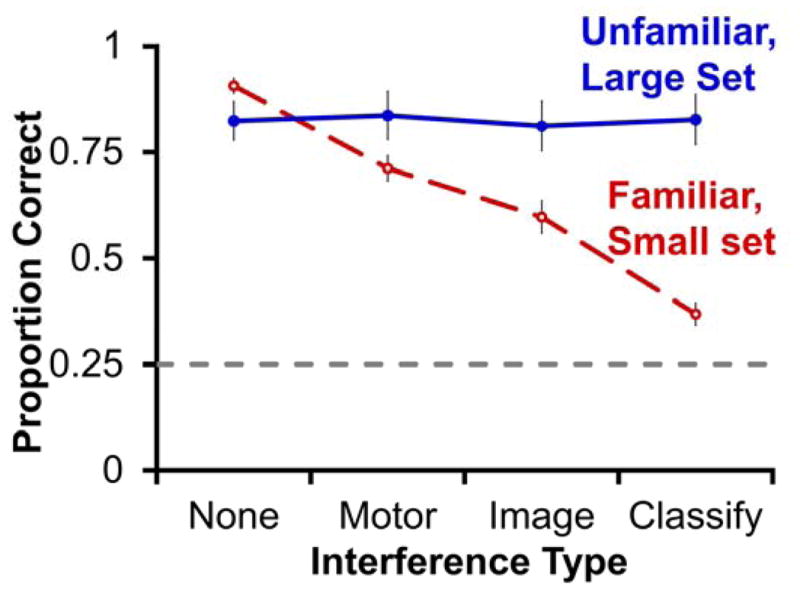
Proportion correct (±SEM) on the final recognition test is graphed for both the familiar small image set (red dashed line) and the unfamiliar large image set (solid blue line) as a function of the four levels of concurrent cognitive demand imposed during the memory interval. The gray horizontal dashed line represents the proportion correct expected by chance.
3. Experiments 2a–2c: Alternative explanations
The results of Experiment 1 suggest that the concurrent tasks impaired performance because holding familiar, but not unfamiliar, images in memory required limited cognitive resources, and the concurrent tasks competed for those resources. Prior to accepting this interpretation, we investigated four alternative explanations. In Experiment 2a, we evaluated whether the decrement occurred because completing the concurrent task lengthened the memory interval. In Experiment 2b, we evaluated whether the decrement was due to the concurrent task occurring immediately after study in the familiar image condition, rather than after a relatively long interval in the unfamiliar image condition. In Experiment 2c, we evaluated whether the decrement occurred only when to-be-remembered samples were classifiable, and also whether the selective decrement was due to the two image sets being tested at different memory delays.
3.1 Methods
All subjects and apparatus were the same as in Experiment 1. All stimuli were the same as in Experiment 1, with the addition of a set of four non-classifiable photographs, already highly familiar from previous testing. For all three sub-experiments, half the trials contained no concurrent task and the other half contained the classification task, which was the most debilitating concurrent task. All other methods were the same as in Experiment 1 unless noted.
3.1.1 Experiment 2a: Lengthened memory interval
We ran one 100-trial session using the familiar set of images with a memory interval of 4 seconds. If the decrement in accuracy was due to the extension of the memory interval by the time taken to complete the secondary task (lengthened from 1 second to 2.5 seconds in the case of the classification task), then an unfilled 4-second interval should produce a decrement of similar or greater magnitude.
3.1.2 Experiment 2b: Timing of concurrent task
We ran two 100-trial sessions using the unfamiliar image set, one with the concurrent task at the end of the 30-second delay and one with the concurrent task at the start of the 30-second delay. If the decrement in accuracy with the small set of familiar items was due to the secondary task following quickly after the sample, then moving the secondary task to the beginning of the delay with the large set of unfamiliar items should produce a similar decrement to that found with the small image set.
3.1.3 Experiment 2c: Image content and constant memory interval
We ran two 100-trial sessions at a consistent memory interval of 4 seconds. The to-be- remembered images for the two sessions were the relatively unfamiliar set of 400 non- classifiable images used in the image condition of Experiment 1 and the set of 4 highly-familiar non-classifiable images, respectively. If the classification task produced a large decrement because the samples were classifiable, then using samples that the monkeys were unable to classify should eliminate the effect. Additionally, if the difference between the two sets was due to them being tested at different memory delays, then testing them at the same memory delay should eliminate the effect.
3.2 Results and Discussion
3.2.1 Experiment 2a: Lengthened memory interval
The performance impairment seen with the familiar images in Experiment 1 was not due to elongation of the memory interval by the addition of time spent completing the concurrent tasks. On average, the concurrent tasks increased the memory interval of the familiar images from 1s to 2.5s in Experiment 1; however, memory performance following the unfilled 4s delay in Experiment 2a was significantly higher than the filled 2.5s delay from the Experiment 1 (mean proportion correct at 4s = .79, t5 = 8.47, p < .001, d = 3.46).
3.2.2 Experiment 2b: Timing of concurrent task
The lack of performance impairment with the unfamiliar images in Experiment 1 was not due to the concurrent task following more quickly after the study phase with the familiar images than with the unfamiliar images in Experiment 1. In Experiment 2b, memory performance with the unfamiliar images was equivalent when the classification task occurred at the end and at the beginning of the 30s delay (mean proportion correct: end of delay = 0.65, beginning of delay = 0.64; t5 = 0.39, p = .709).
3.2.3 Experiment 2c: Image content and constant memory interval
The selective performance impairment seen in Experiment 1 was not due to the to-be-remembered images being classifiable by the monkeys. With non-classifiable images, we again observed selective impairment for the familiar stimuli but not the unfamiliar stimuli (non-classifiable familiar images mean proportion correct: none = .86, concurrent classification task = .51, t5 = 13.08, p < .001, d = 5.34; non-classifiable unfamiliar images mean proportion correct: none = .95, concurrent classification task = .90, t5 = 1.45, p = .208). Nor were the impairments in performance due to the two sets being tested at different memory delays in Experiment 1. The selective impairment seen with the non-classifiable image sets in Experiment 2c was observed at a constant 4s memory delay. Together, Experiments 2a–2c indicate that the concurrent tasks in Experiment 1 impaired performance for familiar images because they imposed different levels of concurrent cognitive demand.
4. General Discussion
Concurrent cognitive demands during the memory delay impaired performance for familiar, but not unfamiliar, images in a demand-dependent manner. This indicates that remembering familiar information is cognitively effortful for monkeys. This establishes a strong parallel with human working memory. It also raises the intriguing possibility that monkeys hold familiar images in working memory via an effortful maintenance process akin to human rehearsal (but see Washburn & Astur, 1998). Primacy, or superior memory for items appearing early in a list, is often due to rehearsal in humans (Marshall & Werder, 1972). We recently found that memory performance for lists of familiar, but not unfamiliar, images showed a primacy effect in monkeys (Basile & Hampton, 2010), again suggesting a rehearsal-like process for familiar information (but see Cook et al., 1991). This difference between processing of familiar and unfamiliar memoranda parallels fMRI results from humans showing that the prefrontal cortex is more active when remembering familiar images (Stern et al., 2001).
The discrepancy of these results, which provide evidence of active maintenance of monkey memory, with previous results, which found no evidence of active maintenance (Cook et al., 1991; Washburn & Astur, 1998), may be due to the relatively high familiarity of the images being remembered. In these previous studies, samples were either drawn from a medium-sized set of 32 photographs (Cook et al., 1991), or an unbounded set of algorithmically-generated grid patterns (Washburn & Astur, 1998). Because target stimuli did not repeat every trial, it is possible that they could be discriminated from distractors at test on the basis of familiarity, and thus monkeys could perform accurately without needing to maintain them in working memory. Although set size appears to be a likely factor considering the current findings, there are too many differences between the current study and the previous ones to draw a firm conclusion without additional experiments.
It is a challenge to select appropriate language to accurately describe cognitive processes in nonhumans. Passive familiarity describes well the immunity to interference we saw in recognition of targets from the large set of unfamiliar images. With the large set, the target had been seen much more recently than the distractors and thus was presumably more familiar, memory for the target was unaffected by concurrent cognitive demands and thus primarily passive, and studies in humans have shown that familiarity judgments are primarily passive (Jacoby, 1991; Yonelinas, 2002). However, one could also describe this as a recency judgment or as a novelty judgment. We cannot distinguish between these descriptions in the current study, and it is not immediately clear whether these are different ways of describing the same type of judgment or different types of judgments. Future studies may help illuminate these distinctions.
Our results indicate that future studies of working memory in nonhumans should contrast performance with familiar and unfamiliar images. Because of the relative ease with which monkeys learn memory tasks with large sets of unfamiliar images (Mishkin & Delacour, 1975), large sets have become the standard in primate memory research; however, the present results show that large and small sets are remembered differently. Failure to recognize this difference may have created the perception of inconsistencies between the nonhuman and human literatures, in which humans are often tested with familiar items and monkeys are often tested with relatively unfamiliar items (Stern et al., 2001). Secondary tasks that manipulate concurrent cognitive load can be used to identify instances of active working memory (Jeneson & Squire, 2012) and may help resolve these apparent inconsistencies. Neurophysiological studies of working memory that contrast performance with large and small stimulus sets (Eacott, Gaffan, & Murray, 1994) or that use other methods to contrast passive familiarity and active maintenance (Miller et al., 1996) will prove especially informative.
Humans often maintain information in working memory through verbal rehearsal, but our results with monkeys indicate that active memory maintenance does not require language. There is evidence that humans engage in nonverbal memory maintenance (Hourihan et al., 2009), but it is difficult to block the human tendency to name visual stimuli, and recoding unfamiliar visual stimuli into familiar words does facilitate memory (Wright et al., 1990). Based on these findings and ours, one intriguing possibility is that the capacity for active control of memory may have more to do with familiarity than with other properties of linguistic material. The ease with which humans recode unfamiliar memoranda into familiar words, an option unavailable to monkeys, may be one of the reasons that cognitive control over memory is more robust in humans than it is in monkeys.
Acknowledgments
We thank Emily K. Brown and Steven L. Sherrin for help running subjects. This work was supported by the National Science Foundation (grant 0745573), the National Center for Research Resources P51RR000165, the Office of Research Infrastructure Programs/OD P51OD011132, the Center for Behavioral Neuroscience under the Science and Technology Center Program of the National Science Foundation (under agreement IBN-9876754), and the National Institute of Mental Health (grant R01M H082819).
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron A, Aron E. Statistics for psychology. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust primacy and recency in memory for lists from small, but not large, image sets. Behavioural Processes. 2010;83(2):183–190. doi: 10.1016/j.beproc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Monkeys show recognition without priming in a classification task. Behavioural Processes. doi: 10.1016/j.beproc.2012.08.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby JA, Leitz JR, Morgan CJA, Curran HV. Decreases in recollective experience following acute alcohol: a dose-response study. Psychopharmacology. 2010;208(1):67–74. doi: 10.1007/s00213-009-1709-y. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nature Neuroscience. 2001;4(3):311–316. doi: 10.1038/85179. [DOI] [PubMed] [Google Scholar]
- Cook RG, Wright AA, Sands SF. Interstimulus-Interval and Viewing Time Effects in Monkey List Memory. Animal Learning & Behavior. 1991;19(2):153–163. [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S, editors. Essence of Memory. Vol. 169. Amsterdam: Elsevier Science Bv; 2008. pp. 323–338. [Google Scholar]
- D’esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved Recognition Memory for Small Sets, and Impaired Stimulus Identification for Large Sets, Following Rhinal Cortex Ablations in Monkeys. European Journal of Neuroscience. 1994;6(9):1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Elmore LC, Ma WJ, Magnotti JF, Leising KJ, Passaro AD, Katz JS, Wright AA. Visual Short-Term Memory Compared in Rhesus Monkeys and Humans. Current Biology. 2011;21(11):975–979. doi: 10.1016/j.cub.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron Activity Related to Short-term Memory. Science. 1971;173(3997):652. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26(32):8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Neonatal Hippocampal Lesions in Rhesus Macaques Alter the Monitoring, but Not Maintenance, of Information in Working Memory. Behavioral Neuroscience. 2011;125(6):859–870. doi: 10.1037/a0025541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyselaar E, Johnston K, Pare M. A change detection approach to study visual working memory of the macaque monkey. Journal of Vision. 2011;11(3) doi: 10.1167/11.3.11. [DOI] [PubMed] [Google Scholar]
- Hourihan KL, Ozubko JD, Macleod CM. Directed forgetting of visual symbols: Evidence for nonverbal selective rehearsal. Memory & Cognition. 2009;37(8):1059–1068. doi: 10.3758/MC.37.8.1059. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A Process Dissociation Framework - Separating Automatic from Intentional Uses of Memory. Journal of Memory and Language. 1991;30(5):513–541. [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO, Squire LR. The role of the hippocampus in retaining relational information across short delays: The importance of memory load. Learning & Memory. 2011;18(5):301–305. doi: 10.1101/lm.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learning & Memory. 2012;19(1):15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie RH. Visuospatial Processing in Working Memory. Quarterly Journal of Experimental Psychology Section a-Human Experimental Psychology. 1986;38(2):229–247. doi: 10.1080/14640748608401596. [DOI] [PubMed] [Google Scholar]
- Marshall PH, Werder PR. Effects of Elimination of Rehearsal on Primacy and Recency. Journal of Verbal Learning and Verbal Behavior. 1972;11(5):649. [Google Scholar]
- Meyer T, Qi XL, Constantinidis C. Persistent discharges in the prefrontal cortex of monkeys naive to working memory tasks. Cerebral Cortex. 2007;17:I70–I76. doi: 10.1093/cercor/bhm063. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Scopolamine Affects Short-Term-Memory but not Inferior Temporal Neurons. Neuroreport. 1993;4(1):81–84. doi: 10.1097/00001756-199301000-00021. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Memory and the medial temporal regions of the brain. In: Pribram KH, Broadbent DE, editors. Biology of Memory. New York: Academic Press; 1970. pp. 29–50. [Google Scholar]
- Mishkin M, Delacour J. Analysis of Short-term Visual Memory in Monkeys. Journal of Experimental Psychology-Animal Behavior Processes. 1975;1(4):326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Christie DFM. Interference with Visualization. Quarterly Journal of Experimental Psychology. 1977;29(V):637–650. doi: 10.1080/14640747708400638. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Jackson WJ, Terry AV, Kille NJ, Arneric SP, Decker MW, Buccafusco JJ. Age-related differences in distractibility and response to methylphenidate in monkeys. Cerebral Cortex. 1998;8(2):164–172. doi: 10.1093/cercor/8.2.164. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Cognition, Evolution, and Behavior. New York: Oxford University Press; 1998. [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11(4):337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Uttal WR. The new phrenology: the limits of localizing cognitive processes in the brain. Cambridge, Mass: MIT Press; 2001. [Google Scholar]
- Washburn DA, Astur RS. Nonverbal working memory of humans and monkeys: Rehearsal in the sketchpad? Memory & Cognition. 1998;26(2):277–286. doi: 10.3758/bf03201139. [DOI] [PubMed] [Google Scholar]
- Wright AA, Cook RG, Rivera JJ, Shyan MR, Neiworth JJ, Jitsumori M. Naming, Rehearsal, and Interstimulus-Interval Effects in Memory Processing. Journal of Experimental Psychology-Learning Memory and Cognition. 1990;16(6):1043–1059. doi: 10.1037//0278-7393.16.6.1043. [DOI] [PubMed] [Google Scholar]
- Wynn T, Coolidge FL. The expert neandertal mind. Journal of Human Evolution. 2004;46(4):467–487. doi: 10.1016/j.jhevol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. Dissociations of processes in recognition memory - Effects of interference and of response speed. Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale. 1994;48(4):516–535. doi: 10.1037/1196-1961.48.4.516. [DOI] [PubMed] [Google Scholar]